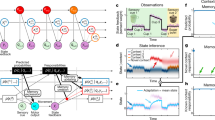
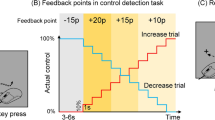
Abstract
Sensorimotor adaptation is essential for keeping our movements well calibrated in response to changes in the body and environment. For over a century, researchers have studied sensorimotor adaptation in laboratory settings that typically involve small sample sizes. While this approach has proved useful for characterizing different learning processes, laboratory studies are not well suited for exploring the myriad of factors that may modulate human performance. Here, using a citizen science website, we collected over 2,000 sessions of data on a visuomotor rotation task. This unique dataset has allowed us to replicate, reconcile and challenge classic findings in the learning and memory literature, as well as discover unappreciated demographic constraints associated with implicit and explicit processes that support sensorimotor adaptation. More generally, this study exemplifies how a large-scale exploratory approach can complement traditional hypothesis-driven laboratory research in advancing sensorimotor neuroscience.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data are available at https://osf.io/5n7jf/.
Code availability
The analysis code is available at https://osf.io/5n7jf/.
References
Krakauer, J., Hadjiosif, A. M., Xu, J., Wong, A. L. & Haith, A. M. Motor learning. Compr. Physiol. 9, 613–663 (2019).
Roemmich, R. T. & Bastian, A. J. Closing the loop: from motor neuroscience to neurorehabilitation. Annu. Rev. Neurosci. 41, 415–429 (2018).
Tsay, J. S. & Winstein, C. J. Five features to look for in early-phase clinical intervention studies. Neurorehabil. Neural Repair 35, 3–9 (2021).
Helmholtz, H. L. F. V. Treatise on Physiological Optics (Dover, 1924).
Stratton, G. M. Some preliminary experiments on vision without inversion of the retinal image. Psychol. Rev. 3, 611–617 (1896).
Ghilardi, M. et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 871, 127–145 (2000).
Krakauer, J., Pine, Z. M., Ghilardi, M. F. & Ghez, C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J. Neurosci. 20, 8916–8924 (2000).
Krakauer, J., Ghez, C. & Ghilardi, M. F. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J. Neurosci. 25, 473–478 (2005).
Ferrea, E., Franke, J., Morel, P. et al. Statistical determinants of visuomotor adaptation along different dimensions during naturalistic 3D reaches. Sci. Rep. 12, 10198 (2022).
Kagerer, F. A., Contreras-Vidal, J. L. & Stelmach, G. E. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp. Brain Res. 115, 557–561 (1997).
Shadmehr, R., Smith, M. A. & Krakauer, J. Error correction, sensory prediction, and adaptation in motor control. Annu. Rev. Neurosci. 33, 89–108 (2010).
Kim, H. E., Avraham, G. & Ivry, R. B. The psychology of reaching: action selection, movement implementation, and sensorimotor learning. Annu. Rev. Psychol. https://doi.org/10.1146/annurev-psych-010419-051053 (2020).
McDougle, S. D., Ivry, R. B. & Taylor, J. A. Taking aim at the cognitive side of learning in sensorimotor adaptation tasks. Trends Cogn. Sci. 20, 535–544 (2016).
Hegele, M. & Heuer, H. Implicit and explicit components of dual adaptation to visuomotor rotations. Conscious. Cogn. 19, 906–917 (2010).
Benson, B. L., Anguera, J. A. & Seidler, R. D. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J. Neurophysiol. 105, 2843–2851 (2011).
Redding, G. M. & Wallace, B. Adaptive spatial alignment and strategic perceptual–motor control. J. Exp. Psychol. Hum. Percept. Perform. 22, 379–394 (1996).
Tsay, J. S. et al. Strategic processes in sensorimotor learning: reasoning, refinement, and retrieval. Preprint at PsyArXiv https://doi.org/10.31234/osf.io/x4652 (2023).
Tsay, J. S. et al. The effect of visual uncertainty on implicit motor adaptation. J. Neurophysiol. https://doi.org/10.1152/jn.00493.2020 (2021).
Kim, H. E., Morehead, R., Parvin, D. E., Moazzezi, R. & Ivry, R. B. Invariant errors reveal limitations in motor correction rather than constraints on error sensitivity. Commun. Biol. 1, 19 (2018).
Herzfeld, D. J., Vaswani, P. A., Marko, M. K. & Shadmehr, R. A memory of errors in sensorimotor learning. Science 345, 1349–1353 (2014).
Albert, S. T. et al. An implicit memory of errors limits human sensorimotor adaptation. Nat. Hum. Behav. https://doi.org/10.1038/s41562-020-01036-x (2021).
Redding, G. M. & Wallace, B. Effects on prism adaptation of duration and timing of visual feedback during pointing. J. Mot. Behav. 22, 209–224 (1990).
Held, R., Efstathiou, A. & Greene, M. Adaptation to displaced and delayed visual feedback from the hand. J. Exp. Psychol. 72, 887–891 (1966).
Kitazawa, S., Kohno, T. & Uka, T. Effects of delayed visual information on the rate and amount of prism adaptation in the human. J. Neurosci. 15, 7644–7652 (1995).
Brudner, S. N., Kethidi, N., Graeupner, D., Ivry, R. B. & Taylor, J. A. Delayed feedback during sensorimotor learning selectively disrupts adaptation but not strategy use. J. Neurophysiol. 115, 1499–1511 (2016).
Tsay, J. S., Irving, C. & Ivry, R. B. Signatures of contextual interference in implicit sensorimotor adaptation. Proc. Biol. Sci. 290, 20222491 (2023).
Tsay, J. S., Kim, H., Haith, A. M., & Ivry, R. B. Understanding implicit sensorimotor adaptation as a process of proprioceptive re-alignment. eLife https://doi.org/10.7554/eLife.76639 (2022).
Martin, T. A., Keating, J. G., Goodkin, H. P., Bastian, A. J. & Thach, W. T. Throwing while looking through prisms: I. Focal olivocerebellar lesions impair adaptation. Brain 119, 1183–1198 (1996).
Tzvi, E., Loens, S. & Donchin, O. Mini-review: the role of the cerebellum in visuomotor adaptation. Cerebellum https://doi.org/10.1007/s12311-021-01281-4 (2021).
Tsay, J. S., Najafi, T., Schuck, L., Wang, T. & Ivry, R. B. Implicit sensorimotor adaptation is preserved in Parkinson’s disease. Brain Commun. 4, fcac303 (2022).
Tsay, J. S., Schuck, L., & Ivry, R. B. Cerebellar degeneration impairs strategy discovery but not strategy recall. Cerebellum https://doi.org/10.1007/s12311-022-01500-6 (2022).
Mutha, P. K., Sainburg, R. L. & Haaland, K. Y. Left parietal regions are critical for adaptive visuomotor control. J. Neurosci. 31, 6972–6981 (2011).
Smith, M. A. & Shadmehr, R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J. Neurophysiol. 93, 2809–2821 (2005).
Henrich, J., Heine, S. J. & Norenzayan, A. The weirdest people in the world? Behav. Brain Sci. 33, 61–83 (2010). Discussion 83–135.
Yarkoni, T. & Westfall, J. Choosing prediction over explanation in psychology: lessons from machine learning. Perspect. Psychol. Sci. 12, 1100–1122 (2017).
Wang, X., Abdullah, B. & Samsudin, S. The effect of contextual interference on motor learning among healthy adolescents: a systematic review. J. Posit. Sch. Psychol. 6, 4545–4580 (2022).
Shewokis, P. A. Is the contextual interference effect generalizable to computer games? Percept. Mot. Skills 84, 3–15 (1997).
Kantner, L. A., Segall, M. H., Campbell, D. T. & Herskovits, M. J. The influence of culture on visual perception. Stud. Art. Educ. 10, 68–71 (1968).
Pitt, B., Carstensen, A., Boni, I., Piantadosi, S. T. & Gibson, E. Different reference frames on different axes: space and language in indigenous Amazonians. Sci. Adv. 8, eabp9814 (2022).
Anderson, D. I., Lohse, K. R., Lopes, T. C. V. & Williams, A. M. Individual differences in motor skill learning: past, present and future. Hum. Mov. Sci. 78, 102818 (2021).
Seidler, R. D. & Carson, R. G. Sensorimotor learning: neurocognitive mechanisms and individual differences. J. Neuroeng. Rehabil. https://doi.org/10.1186/s12984-017-0279-1 (2017).
Ranganathan, R., Cone, S. & Fox, B. Predicting individual differences in motor learning: a critical review. Neurosci. Biobehav. Rev. 141, 104852 (2022).
Ackerman, P. L. Determinants of individual differences during skill acquisition: cognitive abilities and information processing. J. Exp. Psychol. Gen. 117, 288–318 (1988).
Fleishman, E. A. On the relation between abilities, learning, and human performance. Am. Psychol. 27, 1017–1032 (1972).
Tsay, J. S., Lee, A., Ivry, R. B., & Avraham, G. Moving outside the lab: the viability of conducting sensorimotor learning studies online. Neurons Behav. Data Anal. https://doi.org/10.51628/001c.26985 (2021).
Tsay, J. S. et al. OnPoint: a package for online experiments in motor control and motor learning. Preprint at PsyArXiv https://doi.org/10.31234/osf.io/hwmpy (2020).
Germine, L. et al. Is the Web as good as the lab? Comparable performance from Web and lab in cognitive/perceptual experiments. Psychon. Bull. Rev. 19, 847–857 (2012).
Germine, L. T., Duchaine, B. & Nakayama, K. Where cognitive development and aging meet: face learning ability peaks after age 30. Cognition 118, 201–210 (2011).
Wilmer, J. B. et al. Capturing specific abilities as a window into human individuality: the example of face recognition. Cogn. Neuropsychol. 29, 360–392 (2012).
Kim, H. et al. Multiracial Reading the Mind in the Eyes Test (MRMET): an inclusive version of an influential measure. Preprint at OSF https://doi.org/10.31219/osf.io/y8djm (2022).
Wilmer, J. B. How to use individual differences to isolate functional organization, biology, and utility of visual functions; with illustrative proposals for stereopsis. Spat. Vis. 21, 561–579 (2008).
Bond, K. & Taylor, J. A. Flexible explicit but rigid implicit learning in a visuomotor adaptation task. J. Neurophysiol. 113, 3836–3849 (2015).
Shyr, M. C. & Joshi, S. S. A case study of the validity of web-based visuomotor rotation experiments. J. Cogn. Neurosci. 36, 71–94 (2024).
Kim, O. A., Forrence, A. D. & McDougle, S. D. Motor learning without movement. Proc. Natl Acad. Sci. USA 119, e2204379119 (2022).
Taylor, J. A., Krakauer, J. W. & Ivry, R. B. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J. Neurosci. 34, 3023–3032 (2014).
Anwyl-Irvine, A., Dalmaijer, E.S., Hodges, N. et al. Realistic precision and accuracy of online experiment platforms, web browsers, and devices. Behav. Res. 53, 1407–1425 (2021).
Flanagan, J. C. A simplified procedure for determining the reliability of a test by split-halves. J. Educ. Psychol. 28, 99–103 (1937).
Allen, M. J. Introduction to Measurement Theory (Waveland, 1979).
Avraham, G., Morehead, R., Kim, H. E. & Ivry, R. B. Reexposure to a sensorimotor perturbation produces opposite effects on explicit and implicit learning processes. PLoS Biol. 19, e3001147 (2021).
Tsay, J. S., Kim, H. E., Parvin, D. E., Stover, A. R. & Ivry, R. B. Individual differences in proprioception predict the extent of implicit sensorimotor adaptation. J. Neurophysiol. https://doi.org/10.1152/jn.00585.2020 (2021).
Huberdeau, D. M., Krakauer, J. W. & Haith, A. M. Practice induces a qualitative change in the memory representation for visuomotor learning. J. Neurophysiol. https://doi.org/10.1152/jn.00830.2018 (2019).
Haith, A. M., Huberdeau, D. M. & Krakauer, J. W. The influence of movement preparation time on the expression of visuomotor learning and savings. J. Neurosci. 35, 5109–5117 (2015).
Morehead, R., Qasim, S. E., Crossley, M. J. & Ivry, R. Savings upon re-aiming in visuomotor adaptation. J. Neurosci. 35, 14386–14396 (2015).
Schmitz, G. Enhanced cognitive performance after multiple adaptations to visuomotor transformations. PLoS ONE 17, e0274759 (2022).
Tsay, J. S., Irving, C. & Ivry, R. B. Signatures of contextual interference in implicit sensorimotor adaptation. Proc. R. Soc. B 290, 20222491 (2023).
Shea, J. B. & Morgan, R. L. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J. Exp. Psychol. Hum. Learn. 5, 179–187 (1979).
Hadjiosif, A. M. & Smith, M. A. A double dissociation between savings and long-term memory in motor learning. PLoS Biol. 21, e3001799 (2023).
Hadjiosif, A. M., Morehead, J. R. & Smith, M. A. A double dissociation between savings and long-term memory in motor learning. PLoS Biol. 21, e3001799 (2023).
Joiner, W. M. & Smith, M. A. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J. Neurophysiol. 100, 2948–2955 (2008).
Miyamoto, Y. R., Wang, S. & Smith, M. A. Implicit adaptation compensates for erratic explicit strategy in human motor learning. Nat. Neurosci. 23, 443–455 (2020).
Roller, C. A., Cohen, H. S., Kimball, K. T. & Bloomberg, J. J. Effects of normal aging on visuo-motor plasticity. Neurobiol. Aging 23, 117–123 (2002).
Buch, E. R., Young, S. & Contreras-Vidal, J. L. Visuomotor adaptation in normal aging. Learn. Mem. 10, 55–63 (2003).
Vachon, C. M., Modchalingam, S., ’t Hart, B. M. & Henriques, D. Y. P. The effect of age on visuomotor learning processes. PLoS ONE 15, e0239032 (2020).
Wolpe, N. et al. Age-related reduction in motor adaptation: brain structural correlates and the role of explicit memory. Neurobiol. Aging https://doi.org/10.1016/j.neurobiolaging.2020.02.016 (2020).
Wang, T. S. L., Martinez, M., Festa, E. K., Heindel, W. C. & Song, J.-H. Age-related enhancement in visuomotor learning by a dual-task. Sci. Rep. 12, 5679 (2022).
Cressman, E. K., Salomonczyk, D. & Henriques, D. Y. P. Visuomotor adaptation and proprioceptive recalibration in older adults. Exp. Brain Res. 205, 533–544 (2010).
Vandevoorde, K. & Orban de Xivry, J.-J. Why is the explicit component of motor adaptation limited in elderly adults? J. Neurophysiol. 124, 152–167 (2020).
Wong, A. L., Marvel, C. L., Taylor, J. A. & Krakauer, J. W. Can patients with cerebellar disease switch learning mechanisms to reduce their adaptation deficits? Brain https://doi.org/10.1093/brain/awy334 (2019).
Seidler, R. D. Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res. Bull. 70, 337–346 (2006).
Ruitenberg, M. F. L., Koppelmans, V., Seidler, R. D. & Schomaker, J. Developmental and age differences in visuomotor adaptation across the lifespan. Psychol. Res. https://doi.org/10.1007/s00426-022-01784-7 (2023).
Vandevoorde, K. & Orban de Xivry, J.-J. Internal model recalibration does not deteriorate with age while motor adaptation does. Neurobiol. Aging 80, 138–153 (2019).
Morehead, R. & de Xivry, J.-J. O. A synthesis of the many errors and learning processes of visuomotor adaptation. Preprint at bioRxiv https://doi.org/10.1101/2021.03.14.435278 (2021).
Verstynen, T. & Kording, K. P. Overfitting to ‘predict’ suicidal ideation. Nat. Hum. Behav. 7, 680–681 (2023).
Albert, S. T. et al. Competition between parallel sensorimotor learning systems. eLife https://doi.org/10.7554/eLife.65361 (2022).
Tottenham, L. S. & Saucier, D. M. Throwing accuracy during prism adaptation: male advantage for throwing accuracy is independent of prism adaptation rate. Percept. Mot. Skills 98, 1449–1455 (2004).
Zar, J. H. Biostatistical Analysis: International Edition 5th edn (Pearson, 2007).
Gajda, K., Sülzenbrück, S. & Heuer, H. Financial incentives enhance adaptation to a sensorimotor transformation. Exp. Brain Res. 234, 2859–2868 (2016).
Tsay, J. S., Tan, S., Chu, M., Ivry, R. B. & Cooper, E. A. Low vision impairs implicit sensorimotor adaptation in response to small errors, but not large errors. J. Cogn. Neurosci. https://doi.org/10.1162/jocn_a_01969 (2023).
Burge, J., Ernst, M. O. & Banks, M. S. The statistical determinants of adaptation rate in human reaching. J. Vis. 8, 20 (2008).
Körding, K. P. & Wolpert, D. M. Bayesian integration in sensorimotor learning. Nature 427, 244–247 (2004).
McDougle, S. D. & Taylor, J. A. Dissociable cognitive strategies for sensorimotor learning. Nat. Commun. 10, 40 (2019).
Fernandez-Ruiz, J., Wong, W., Armstrong, I. T. & Flanagan, J. R. Relation between reaction time and reach errors during visuomotor adaptation. Behav. Brain Res. 219, 8–14 (2011).
Morehead, J. R. & Ivry, R. Intrinsic Biases Systematically Affect Visuomotor Adaptation Experiments (Society for Neural Control of Movement, 2015); http://ivrylab.berkeley.edu/uploads/4/1/1/5/41152143/morehead_ncm2015.pdf
Vindras, P., Desmurget, M., Prablanc, C. & Viviani, P. Pointing errors reflect biases in the perception of the initial hand position. J. Neurophysiol. 79, 3290–3294 (1998).
Wilson, E. T., Wong, J. & Gribble, P. L. Mapping proprioception across a 2D horizontal workspace. PLoS ONE 5, e11851 (2010).
McNay, E. C. & Willingham, D. B. Deficit in learning of a motor skill requiring strategy, but not of perceptuomotor recalibration, with aging. Learn. Mem. 4, 411–420 (1998).
Fernández-Ruiz, J., Hall, C., Vergara, P. & Díiaz, R. Prism adaptation in normal aging: slower adaptation rate and larger aftereffect. Brain Res. Cogn. Brain Res. 9, 223–226 (2000).
Dhawale, A. K., Smith, M. A. & Ölveczky, B. P. The role of variability in motor learning. Annu. Rev. Neurosci. 40, 479–498 (2017).
He, K. et al. The statistical determinants of the speed of motor learning. PLoS Comput. Biol. 12, e1005023 (2016).
Wu, H. G., Miyamoto, Y. R., Castro, L. N. G., Ölveczky, B. P. & Smith, M. A. Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat. Neurosci. 17, 312–321 (2014).
Singh, P., Jana, S., Ghosal, A. & Murthy, A. Exploration of joint redundancy but not task space variability facilitates supervised motor learning. Proc. Natl Acad. Sci. USA 113, 14414–14419 (2016).
Behrens, T. E. J., Woolrich, M. W., Walton, M. E. & Rushworth, M. F. S. Learning the value of information in an uncertain world. Nat. Neurosci. 10, 1214–1221 (2007).
Tsay, J. S., Kim, H., Haith, A. M. & Ivry, R. B. Understanding implicit sensorimotor adaptation as a process of proprioceptive re-alignment. eLife https://doi.org/10.7554/eLife.76639 (2022).
Bönstrup, M., Iturrate, I., Hebart, M. N., Censor, N. & Cohen, L. G. Mechanisms of offline motor learning at a microscale of seconds in large-scale crowdsourced data. NPJ Sci. Learn. 5, 7 (2020).
Taylor, J. A. & Ivry, R. B. Flexible cognitive strategies during motor learning. PLoS Comput. Biol. 7, e1001096 (2011).
Hebiri, M. & Lederer, J. How correlations influence lasso prediction. IEEE Trans. Inf. Theory 59, 1846–1854 (2013).
Burgoyne, A. P., Harris, L. J. & Hambrick, D. Z. Predicting piano skill acquisition in beginners: the role of general intelligence, music aptitude, and mindset. Intelligence 76, 101383 (2019).
McGregor, H. R. & Gribble, P. L. Functional connectivity between somatosensory and motor brain areas predicts individual differences in motor learning by observing. J. Neurophysiol. 118, 1235–1243 (2017).
Roberts, R. E., Bain, P. G., Day, B. L. & Husain, M. Individual differences in expert motor coordination associated with white matter microstructure in the cerebellum. Cereb. Cortex 23, 2282–2292 (2013).
Landi, S. M., Baguear, F. & Della-Maggiore, V. One week of motor adaptation induces structural changes in primary motor cortex that predict long-term memory one year later. J. Neurosci. 31, 11808–11813 (2011).
Koppelmans, V., Bloomberg, J. J., Mulavara, A. P. & Seidler, R. D. Brain structural plasticity with spaceflight. NPJ Microgravity https://doi.org/10.1038/s41526-016-0001-9 (2016).
Pearson-Fuhrhop, K. M., Minton, B., Acevedo, D., Shahbaba, B. & Cramer, S. C. Genetic variation in the human brain dopamine system influences motor learning and its modulation by l-DOPA. PLoS ONE 8, e61197 (2013).
Listman, J. B., Tsay, J. S., Kim, H. E., Mackey, W. E. & Heeger, D. J. Long-term motor learning in the ‘wild’ with high volume video game data. Front. Hum. Neurosci. 15, 777779 (2021).
Aung, M. et al. Predicting skill learning in a large, longitudinal MOBA dataset. In IEEE Conference on Computational Intelligence and Games (CIG) 1–7 (IEEE, 2018).
Brookes, J., Warburton, M., Alghadier, M., Mon-Williams, M. & Mushtaq, F. Studying human behavior with virtual reality: the Unity Experiment Framework. Behav. Res Methods 52, 455–463 (2020).
Chen, X. et al. Age-dependent Pavlovian biases influence motor decision-making. PLoS Comput. Biol. 14, e1006304 (2018).
Donovan, I., Saul, M. A., DeSimone, K., Listman, J. B., Mackey, W. E., & Heeger, D. J. Assessment of human expertise and movement kinematics in first-person shooter games. Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2022.979293 (2022).
Stafford, T. & Dewar, M. Tracing the trajectory of skill learning with a very large sample of online game players. Psychol. Sci. 25, 511–518 (2014).
Stafford, T., & Vaci, N. Maximizing the potential of digital games for understanding skill acquisition. Curr. Dir. Psychol. https://doi.org/10.1177/09637214211057841 (2022).
Balestrucci, P., Wiebusch, D. & Ernst, M. O. ReActLab: a custom framework for sensorimotor experiments ‘in-the-wild’. Front. Psychol. https://doi.org/10.3389/fpsyg.2022.906643 (2022).
Kaur, J. & Balasubramaniam, R. Sequence learning in an online serial reaction time task: the effect of task instructions. J. Mot. Learn. Dev. 1–17 (2022).
Brantley, J. A. & Kording, K. P. Bayesball: Bayesian integration in professional baseball batters. Preprint at bioRxiv https://doi.org/10.1101/2022.10.12.511934 (2022).
Drazan, J. F., Phillips, W. T., Seethapathi, N., Hullfish, T. J. & Baxter, J. R. Moving outside the lab: markerless motion capture accurately quantifies sagittal plane kinematics during the vertical jump. J. Biomech. 125, 110547 (2021).
Hausmann, S. B., Vargas, A. M., Mathis, A. & Mathis, M. W. Measuring and modeling the motor system with machine learning. Preprint at arXiv https://doi.org/10.48550/arXiv.2103.11775 (2021).
Hooyman, A. & Schaefer, S. Y. Age and sex effects on Super G performance are consistent across internet devices. Int. J. Serious Games 10, 25–36 (2023).
Yin, C. & Wei, K. Savings in sensorimotor adaptation without explicit strategy. J. Neurophysiol. https://doi.org/10.1152/jn.00524.2019 (2020).
Coltman, S. K., Cashaback, J. G. A. & Gribble, P. L. Both fast and slow learning processes contribute to savings following sensorimotor adaptation. J. Neurophysiol. 121, 1575–1583 (2019).
Morehead, R., Taylor, J. A., Parvin, D. E. & Ivry, R. B. Characteristics of implicit sensorimotor adaptation revealed by task-irrelevant clamped feedback. J. Cogn. Neurosci. 29, 1061–1074 (2017).
Maresch, J., Werner, S. & Donchin, O. Methods matter: your measures of explicit and implicit processes in visuomotor adaptation affect your results. Eur. J. Neurosci. https://doi.org/10.1111/ejn.14945 (2020).
Hooyman, A., Huentelman, M. J., De Both, M., Ryan, L. & Schaefer, S. Y. Establishing the validity and reliability of an online motor learning game: applications for Alzheimer’s disease research within MindCrowd. Games Health J. https://doi.org/10.1089/g4h.2022.0042 (2023).
Allen, K. R., Smith, K. A. & Tenenbaum, J. B. Rapid trial-and-error learning with simulation supports flexible tool use and physical reasoning. Proc. Natl Acad. Sci. USA 117, 29302–29310 (2020).
Tsay, J. S., Schuck, L. & Ivry, R. B. Cerebellar degeneration impairs strategy discovery but not strategy recall. Cerebellum https://doi.org/10.1007/s12311-022-01500-6 (2022).
Saban, W. & Ivry, R. B. PONT: a Protocol for Online Neuropsychological Testing. J. Cogn. Neurosci. 1–13 (2021).
Chakraborty, S. & Wong, S. W. K. BAMBI: An R package for fitting bivariate angular mixture models. J. Stat. Softw. 99, 1–69 (2021).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, 1–22 (2010).
Choi, Y., Park, R. & Seo, M. Lasso on Categorical Data (CiteSeerX, 2012); https://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.278.5439
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. B 58, 267–288 (1996).
McDougle, S. D., Bond, K. & Taylor, J. A. Implications of plan-based generalization in sensorimotor adaptation. J. Neurophysiol. 118, 383–393 (2017).
Day, K. A., Roemmich, R. T., Taylor, J. A. & Bastian, A. J. Visuomotor learning generalizes around the intended movement. eNeuro https://doi.org/10.1523/ENEURO.0005-16.2016 (2016).
Acknowledgements
This project was supported by two NIH grants (no. 1F31NS120448 awarded to J.S.T. and no. R35NS116883-01 awarded to R.B.I.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.S.T.: conceptualization, resources, data curation, software, formal analysis, funding acquisition, validation, investigation, visualization, methodology, project administration, writing—original draft, writing—review and editing. H.A.: software, formal analysis, validation, investigation, visualization, methodology, writing—review and editing. L.T.G.: data curation, visualization, writing—review and editing. J.W.: data curation, visualization, writing—review and editing. R.B.I.: data curation, writing—review and editing, funding acquisition, validation, investigation, visualization. K.N.: data curation, writing—review and editing, validation, investigation, visualization, project administration.
Corresponding authors
Ethics declarations
Competing interests
R.B.I. is a co-founder with equity in Magnetic Tides, Inc., a biotechnology company created to develop a novel method of non-invasive brain stimulation. The other authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Joshua Cashaback, Scott T Albert, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Correlations between different phases of motor adaptation.
Correlation between early and late adaptation (a), aftereffect and early adaptation (b), and aftereffect and late adaptation (c). rp denotes Pearson’s correlation (# of sessions = 1,747); pbf denotes the p-value for a two-tailed t-test (Bonferroni-corrected for three comparisons).
Extended Data Fig. 2 Age distribution.
Blue shading denotes different age groups (that is, rounded to the nearest decade). 107 individuals are closest to age 10, 1068 to age 20, 269 to age 30, 126 to age 40, 61 to age 50, 59 to age 60, 24 to age 70, 28 to age 80, and 5 to age 90. The oldest group was excluded in our aging analyses due to its limited sample size (n = 5).
Extended Data Fig. 3 Correlation matrix.
Color denotes the direction of the Pearson’s correlations (# of sessions = 1,747), and square size denotes correlation magnitude.
Extended Data Fig. 4 Results from our post-hoc Lasso regression were robust to changes in number of folds and percent of data used for training.
a–c, Shading and numbers denote the cross-validated coefficient of determination (\({R}_{{cv}}^{2}\)). The red box denotes the model used in this manuscript (that is, 80% training data split across 10-folds).
Extended Data Fig. 5 Features corresponding to Pattern 3 (continued).
(a, b) Baseline reaction time, (c, d) average amount of sleep every night, and (e, f) computer screen size. Left column: Data are presented as median values ± SEM. Right column: The width of the violin plot represents data density. Vertical black lines represent median values ± 1st/3rd IQR. rs denotes Spearman’s correlation; p value is obtained from a two-tailed t-test. We used data from 1,747 sessions (naïve participants who completed the one-target version of the task).
Extended Data Fig. 6 Features corresponding to Pattern 5.
(a, b) Perturbation direction, (c, d) device used, (e, f) self-reported neurological disease, and (g, h) amount of average computer usage. Left column: Data are presented as median values ± SEM. Right column: The width of the violin plot represents data density. Vertical black lines represent median values ± 1st/3rd IQR. rs denotes Spearman’s correlation; p value is obtained from a two-tailed t-test. We used data from 1,747 sessions (naïve participants who completed the one-target version of the task).
Extended Data Fig. 7 Features corresponding to Pattern 5 (continued).
(a, b) Internet browser used, (c, d) undergraduate major, and (e, f) self-reported ratings of clumsiness. Left column: Data are presented as median values ± SEM. Right column: The width of the violin plot represents data density. Vertical black lines represent median values ± 1st/3rd IQR. We used data from 1,747 sessions (naïve participants who completed the one-target version of the task).
Extended Data Fig. 8 Self-reported neurological disease.
Among the 313 individuals reporting a neurological disease, only 12 described their specific disease, which we categorized into five main categories. AD: Alzheimer′s Disease. CD: Cerebellar Degeneration. MS: Multiple Sclerosis. PD: Parkinson’s Disease. ST: Stroke.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsay, J.S., Asmerian, H., Germine, L.T. et al. Large-scale citizen science reveals predictors of sensorimotor adaptation. Nat Hum Behav 8, 510–525 (2024). https://doi.org/10.1038/s41562-023-01798-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-023-01798-0
This article is cited by
-
Ouvrai opens access to remote virtual reality studies of human behavioural neuroscience
Nature Human Behaviour (2024)