Abstract
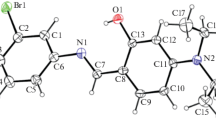
The DFT computational studies, crystal structures and Hirshfeld surface analysis of (E)-4-(2-chlorostyryl)-2,2-dimethyl-2,3-dihydro-1H-benzo[b][1,4]diazepine (1) and (E)-4-(2-(2,2-dimethyl-2,3-dihydro-1H-benzo[b][1,4]diazepin-4-yl)vinyl)phenol (2) have been presented. The compounds crystallized in the monoclinic space group P21/c with 4 molecules in their unit cells each. The experimental and computed bond lengths and bond angles deviated from each other to some extent but also showed good agreement with each other in some cases. Hirshfeld surface analysis of the compounds provided further information about the structural properties of the compounds.















Similar content being viewed by others
REFERENCES
S. Sevvanthi, S. Muthu, S. Aayisha, P. Ramesh, and M. Raja. Spectroscopic (FT-IR, FT-Raman and UV-Vis), computational (ELF, LOL, NBO, HOMO–LUMO, Fukui, MEP) studies and molecular docking on benzodiazepine derivatives-heterocyclic organic arenes. Chem. Data Collect., 2020, 30, 100574 https://doi.org/10.1016/j.cdc.2020.100574
D. Jeroundi, A. Mazzah, T. Hökelek, E. M. E. Hadrami, C. Renard, A. Haoudi, and E. M. Essassi. Crystal structure, Hirshfeld surface analysis and interaction energy and DFT studies of (S)-10-propargylpyrrolo[2,1-c][1,4]benzodiazepine-5,11-dione. Acta Crystallogr., Sect. E: Crystallogr Commun., 2020, 76, 467-472. https://doi.org/10.1107/S2056989020002698
K. Chkirate, K. Karrouchi, N. Dege, N. K. Sebbar, A. Ejjoummany, S. Radi, N. N. Adarsh, A. Talbaoui, M. Ferbinteanu, El M. Essassi, and Y. Garcia. Co(II) and Zn(II) pyrazolyl-benzimidazole complexes with remarkable antibacterial activity. New J. Chem., 2020, 44, 2210-2221. https://doi.org/10.1039/C9NJ05913J
S. Valenti, M. Barrio, P. Negrier, M. Romanini, R. Macovez, and J. L. Tamarit. Comparative physical study of three pharmaceutically active benzodiazepine derivatives: Crystalline versus amorphous state and crystallization tendency. Mol. Pharmaceutics, 2021, 1819-1832. https://doi.org/10.1021/acs.molpharmaceut.1c00081
T. Hiroyuki, O. Kazushige, I. Takayoshi, and N. Umpei. Molecular structure optimization and molecular dynamics using Hamiltonian algorithm: Structure of benzodiazepine minor tranquilizers - towards non-empirical drug design. Bull. Chem. Soc. Jpn., 2008, 81, 1094-1102. https://doi.org/10.1246/bcsj.81.1094
E. Sigel. Mapping of the benzodiazepine recognition site on GABAA receptors. Curr. Top. Med. Chem., 2002, 2, 833-839. https://doi.org/10.2174/1568026023393444
P. Garbacz and M. Wesolowski. Benzodiazepines co-crystals screening using FTIR and Raman spectroscopy supported by differential scanning calorimetry. Spectrochim. Acta, Part A, 2020, 234, 118242. https://doi.org/10.1016/j.saa.2020.118242
S. D. Tupare and R. P. Pawar. Highly efficient synthesis and antibacterial of 1,5-benzodiazepines under microwave irradiation. Int. J. Appl. Chem., 2017, 13, 369-376, http://www.ripublication.com (accessed Sept 20, 2023).
M. Hawash, N. Jaradat, S. Hameedi, and A. D. Mousa. Design, synthesis and biological evaluation of novel benzodioxole derivatives as COX inhibitors and cytotoxic agents. BMC Chem., 2020, 14(54), 1-9. https://doi.org/10.1186/s13065-020-00706-1
M. M. K. Kumar, T. Mohan, G. K. Mai, G. P. V Sangeeta, and K. P. Nagasree. Synthesis, characterization and biological evaluation of novel 1,4-benzodiazepine derivatives as potent anti-tubercular agents. J. Young Pharm., 2018, 10, 267-271. https://doi.org/10.5530/jyp.2018.10.60
A. M. Taha and M. K. Rasheed. 2nd International Conference of Al-Esraa University College for Engineering Sciences (ICAUC_ES). IOP Conf., Ser.: Earth Environ. Sci., 2022, 961(1), 011001. https://doi.org/10.1088/1755-1315/961/1/011001
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox. Gaussian16, Revision G09. Wallingford, CT, USA: Gaussian, Inc., 2016.
K. P. Safna Hussan, M. S. Thayyil, V. K. Rajan, and K. Muraleedharan. DFT studies on global parameters, antioxidant mechanism and molecular docking of amlodipine besylate. Comput. Biol. Chem., 2019, 80, 46-53. https://doi.org/10.1016/j.compbiolchem.2019.03.006
K. Bronisz, M. Ostafin, O. Kh. Poleshchuk, J. Mielcarek, and B. Nogaj. Studies of the electronic structure and biological activity of chosen 1,4-benzodiazepines by 35Cl NQR spectroscopy and DFT calculations. Chem. Phys., 2006, 330, 301-306. https://doi.org/10.1016/j.chemphys.2006.09.001
S. Sarala, S. K. Geetha, S. Muthu, and A. Irfan. Computational investigation, comparative approaches, molecular structural, vibrational spectral, non-covalent interaction (NCI), and electron excitations analysis of benzodiazepine derivatives. J. Mol. Model., 2021, 27, 266. https://doi.org/10.1007/s00894-021-04877-z
L. El Ghayati, Y. Sert, N. K. Sebbar, Y. Ramli, N. H. Ahabchane, A. Talbaoui, J. T. Mague, B. El Ibrahimi, M. L. Taha, E. M. Essassi, N. Al-Zaqri, and A. Alsalme. Syntheses of novel 1,5-benzodiazepine derivatives: Crystal structures, spectroscopic characterizations, Hirshfeld surface analyses, molecular docking studies, DFT calculations, corrosion inhibition anticipation, and antibacterial activities. J. Heterocycl. Chem., 2021, 58, 270-289. https://doi.org/10.1002/jhet.4167
N. Elangovan, R. Thomas, and S. Sowrirajan. Synthesis of Schiff base (E)-4-((2-hydroxy-3,5-diiodobenzylidene)amino)-N-thiazole-2-yl)benzenesulfonamide with antimicrobial potential, structural features, experimental biological screening and quantum mechanical studies. J. Mol. Struct., 2022, 1250, 131762. https://doi.org/10.1016/j.molstruc.2021.131762
M. A. Flores-Hidalgo, D. Barraza-Jiménez, and D. Glossman-Mitnik. Effects of sulfur substitutional impurities on (ZnO)n clusters (n = 4-12) using density functional theory. Comput. Theor. Chem., 2011, 965, 154-162. https://doi.org/10.1016/j.comptc.2011.01.037
K. Selvaraju and P. Kumaradhas. Charge density analysis and transport properties of TTF based molecular nanowires: A DFT approach. J. Nanosci., 2015, 2015, 806181. https://doi.org/10.1155/2015/806181
APEX2, SADABS and SAINT. Madison, WI, USA: Bruker AXS, 2010.
G. M. Sheldrick. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64(1), 112-122. https://doi.org/10.1107/s0108767307043930
C. B. Hubschle, G. M. Sheldrick, and B. Dittrich. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr., 2011, 44, 1281. https://doi.org/10.1107/S0021889811043202
F. Odame, R. Schoeman, J. Krause, E. C. Hosten, Z. R. Tshentu, and C. L. Frost. Synthesis, characterization, crystal structures, and anticancer activity of some new 2,3-dihydro-1,5-benzoxazepines. Med. Chem. Res., 2021, 30, 987-1004. https://doi.org/10.1007/s00044-021-02706-9
F. Odame, E. C. Hosten, R. Betz, J. Krause, C. L. Frost, K. Lobb, and Z. R. Tshentu. Synthesis, characterization, computational studies and DPPH scavenging activity of some triazatetracyclic derivatives. J. Iran. Chem. Soc., 2021, 18, 1979-1995. https://doi.org/10.1007/s13738-021-02158-3
F. Odame, P. Kleyi, E. Hosten, R. Betz, K. Lobb, and Z. R. Tshentu. The formation of 2,2,4-trimethyl-2,3-dihydro-1H-1,5-benzodiazepine from 1,2-diaminobenzene in the presence of acetone. Molecules, 2013, 18, 14293-14305. https://doi.org/10.3390/molecules181114293
F. Odame, E. Hosten, R. Betz, K. Lobb, and Z. Tshentu. Synthesis, characterization and computational studies of 2-(benzamido)thiazol-5-yl benzoate. J. Struct. Chem., 2019, 60(1), 136-142. https://doi.org/10.1134/S0022476619010190
F. Odame, E. C. Hosten, and Z. R. Tshentu. Synthesis, characterization and computational studies on N-[(9E)-8,10,17-triazatetracyclo[8.7.0.02,7.011,16]heptadeca-1(17),2,4,6,11(16),12,14-heptaen-9-ylidene]benzamide. J. Struct. Chem., 2020, 61(8), 1177-1185. https://doi.org/10.1134/S0022476620080016
F. Odame, E. C. Hosten, R. Betz, K. Lobb, and Z. R. Tshentu. Synthesis characterization and computational studies of a co-crystal of 2-aminobenzimidazole and 2-[(benzoylcarbamothioyl) amino]propanoic acid. J. Struct. Chem., 2018, 59(5), 1240-1244. https://doi.org/10.1134/S0022476618050268
J. Sebhaoui, Y. El Bakri, Y. El Aoufir, E. H. Anouar, A. Guenbour, A. A. Nasser, and E. Mokhtar Essassi. Synthesis, NMR characterization, DFT and anti-corrosion on carbon steel in 1M HCl of two novel 1,5-benzodiazepines. J. Mol. Struct., 2019, 1182, 123-130. https://doi.org/10.1016/j.molstruc.2019.01.037
M. A. Spackman and J. J. McKinnon. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm, 2002, 4, 378-392. https://doi.org/10.1039/B203191B
M. A. Spackman and D. Jayatilaka. Hirshfeld surface analysis. CrystEngComm, 2009, 11, 19-32. https://doi.org/10.1039/B818330A
S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka, and M. A. Spackman. Crystal Explorer 3.0. Perth, Australia: University of Western Australia, 2012, https://www.scirp.org (accessed Sept 20, 2023).
N. E. Eltayeb, F. Şen, J. Lasri, M. A. Hussien, S. E. Elsilk, B. A. Babgi, H. Gökce, and Y. Sert. Hirshfeld surface analysis, spectroscopic, biological studies and molecular docking of (4E)-4-((naphthalen-2-yl)methyleneamino)-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one. J. Mol. Struct., 2020, 1202, 127315. https://doi.org/10.1016/j.molstruc.2019.127315
R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, and M. A. Spackman. CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr., 2021, 54, 1006-1011. https://doi.org/10.1107/S1600576721002910
V. Choudhary, A. Bhatt, D. Dash, and N. Sharma. DFT calculations on molecular structures, HOMO–LUMO study, reactivity descriptors and spectral analyses of newly synthesized diorganotin(IV) 2-chloridophenylacetohydroxamate complexes. J. Comput Chem., 2019, 40, 2354-2363. https://doi.org/10.1002/jcc.26012
P. Matunová, V. Jirásek, and B. Rezek. DFT calculations reveal pronounced HOMO–LUMO spatial separation in polypyrrole–nanodiamond systems. Phys. Chem. Chem., 2019, 21, 11033-11042. https://doi.org/10.1039/C8CP07622G
A. Poiyamozhi, N. Sundaraganesan, M. Karabacak, O. Tanrıverdi, and M. Kurt. The spectroscopic (FTIR, FT-Raman, UV and NMR), first-order hyperpolarizability and HOMO–LUMO analysis of 4-amino-5-chloro-2-methoxybenzoic acid. J. Mol. Struct., 2012, 1024, 1-12. https://doi.org/10.1016/j.molstruc.2012.05.008
P. Udhayakala, A. Jayanthi, T. V. Rajendiran, and S. Gunasekaran. Molecular structure, FT-IR and FT-Raman spectra and HOMO-LUMO analysis of 2-methoxy-4-nitroaniline using ab initio HF and DFT (B3LYP/B3PW91) calculations. Arch. Appl. Sci. Res., 2011, 3, 424-439, https://www.scholarsresearchlibrary.com (accessed Sept 15, 2023).
M. Govindarajan, S. Periandy, and K. Carthigayen. FT-IR, FT-Raman and UV spectral investigation: computed frequency estimation analysis and electronic structure calculations on chlorobenzene using HF and DFT. Spectrochim. Acta, Part A, 2012, 97, 411-422. https://doi.org/10.1016/j.saa.2011.11.052
R. G. Parr and P. K. Chattaraj. Principle of maximum hardness. J. Am. Chem. Soc., 1991, 113, 1854-1855. https://doi.org/10.1021/ja00005a072
R. G. Parr, L. V. Szentpaly, and S. Liu. Electrophilicity index. J. Am. Chem. Soc., 1999, 121, 1922-1924. https://doi.org/10.1021/ja983494x
R. Parthasarathi, J. Padmanabhan, V. Subramanian, B. Maiti, and P. K. Chattaraj. Chemical reactivity profiles of two selected polychlorinated biphenyls. J. Phys. Chem. A, 2003, 107, 10346-10352. https://doi.org/10.1021/jp035620b
R. Parthasarathi, J. Padmanabhan, V. Subramanian, B. Maiti, and P. K. Chattaraj. Toxicity analysis of 33′44′5-pentachloro biphenyl through chemical reactivity and selectivity profiles. Curr. Sci., 2004, 86, 535-542, https://www.jstor.org/stable/i24103934 (accessed Sept 15, 2023).
M. T. de Oliveira, J. M. A. Alves, A. A. C. Braga, D. J. D. Wilson, and C. A. Barboza. Do double-hybrid exchange-correlation functionals provide accurate chemical shifts? A benchmark assessment for proton NMR. Chem. Theory Comput., 2021, 17, 6876-6885. https://doi.org/10.1021/acs.jctc.1c00604
T. Lu and F. Chen. Multiwfn: a multifunctional wavefunction analyser. J. Comput. Chem., 2012, 33, 580-592. https://doi.org/10.1002/jcc.22885
S. Grimme, C. Bannwarth, S. Dohm, A. Hansen, J. Pisarek, P. Pracht, J. Seibert, and F. Neese. Fully automated quantum-chemistry-based computation of spin-spin-coupled nuclear magnetic resonance spectra. Angew. Chem., Int. Ed. Engl., 2017, 56, 14763-14769. https://doi.org/10.1002/anie.201708266
F. Neese, F. Wennmohs, U. Becker, and C. Riplinger. The ORCA quantum chemistry program package. J. Chem. Phys., 2020, 152, 224108. https://doi.org/10.1063/5.0004608
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interests.
Additional information
Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 12, 119104.https://doi.org/10.26902/JSC_id119104
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Rights and permissions
About this article
Cite this article
Odame, F., Madanhire, T., Hosten, E.C. et al. Crystal Structure, Hirshfeld Surface Analysis and Computational Studies of Two Benzo[b][1,4]Diazepine Derivatives. J Struct Chem 64, 2326–2342 (2023). https://doi.org/10.1134/S0022476623120041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623120041