Abstract
Thermal barrier coatings (TBCs) are essential for protecting the high-temperature components in gas turbines. However, the durability of TBCs is limited, and new technologies to improve their lifetime are necessary. This study focuses on the pre-oxidation treatment of the NiCoCrAlY bond coat in TBCs to reduce the growth rate of the thermally grown oxide (TGO) and improve the TBC lifetime. The mechanism of the TGO growth rate reduction by the pre-oxidation treatment of NiCoCrAlY was investigated. Oxidation behaviors of the surface of NiCoCrAlY coating samples with or without pre-oxidation treatment were evaluated in air at 800 °C and 900 °C. In-situ X-ray diffraction (XRD) measurements and isothermal oxidation tests were performed. The obtained results revealed that the growth rate of TGO is significantly suppressed by α-Al2O3 layer formed by the pre-oxidation treatment. The generation of γ-Al2O3 and θ-Al2O3, which are typically formed below 900 °C, was significantly reduced by pre-oxidation. It is suggested that this reduction suppresses of the TGO growth.
Similar content being viewed by others
Introduction
Recently, there has been a growing demand to increase the efficiency of industrial gas turbines to reduce carbon emissions. A higher turbine inlet temperature (TIT) is required to improve the gas turbine efficiency. The high-temperature components of gas turbines, such as combustors, turbine blades, and turbine nozzles, are directly exposed to high-temperature combustion gases. Therefore, these components require excellent high-temperature strength and oxidation resistance. Ni-based superalloys are mainly used in turbine blades and nozzles. In recent years, directionally solidified alloys and single-crystal alloys with excellent high-temperature creep strengths have become commonly used [1]. However, even these alloys cannot completely satisfy the demand for increasing the TIT, and the application of thermal barrier coatings (TBCs) on superalloys to reduce component temperatures is becoming indispensable. Generally, TBCs consist of two layers: a bond coat and top coat [2]. The bond coat is applied to improve the oxidation resistance and adhesion to the top coat. MCrAlY (M = Ni, Co, or both) alloys are normally used as bond coats and are generally sprayed using low-pressure plasma spray (LPPS) or high-velocity oxy-fuel (HVOF) processes. The top coat provides thermal insulation from the combustion gas. The top coat is usually made of yttria-stabilized zirconia (YSZ) and sprayed with atmospheric plasma spray (APS).
The use of TBCs has enabled increasing the TIT by reducing the heat input from the combustion gas to the component. However, for even higher TIT values, existing TBCs are not sufficiently durable. Thus, technologies that improve the durability of TBCs are required.
Many mechanisms have been proposed to explain the damage caused to TBCs. Examples include erosion, foreign object damage (FOD), phase transformation of the YSZ used as the top coat, and infiltration of calcium-magnesium aluminosilicates (CMAS) [3, 4]. Generally, spallation of the top coat limits the lifetime of TBCs. A thermally grown oxide (TGO) is formed at the interface between the top coat and the bond coat owing to the high-temperature oxidation of the bond coat. The TGO has a lower thermal expansion coefficient than the top and bond coats. This mismatch in thermal expansion generates thermal stress in the TGO and top coat, which leads to cracks in the TGO and top coat, eventually causing the spallation of the top coat [5]. Therefore, it is essential to suppress the TGO growth rate to improve the durability of TBCs [6, 7].
Various methods have been proposed to reduce the TGO growth rate [8,9,10,11,12,13,14,15,16,17,18,19,20]. Addition of elements such as Pt and Re to the bond coat improves the oxidation resistance [8,9,10]. Another approach is applying α-Al2O3 diffusion barrier layer on the bond coat surface by dry coating processes (such as chemical vapor deposition (CVD) or sputtering) [11, 12]. In this method, the α-Al2O3 layer acts as a diffusion barrier for the outward diffusion of Al from the bond coat to the surface. This method inhibits further oxide growth and suppresses the TGO growth rate. However, the high cost of α-Al2O3 layer deposition by dry coating remains a significant challenge associated with the use of this method in industrial applications.
A method of forming the α-Al2O3 barrier layer by pre-oxidation treatment of the bond coat has been proposed [10, 13,14,15,16,17,18,19,20]. The pre-oxidation process is a much simpler approach to form α-Al2O3 barrier layer compared to dry coating processes such as CVD or spattering.
In the pre-oxidation treatment, heat treatment is conducted before the actual use of the TBC. It is typically conducted in low oxygen partial pressure conditions (in inert gas or vacuum) at approximately 1100 ℃ for a few hours. Previous studies have demonstrated that pre-oxidation effectively reduces the TGO growth rate. Matsumoto et al. reported that pre-oxidation treatment reduced the TGO thickness to less than half after 50 h oxidation in air at 1200 ℃ [15].
The previous studies of current authors focused on the pre-oxidation method and investigated the suitable conditions (temperature, oxygen partial pressure, etc.) of pre-oxidation treatment to form the α-Al2O3 layer [21, 22]. An isothermal oxidation test was conducted at 1050 ℃, and the results showed that the pre-oxidation approach reduced the TGO thickness to approximately two-thirds of that observed in TBC without pre-oxidation.
Regarding Al2O3-former alloys, which include MCrAlY used for the bond coat of TBCs, it has been pointed out that the crystal structure of Al2O3 formed during high-temperature oxidation is dependent on the oxidation temperature. Generally, γ-Al2O3, θ-Al2O3 (transition phase) and α-Al2O3 (stable phase) are formed [23]. θ-Al2O3 or α-Al2O3 is formed above 1000 °C, and θ-Al2O3 transforms to α-Al2O3 at an early stage of oxidation. On the other hand, γ- and θ-Al2O3 are formed and these transition phases last longer at temperatures below 900 ℃ [24]. Importantly, γ- and θ-Al2O3 grow much more rapidly than α-Al2O3 [25] and might lead to spallation of the top coat.
If the α-Al2O3 layer is formed on the MCrAlY surface by pre-oxidation before high-temperature oxidation, it can be expected that the formation of γ- and θ-Al2O3 at temperatures below 900 °C will be suppressed. This would significantly reduce the scale growth rate. It should be noted that many gas turbine components are operated at temperatures near or below 900 ℃. Therefore, evaluating oxidation behavior in this temperature range is indispensable. However, most previous studies on the TGO reduction effect of α-Al2O3 pre-oxidation layer have been evaluated by oxidation tests at temperatures above 1000 °C. The TGO suppression effect of the α-Al2O3 layer at temperatures below 900 °C, at which γ- and θ-Al2O3 are the main phases of TGO, has not been sufficiently investigated.
In this study, the oxidation behaviors of NiCoCrAlY, which is used as a bond coat of TBCs, were investigated at 800 ℃ and 900 °C to evaluate the effect of TGO suppression by pre-oxidation. Specifically, we prepared NiCoCrAlY bond coat samples with the α-Al2O3 layer formed by pre-oxidation treatment, and their oxidation behavior in air was compared with the samples without an α-Al2O3 layer. X-ray diffraction (XRD) experiments using a synchrotron radiation facility were also conducted to evaluate the initial oxidation behavior because the initial oxide layer at low temperatures is expected to be very thin, and synchrotron radiation X-rays help identify thin layers [26].
Experimental Procedures
Preparation of Samples
A Ni-based superalloy (CMSX-4) was used as the substrate. NiCoCrAlY (Ni-23Co-17Cr-13Al-0.45Y, mass %) was sprayed onto the substrate using the HVOF method. After NiCoCrAlY deposition, the samples were cut into predetermined sizes (for in-situ XRD: 15 × 10 × 1.5 mm3, for the isothermal oxidation test: 10 × 20 × 5 mm3). The sample surfaces were mirror polished, and the thickness of NiCoCrAlY layer after polishing was approximately 100 μm. Pre-oxidation treatment was performed on this sample under the conditions described in the previous report [21] to form an α-Al2O3 layer on the NiCoCrAlY surface. The conditions of pre-oxidation were 1080 °C for 4 h under vacuum (oxygen partial pressure is approximately 10–14 Pa). The samples subjected to this pre-oxidation treatment were labeled as “with pre-oxidation.” The samples without the pre-oxidation treatment, which only had their surfaces mirror-polished, were labeled as “without pre-oxidation” for comparison.
Evaluation of Initial Oxidation Behavior Using In-situ XRD Measurements
The oxidation behavior during heating the samples to the peak temperature and maintaining that state for a short time (~ 1 h) was evaluated using in-situ XRD measurements. The measurements were carried out at beamline BL16XU, SPring-8. 10 keV X-rays with a wavelength of 1.24 Å were used. The samples were then placed on a heating stage (Anton Paar DHS1100). Dry air was used as the atmosphere in the sample chamber. The sample was gradually heated at a rate of 10 °C/min till it reached 800 °C or 900 °C, where the temperature was maintained for 1 h. To detect the thin oxide layer on the sample surface, XRD measurements were performed at a small X-ray incident angle of 0.5°, and the diffraction (2θ) angle was fixed at 1.0°. X-ray diffraction to in-plane direction was measured by scanning the 2θH axis. The XRD profiles were obtained at intervals of 7.5 min.
Isothermal Oxidation Test
Isothermal oxidation tests were conducted in an electric furnace to investigate the oxidation behavior during long-term oxidation, which is difficult to evaluate using in-situ XRD measurements. In this test, the samples were heated at a heating rate of 10 °C/min until they reached the test temperatures (800 °C or 900 °C), where they were soaked for different testing times of 1, 10, and 100 h. Then, they were cooled down to room temperature in the furnace. This test was conducted in normal laboratory air atmosphere.
After the oxidation tests, the samples were evaluated using an XRD instrument (SmartLab, Rigaku), and cross-sectional observations were made using a scanning electron microscope (SEM, SU5000, Hitachi). The grazing incidence XRD (GIXRD) method was applied to evaluate the thin oxide film on the surface of the samples to obtain the XRD patterns. For SEM observations, cross-sectional samples were prepared as follows: First, an Au–Pd layer was sputtered onto the sample surface. Then, a protective layer was formed by Ni plating. Subsequently, the cross sections of the samples were polished by ion milling.
The TGO thickness was measured from cross-sectional SEM images using the following method: Two 25 μm-wide SEM images were taken for each oxidation condition, and then a TGO thickness of 5 points with equal spacing was measured from each image. As described in results and discussion, localized formation of needle-like oxides was found in some conditions. The areas where needle-like oxides exist were excluded from the measurement points of TGO because accurately evaluation of TGO thickness was difficult. For each oxidation condition, the TGO thickness was determined by averaging the thickness values obtained at ten observed points.
SEM electron backscatter diffraction (EBSD; GS 460; Carl Zeiss and Symmetry S2; Oxford Instruments) measurements were conducted to evaluate the distribution of each Al2O3 crystalline phase in the TGO after the isothermal oxidation test. A pattern-matching method was used for analysis.
The experimental conditions for in-situ XRD measurement and isothermal oxidation test are summarized in Table 1.
Results and Discussion
Evaluation of α-Al2O3 Layer After Pre-Oxidation Treatment
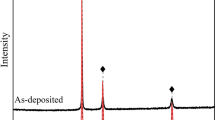
Figure 1 shows a cross-sectional SEM image of NiCoCrAlY after pre-oxidation treatment. A continuous oxide layer of approximately 0.3 μm thickness was formed on the NiCoCrAlY surface. Figure 2 shows the GIXRD pattern of the NiCoCrAlY surface after pre-oxidation treatment, and only α-Al2O3 was detected as the oxide. This XRD results that the oxide layer in was only α-Al2O3. Furthermore, this observation is the same as the results reported in our previous studies [21, 22].
In-situ XRD Analysis During Oxidation in Air
Figure 3a shows the XRD patterns of the oxidation behavior of the NiCoCrAlY surface without pre-oxidation during the heating the samples to 800 °C and maintaining them at 800 °C. γ-Al2O3 peaks appeared around 800 ℃ during temperature rising. The intensity of the γ-Al2O3 peaks increased gradually at 800 ℃, and no peaks of other oxides appeared. Figure 3b shows the XRD patterns of the oxidation behavior of the NiCoCrAlY surface without pre-oxidation during heating the samples to 900 °C and maintaining them at 900 °C. Its oxidation behavior was very similar to that at 800 °C, γ-Al2O3 peak appeared at around 800 °C, and no peaks corresponding to other oxides appeared.
Figure 4 shows the results of the in-situ XRD measurements of NiCoCrAlY with pre-oxidation. Only α-Al2O3 was detected as oxide during heating up to and maintaining at the peak temperature. Increases in α-Al2O3 peak intensity were also observed during oxidation test. These results indicate that the α-Al2O3 layer, formed during pre-oxidation, grew at 800 ℃ and 900 ℃, instead of forming γ-Al2O3.
Isothermal Oxidation Test
Figure 5 shows the XRD patterns of NiCoCrAlY without pre-oxidation after isothermal oxidation tests at 800 °C for 1, 10, and 100 h. Only γ-Al2O3 was detected as oxide on the NiCoCrAlY surface after 10 h (Fig. 5a), but no oxide was detected after 1 h. The formation of γ-Al2O3 was detected after 1 h by in-situ synchrotron XRD (Fig. 3a). This is because the detection limit of the laboratory XRD was higher than that of synchrotron XRD. As described later, the thickness of TGO formed at 800 °C for 1 h was less than 100 nm. In the isothermal oxidation test at 900 °C for 1 h, only γ-Al2O3 was detected, but θ-Al2O3 was detected at oxidation times of 10 h and 100 h (Fig. 5b). This might have occurred because the γ → θ phase transformation of Al2O3 happens between 1 and 10 h [27].
Figure 6 shows the XRD patterns of NiCoCrAlY with pre-oxidation after the isothermal oxidation tests. At 800 ℃, only α-Al2O3 was detected as oxide phase for all test times of 1, 10, and 100 h (Fig. 6a). γ-Al2O3, which was formed on the surface of NiCoCrAlY without pre-oxidation after the isothermal oxidation test at 800 ℃, was not detected. Similar to the results obtained at 800 °C, at 900 °C, only α-Al2O3 was detected as oxide phase for 1 and 10 h. However, θ-Al2O3 was also detected along with α-Al2O3 on the NiCoCrAlY after the isothermal oxidation test for 100 h (Fig. 6b).
Figures 7 and 8 show the cross-sectional SEM images of NiCoCrAlY without and with pre-oxidation treatment, respectively, after the isothermal oxidation test. The Al2O3 phases shown in the figures indicate the results of the XRD measurements as shown in Figs. 6 and 7. After the isothermal oxidation tests at 800 °C, a continuous TGO layer was formed on the NiCoCrAlY surface without pre-oxidation (Fig. 7a–c). The thickness of the TGO layer is uniform and increases over time. After 100 h of oxidation, the thickness of the TGO layer was approximately 0.3 μm.
On the other hand, during isothermal oxidation at 800 ℃, NiCoCrAlY surface with pre-oxidation showed negligible increase in the TGO thickness over time. (Figs. 8a–c) The TGO thickness was approximately 0.3 μm and remained nearly constant during the isothermal oxidation test. In other words, the TGO thickness was approximately the same as that of α-Al2O3 layer formed by pre-oxidation treatment (Fig. 1).
As for the isothermal oxidation tests at 900 °C, the thickness of the TGO increased with time for both NiCoCrAlY without and with pre-oxidation (Figs. 7d–f, 8d–f). However, the growth rate of the TGO on NiCoCrAlY with pre-oxidation was lower than on that without pre-oxidation. The thickness of the TGO layer after 100 h was clearly smaller for NiCoCrAlY with pre-oxidation than for NiCoCrAlY without pre-oxidation. In other words, the pre-oxidation treatment suppressed the growth of TGO.
Additionally, needle-like oxides were partially formed on the NiCoCrAlY with pre-oxidation after the isothermal oxidation tests at 900 °C for 10 and 100 h (Fig. 8e, f). The low-magnification SEM images of Fig. 8d–f are shown in Fig. 9a–c, respectively. These needle-like oxides were formed locally, not on the entire surface of the sample, and were generally spaced at intervals of 1 ~ 5 μm.
The XRD results indicated that the TGO of the NiCoCrAlY with pre-oxidation after the isothermal oxidation at 900 °C for 100 h (Fig. 8f) contained both α-Al2O3 and θ-Al2O3. It was reported that θ-Al2O3 grew as needle-like oxide during high-temperature oxidation of Al2O3-former alloys [27]. Thus, the needle-like oxides indicated in Fig. 8f are considered to be θ-Al2O3. Additionally, EBSD analysis was performed to accurately evaluate the distribution of α-Al2O3 and θ-Al2O3 in the TGO. The obtained results are shown in Fig. 10. The continuous oxide layer, which was approximately 1 μm thick, was identified as α-Al2O3, and the needle-like oxides formed above the α-Al2O3 layer were identified as θ-Al2O3.
The crystal structure of Al2O3 formed by high-temperature oxidation of Al2O3-former alloys changes depending on the temperature, time, and alloy composition [24, 25]. Generally, γ- and θ-Al2O3 are formed in a relatively low-temperature range or as an initial oxidation phase.
Our experiment for the NiCoCrAlY without pre-oxidation revealed that the γ- or θ-Al2O3, which are transition phases of Al2O3, formed on the NiCoCrAlY at both 800 ℃ and 900 ℃ up to 100 h, and no α-Al2O3, which is the only stable phase of Al2O3, was formed. Brumm et al. reported that they conducted the oxidation test for NiAl-15Cr alloy, and θ → α transformation had not occurred under 900 ℃ [25]. Although their alloy compositions differed slightly from ours, the results are comparable.
In contrast, α-Al2O3 was observed to be the dominant oxide species in the NiCoCrAlY with pre-oxidation in our isothermal oxidation tests. Figure 11a shows the TGO thickness during the isothermal oxidation test in ambient air at 900 ℃. It should be noted that needle-like oxides on the NiCoCrAlY with pre-oxidation was excluded from the measurement of the TGO thickness. The growth rate of the TGO was significantly lower in NiCoCrAlY with pre-oxidation than in NiCoCrAlY without pre-oxidation. The TGO thickness is plotted against 1/2 power of the oxidation time in Fig. 11b. The TGO thickness is proportional to 1/2 power of the oxidation time, indicating that TGO growth follows a parabolic row. The TGO growth rate constant k in Eq. (1) was calculated to be approximately 0.17 μm/h1/2 for the NiCoCrAlY without pre-oxidation and 0.03 μm/h1/2 for the NiCoCrAlY with pre-oxidation.
where x denotes TGO thickness in μm and t denotes exposure time in h.
The difference in the growth rate of the TGO with and without pre-oxidation can be attributed to the difference in the crystal structure of Al2O3 grown during the isothermal oxidation tests. It is well known that the growth rate of γ- and θ-Al2O3 formed on the Al2O3-former alloy is more than two orders higher than that of α-Al2O3 [25]. Because γ- and θ-Al2O3 are looser packing than the α-Al2O3 structure [28], and γ- or θ-Al2O3 grow as needle-like oxides [27], the diffusion barrier effect of γ- and θ-Al2O3 should be lower than that of α-Al2O3. In the NiCoCrAlY with pre-oxidation, α-Al2O3 which first formed by pre-oxidation treatment seemed to continue growing during the isothermal oxidation test. As shown in Fig. 10b, the α-Al2O3 layer consisted of a single layer after isothermal oxidation, and the grain size of α-Al2O3 is almost equal to the TGO thickness. This is evidence that the α-Al2O3 layer formed by pre-oxidation process continued to grow during the isothermal oxidation test.
However, during the isothermal oxidation of the NiCoCrAlY with pre-oxidation at 900 °C, formation of θ-Al2O3 was observed. This might be due to the morphology of α-Al2O3 layer after the pre-oxidation treatment. As shown in Fig. 1, the α-Al2O3 layer was mostly uniform; however, there were some areas where the α-Al2O3 thickness was locally thin, which can be called the defects. It is thought that these defect areas showed oxidation behavior similar to that in the NiCoCrAlY without pre-oxidation, leading to the formation of θ-Al2O3.
It should be noted that no γ- or θ-Al2O3 were detected in the in-situ XRD test (Fig. 4b) and in the XRD measurements after the isothermal oxidation tests at 1 and 10 h (Fig. 6b); however, this is because the amount of transient Al2O3 (including γ- and θ-Al2O3) was below the detection limit. Small amounts of γ- or θ-Al2O3 can exist before 100 h.
Nevertheless, the growth of θ-Al2O3 was not expected to proceed after 100 h. As shown in Fig. 10b, α-Al2O3 had already formed below θ-Al2O3 at 100 h, and a continuous α-Al2O3 layer was completely formed. This might be the result of the growth of α-Al2O3 near the defective regions in α-Al2O3 layer formed during pre-oxidation. The continuous α-Al2O3 layer should prevent further growth of θ-Al2O3.
As understood from the comparison of Figs. 7f and 8f, the TGO can be significantly reduced by the current pre-oxidation treatment. The results of this research suggest that the TGO growth rate up to 100 h can be further reduced if the uniformity of the α-Al2O3 layer formed by pre-oxidation treatment is improved and the defects are eliminated. The development of a process to form a uniform α-Al2O3 layer will be studied in future.
Conclusions
To reduce the growth rate of the TGO in TBCs, we focused on pre-oxidation treatment of the NiCoCrAlY bond coat and evaluated the effects of pre-oxidation on the oxidation behavior of NiCoCrAlY at 800 °C and 900 °C. Pre-oxidation treatment was conducted in low oxygen partial pressure condition for the NiCoCrAlY, and α-Al2O3 layer was successfully formed on the NiCoCrAlY surface.
The results of in-situ XRD measurement tests and isothermal oxidation tests revealed that the formation of α-Al2O3 on the NiCoCrAlY surface by pre-oxidation treatment reduced the growth rate of TGO at 800 °C and 900 °C. γ- and θ-Al2O3 were the main phases of the TGO formed on the NiCoCrAlY without pre-oxidation during isothermal oxidation until the oxidation time reached 100 h. Whereas, on the NiCoCrAlY with pre-oxidation, α-Al2O3 was main phase of the TGO. The α-Al2O3 layer formed by pre-oxidation prevents the formation of γ and θ-Al2O3, which have high scale growth rates. Thus, a reduction in the TGO growth rate was achieved.
Data availability
No datasets were generated or analysed during the current study.
References
M. Hetmańczyk, L. Swadźba, and B. Mendala, Journal of achievements in Materials and manufacturing Engineering 24, 372 (2007).
J. Nicholls, MRS Bulletin 28, 659 (2003).
C. G. Levi, J. W. Hutchinson, M. H. Vidal-Sétif, et al., MRS Bulletin 37, 932 (2012).
R. Darolia, International Materials Reviews 58, 315 (2013).
N. P. Padture, M. Gell, and E. H. Jordan, Science 296, 280 (2002).
J. T. Demasimarcin, K. D. Sheffler, and S. Bose, Journal of Engineering for Gas Turbines and Power 112, 521 (1990).
A. M. Freborg, B. L. Ferguson, W. J. Brindley, et al., Materials Science and Engineering A 245, 182 (1998).
H. Lan, P. Y. Hou, Z. G. Yang, Y. D. Zhang, et al., Oxidation of Metals 75, 77 (2011).
S. Salam, P. Y. Hou, Y. D. Zhang, et al., Corrosion Science 95, 143 (2015).
A. Vande Put, D. Oquab, E. Péré, A. Raffaitin, et al., Oxidation of Metals 75, 247 (2011).
K. G. Schmitt-Thomas and U. Dietl, Surface and Coatings Technology 68, 113 (1994).
J. H. Sun, E. Chang, B. C. Wu, et al., Surface and Coatings Technology 58, 93 (1993).
S. Takahashi, M. Yoshiba, and Y. Harada, Materials Transactions 44, 1181 (2003).
S. Takahashi, M. Yoshiba, and Y. Harada, Materials Science Forum 461–464, 367 (2004).
M. Matsumoto, K. Hayakawa, S. Kitaoka, et al., Materials Science and Engineering A 441, 119 (2006).
S. Kitaoka, T. Kuroyama, M. Matsumoto, et al., Corrosion Science 52, 429 (2010).
M. Matsumoto, T. Kato, K. Hayakawa, et al., Surface and Coatings Technology 202, 2743 (2008).
T. J. Nijdam and W. G. Sloof, Surface and Coatings Technology 201, 3894 (2006).
W. R. Chen, X. Wu, B. R. Marple, et al., Surface and Coatings Technology 202, 3787 (2008).
V. Tolpygo and D. R. Clarke, Surface and Coatings Technology 200, 1276 (2005).
M. Negami, S. Hibino, A. Kawano, et al., Journal of Engineering for Gas Turbines and Power 140, 082101 (2018).
M. Negami, S. Hibino, A. Kawano, et al., Journal of the Gas Turbine Society of Japan 48, 321 (2020).
J. Doychak and M. Ruhle, Oxidation of Metals 31, 431 (1989).
S. Taniguchi and A. Andoh, Oxidation of Metals 58, 545 (2002).
M. W. Brumm and H. J. Grabke, Corrosion Science 33, 1677 (1992).
S. Hayashi, Y. Takada, S. Yoneda, et al., Oxidation of Metals 86, 151 (2016).
A. Andoh, S. Taniguchi, and T. Shibata, Tetsu-to-Hagane 84, 285 (1998).
J. Y. David, High Temperature Oxidation and Corrosion of Metals, (Elsevier, Amsterdam, 2016).
Acknowledgements
The synchrotron radiation experiments were performed at the BL16XU of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2022A5012, 2022B5012).
Funding
Open Access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
MN: conceptualization, investigation, data curation, writing—original draft. YYM: conceptualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Negami, M., Yamabe-Mitarai, Y. The Oxidation Behaviors of NiCoCrAlY Coatings After Pre-Oxidation Treatment During High-Temperature Oxidation at 800 ℃ and 900 ℃. High Temperature Corrosion of mater. (2024). https://doi.org/10.1007/s11085-024-10221-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11085-024-10221-6