Abstract
Attempts to geochemically distinguish between metamorphic-hydrothermal systems that form orogenic gold deposits and both reduced and oxidized magmatic-hydrothermal systems using isotopes or metal associations have proven ambiguous, particularly for orogenic gold and reduced intrusion-related gold systems. The absence of conclusive geochemical discriminators and the overlap in geologic characteristics have led to gold deposit models being potentially incorrectly applied, which in turn negatively affect regional mineral exploration and mine planning. In this study, in situ electron microprobe geochemical analyses of hydrothermal monazite and xenotime crystals associated with different types of gold-bearing deposits are shown to be effective geochemical discriminators. There are notable differences in mineral chemistry such as rare earth element (REE) profiles, total light REE, Dy, Er, Pr, Y, Nd/Sm, and La/Sm that distinguish monazite precipitated from metamorphic-hydrothermal fluids that form orogenic gold deposits and those precipitated from magmatic-hydrothermal fluids that form both porphyry Cu-Mo-Au and reduced intrusion-related gold deposits. Notable differences in overall xenotime abundances and concentrations of heavy REEs, Ca, and Sc are distinctive between the different deposit classes for xenotime. The origin of the controversially classified Pogo gold deposit, Tintina gold province, Alaska, which has been characterized as both a reduced intrusion-related and an orogenic gold deposit, is tested based upon the noted chemical differences associated with these hydrothermal phosphates. The findings of this study have implications for exploration and mine development in the Tintina gold province and other areas that contain deposits that are controversially classified as either orogenic or as magmatic-hydrothermal gold deposits.
Similar content being viewed by others
Introduction
Accreted metamorphic terranes, such as those found in the North American Cordillera, contain three important types of lode gold-bearing mineralizing systems. Following the mineral systems classification scheme of Hofstra and Kreiner (2020), these include orogenic gold, reduced intrusion-related gold, and porphyry Cu-Mo-Au systems which can all contain substantial gold resources as well as critical minerals. These system types share many geologic and geochemical features but are ultimately formed by different processes and with different types of hydrothermal fluids.
Orogenic gold deposits are the products of metamorphic-hydrothermal fluids derived from metamorphic devolitization reactions in the middle crust or deeper levels (e.g., Goldfarb et al. 2005). Magmatic-hydrothermal gold deposits form from fluids exsolved from a crystallizing intrusion emplaced into the shallower crust (e.g., John et al. 2010), although the hydrous magma may be sourced from as deep as the mantle (Loucks 2021). Magmatic-hydrothermal gold systems can be further subdivided into porphyry Cu-Mo-Au deposits formed from oxidized magnetite-series melts intruded into the shallow crust and reduced intrusion-related gold deposits formed from reduced ilmenite-series melts. No conclusive geochemical method for differentiating between metamorphic-hydrothermal and magmatic-hydrothermal fluid types is known, and classification of individual deposits can be controversial. This is especially true for differentiating reduced intrusion-related gold deposits from orogenic gold deposits, since both form at similar crustal depths, from low-salinity aqueous carbonic fluids, and have similar metal associations and alteration assemblages (Table 1; Hart 2005). Instead, geologic features are typically invoked to characterize the genetic origin of a deposit although this can be ambiguous at times and lead to controversial classifications.
Successfully locating deposits formed by these distinctive genetic models requires fundamentally different approaches to exploration. Exploration for orogenic gold deposits should focus on areas proximal to large-scale faults whereas exploration for magmatic-hydrothermal gold deposits demands an emphasis on the location of plutonic bodies of favorable age and chemistry. Although both large-scale faults and intrusions are common in accreted terranes and can coincide with each other, they can also form independently. Applying a correct genetic model during mineral exploration would expose potential mineral resources and favorable tracts of land for new deposit discoveries along with refining existing mine plans.
Rare earth element (REE) phosphate minerals such as monazite and xenotime are trace hydrothermal products found in both orogenic and magmatic-hydrothermal deposits that have proven important for U-Th-Pb geochronological investigations of ore formation (e.g., Vielreicher et al. 2003; Li et al. 2011; Taylor et al. 2015; Qiu et al. 2019). The chemistry of these phases has also been successfully used to differentiate between magmatic phosphates found in granitic rocks, metamorphic phosphates formed by metamorphic reactions, diagenetic phosphates formed during the lithification process of sediments, and phosphates of generic hydrothermal formation (Kositcin et al. 2003; Schandl and Gorton 2004; Taylor et al. 2015), but never between different types of hydrothermal fluids responsible for gold deposit formation.
This study examines chemical data for hydrothermal monazite and xenotime from unequivocal examples of porphyry Cu-Mo-Au deposits (Butte, Montana, and Pebble, Alaska), reduced intrusion-related gold deposits (Clear Creek, Yukon, and Shotgun, Alaska), and both metamorphic rock-hosted and granite-hosted orogenic gold deposits from the Klamath Mountains and Sierra Nevada foothills gold provinces of California (Fig. 1). We demonstrate chemical differences in hydrothermal monazite and xenotime related to these different deposit types, inferred to relate to the distinct sources and types of hydrothermal fluids responsible for metal precipitation. In addition, this study demonstrates their application as genetic discriminators for the controversially classified Pogo gold deposit, Alaska, and can aid in vectoring toward favorable geological features within exploration tracts.

Modified from Goldfarb et al. (2008)
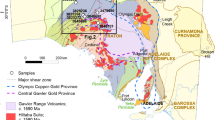
Simplified map of the Western Cordillera of North America showing the extent of terranes accreted to the North American craton and sample locations from this study. The approximate location of the Tintina gold province of Alaska and the Yukon is outlined as it contains numerous gold deposits that are classified as both orogenic and reduced intrusion-related gold systems. The Klamath Mountain orogenic gold province includes the McKeen, Hickey, McKinley, and Schroeder deposits. The Sierra Nevada foothills orogenic gold province includes the Empire, Sixteen-to-One, and Harvard deposits. Au, gold; RIRG, reduced intrusion-related gold.
Magmatic-hydrothermal versus orogenic gold systems
The tectonic evolution of accreted terranes produces spatially and temporally related metamorphism and magmatism, both of which can generate hydrothermal fluids. Because of this, it is incorrect to assume that the gold mineralization is genetically associated with one or the other based solely on a spatial relation with either a pluton or a large-scale fault.
Metamorphic-hydrothermal fluids derived from depth form orogenic gold deposits. Metamorphic devolatilization reactions drive off CO2, H2O, and other volatiles from a buried rock pile as it undergoes the metamorphic transition from greenschist- to amphibolite-facies (Goldfarb et al. 1988; Phillips and Powell 2010; Goldfarb and Pitcairn 2022). Metals, including gold, are also driven from the rock package and incorporated into these metamorphic fluids (Pitcairn et al. 2006). The fluids can be focused into large-scale crustal faults where they are seismically pumped upwards during earthquake events (Sibson et al. 1988; Sibson 1990). Quartz and other minerals precipitate from the metamorphic fluids at shallower depths in attendant subsidiary faults. The REE budget of a metamorphic fluid is dependent on the composition of the original rock package being metamorphosed, the degree of metamorphism, and the metamorphic reactions that result in specific residual and recrystallized mineral assemblages. In metamorphic environments, garnet, amphibole, epidote, and phosphate minerals are key REE reservoirs.
Magmatic-hydrothermal fluids are generated through igneous processes. Volatiles and other incompatible species such as REE are concentrated in the remnant magma of a hydrous magma as it undergoes fractional crystallization. Eventually as the volatiles and metals reach saturation, they will immiscibly separate from the remaining magma. If the pressure from the buildup of this separated volatile phase exceeds the tensile strength of the overlying rock, it will fracture allowing formation of sheeted and stockwork veins.
Magmatic-hydrothermal gold deposits have both oxidized and reduced endmembers (Thompson et al. 1999; Hart 2005, 2007). The oxidized and reduced granite-series are geochemically similar but differ in their magnetite contents due to differences in ferrous/ferric iron contents and SO2 contents which appreciably affect the oxidation state but not whole rock geochemistry (Ishihara 1981; Takagi and Tsukimura 1997). They are therefore differentiated based upon oxide mineralogy of the pluton, ore, and alteration assemblages (Smith et al. 2012). The presence of water in the latest stages of emplacement may be an important oxidizing agent in magnetite-series granites, whereas incorporation of crustal carbon may be an important reducing agent in ilmenite-series granites (Ishihara 1981). The oxidized nature of many porphyry Cu-Mo-Au deposits is noted in the abundance of hydrothermal magnetite associated with the potassic alteration assemblages (Sillitoe 1993); reduced deposits will have associated ilmenite and/or pyrrhotite. Examples of reduced intrusion-related gold deposits include the Tintina gold province (Hart 2005) and the Cascade arc (Smithson and Rowins 2005; Smith et al. 2012). Examples of more oxidized, magnetite-bearing gold systems include eastern Australia, Spain, Kazakstan, Japan, and also the Tintina gold province (Ishihara 1981; Blevins and Chappell 1992; Thompson et al. 1999). Gold-rich porphyry Cu-Mo-Au deposits tend to be derived from more mafic plutons, whereas plutons responsible for forming reduced intrusion-related gold deposits tend to be more felsic (Hart 2007).
The redox state of a magma affects the solubility and partitioning of multivalent metals into the separated volatile phase. Redox-sensitive lithophile elements include Eu, Ce, U, Th, and Pb. The remainder of the REEs and Sc is not redox-sensitive because these elements have one oxidation state. Ilmenite-series igneous rocks are generally enriched in lithophile elements relative to magnetite-series rocks (Ishihara 1981).
Despite the genetic differences, magmatic-hydrothermal gold (especially reduced intrusion-related) and orogenic gold deposits can display similar geologic and geochemical characteristics (Table 1). Commonly observed characteristics include Au-As-Sb-Bi-Te-As metal associations, low-salinity aqueous-carbonic fluids presumably responsible for vein formation, common quartz-sericite-pyrite and carbonate alteration assemblages associated with the veins, mesozonal to epizonal depths of deposit formation, and mineralization in both metamorphic country rock and granitic intrusions subsequent to peak metamorphism of the host rocks (Goldfarb et al. 2005).
An additional complication arises by the inherent general colocation of plutons and major faults within an accreted arc setting, as mentioned above. Consequently, there is the potential for generation of both metamorphic and igneous-derived mineralizing fluids. Plutons are commonly found near faults which can act as the path of least resistance for a magma to intrude. Additionally, many orogenic gold deposits are also temporally and spatially associated with plutons because they are effective structural and chemical traps, even when they are not genetically related (Goldfarb et al. 2005; Taylor et al. 2015).
Deposit backgrounds
Porphyry Cu-Mo-Au deposits—magmatic-hydrothermal
The Butte porphyry deposit located in Montana (Fig. 1) consisted of pre-mining resources of 5200 Mt grading at 0.673% Cu, 0.028% Mo, and 0.042 g/t Au (Singer et al. 2008). The deposit is entirely hosted by the 74.5 ± 0.9 Ma Butte Quartz Monzonite of the Boulder batholith which intruded Mesoproterozoic sedimentary rocks of the Belt Supergroup (Lund et al. 2002). To the south of Butte is Archean crystalline basement. Stockwork porphyry mineralization occurred at ~ 66–64 Ma likely related to dike emplacement (Lund et al. 2002, 2018; Dilles et al. 2003).
The Butte porphyry mineralization formed at greater depths than many typical porphyry deposits, with surficial outcrops calculated to have formed at paleodepths of 5–9 km (Houston and Dilles 2013). Fluid inclusions are composed of low-salinity, CO2-bearing, single aqueous phase magmatic fluids (Rusk et al. 2004). Hydrothermal monazite and xenotime are minor components of the potassic alteration assemblage and are associated with potassium feldspar, biotite, magnetite, rutile, apatite, pyrite, and chalcopyrite (Fig. 2a).
Back-scattered electron images of hydrothermal phosphates analyzed in this study. A Xenotime and monazite associated with hydrothermal pyrite and biotite from the Butte porphyry Cu-Mo-Au deposit. B Monazite growing around hydrothermal rutile from the Pebble porphyry Cu-Mo-Au deposit. C A cluster of monazite crystals within a sericitized vein selvage from the Shotgun reduced intrusion-related gold deposit. D A large elongate monazite crystal growing between pre-existing quartz crystals and an associated hydrothermal scheelite grain from the Clear Creek reduced intrusion-related gold deposit. E Monazite and xenotime within a quartz vein from the Empire mine, Grass Valley district, granite-hosted orogenic gold deposit. F Monazite with hydrothermal calcite and biotite from the McKinley greenschist-hosted orogenic gold deposit. G–H Monazite and xenotime associated with a variety of hydrothermal ore minerals from the Pogo gold deposit. Mineral abbreviations are as follows: ap, apatite; asp, arsenopyrite; bt, biotite; cal, calcite; gn, galena; kspar, potassium feldspar; mon, monazite; py, pyrite; qtz, quartz; rt, rutile; sch, scheelite; ser, sericite; and xen, xenotime
The Pebble porphyry deposit, located in southwestern Alaska (Fig. 1), has measured and indicated resources of 5942 Mt grading at 0.42% Cu, 0.025% Mo, and 0.35 g/t Au, plus an inferred resource of 4835 Mt grading 0.24% Cu, 0.022% Mo, and 0.26 g/t Au (Kelley et al. 2013; Lang et al. 2013). The total gold endowment of 107.4 Moz is the largest of any porphyry deposit worldwide. Magmatic-hydrothermal mineralization occurred at ~ 90 Ma related to hornblende granodiorite porphyry intrusions emplaced within Jurassic–Cretaceous rocks, including the extensive Kahiltna flysch (Lang et al. 2013).
The Pebble deposit likely formed at shallow paleodepths of less than 2 km (Lang et al. 2013). Ore deposition occurred with potassic and sodic-potassic alteration and in a high-grade, high-sulfidation zone along its eastern fault (Lang et al. 2013). Abundant hydrothermal monazite and xenotime are part of the potassic alteration assemblage and are associated with rutile, pyrite, chalcopyrite, apatite, potassium feldspar, quartz, and biotite (Fig. 2b). Hydrothermal monazite was also found as part of the sericite and late illite alteration packages.
The Butte and Pebble calc-alkaline porphyry Cu-Mo-Au systems concentrated abundant Au, but also have strong Cu mineralization that is absent from reduced intrusion-related gold systems. Potential associated critical minerals also differ between porphyry Cu-Mo-Au systems and reduced intrusion-related deposits (Hofstra and Kreiner 2020).
Reduced intrusion-related gold deposits—magmatic-hydrothermal
The Shotgun reduced intrusion-related gold deposit is located in southwestern Alaska (Fig. 1) and has a resource of 20.7 Mt grading at 1.06 g/t Au (Van Wyck and Armitage 2013). Gold mineralization occurred in quartz stockwork veins and breccias associated with a 69.7 ± 0.3 Ma granite porphyry within hornfelsed sedimentary rocks of the Late Cretaceous Kuskokwim Group (Berry et al. 1979; Rombach and Newberry 2001). The mineralizing porphyry is an ilmenite-series I-type intrusion, which is considered a defining characteristic for reduced intrusion-related gold deposits (Thompson et al. 1999). It was emplaced at shallow depths of less than 3 km that permitted boiling of the hydrothermal fluid (Rombach and Newberry 2001; Baker et al. 2006).
The Shotgun mineral system lacks potassic alteration but has a pervasive core of albite—quartz ± sericite ± carbonate alteration associated with gold that is capped by a broader zone of sericite ± carbonate alteration assemblages (Rombach and Newberry 2001; Goldfarb et al. 2007); tourmaline is locally abundant. Fluid inclusions are dominated by populations of vapor-rich H2O-CO2 and saline aqueous inclusions with traces of CH4 (Rombach and Newberry 2001). Hydrothermal monazite and xenotime are located within veins associated with arsenopyrite, within altered host rock vein selvages, and within altered host rock slivers incorporated into the veins (Fig. 2c).
The Clear Creek reduced intrusion-related gold deposit is located in west-central Yukon, Canada (Fig. 1), as part of the Tombstone gold belt in the Tintina gold province (Marsh et al. 2003). Sheeted vein and stockwork lode gold deposits are the source of more than 129,000 oz of past placer gold production (Allen et al. 1998). The auriferous quartz veins cut six Cretaceous stocks and the surrounding hornfelsed greenschist-facies metasedimentary rocks of the Selwyn basin (Marsh et al. 2003). Multiple geochronometers have shown that mineralization and plutonism are coincident at 92 ± 2 Ma (Murphy 1997; Marsh et al. 2003; Selby et al. 2003). Current exposed outcrops of auriferous quartz veins are suggested to have formed at paleodepths of 5–7 km based upon fluid inclusion geobarometry, with the fluids being characterized as H2O-CO2 containing 4 mol% NaCl ± KCl and 1 mol% CH4 (Marsh et al. 2003). Moderate to weakly developed potassic alteration forms selvages surrounding the veins; tourmaline is locally abundant. Most of the hydrothermal monazite and xenotime occurs in altered host rock selvages and slivers incorporated into the veins (Fig. 2d), with lesser amounts located within the quartz veins.
Orogenic gold deposits—metamorphic-hydrothermal systems
The Sierra Nevada foothills and the Klamath Mountains gold provinces of California contain both granitoid- and metamorphic rock-hosted orogenic gold deposits. These deposits formed from metamorphic-hydrothermal fluids generated at depth that were pumped upwards along large-scale crustal faults and crystallized quartz-sulfide veins at mesozonal to epizonal depths.
The Empire mine in the Sierra Nevada foothills gold province is a magnetite-series granodiorite-hosted gold mine that produced 5.8 Moz of Au, making it the most productive lode gold mine in the Grass Valley district (Pease 2009). The gold-bearing quartz veins formed at 162 ± 5 Ma, shortly after solidification and cooling of the hosting calc-alkaline, peraluminous granodiorite (Taylor et al. 2015). Taylor et al. (2015) demonstrated that hydrothermal phosphates associated with sulfide minerals, gold, hydrothermal mica, and quartz-sericite-pyrite–altered wall rock slivers (Fig. 2e) are geochemically distinct from igneous phosphate from the hosting granodiorite intrusion and thus are not genetically related. Vein formation is estimated to have occurred at ~ 8 km depth based upon fluid inclusion evidence (Taylor et al. 2021).
The 141.4 ± 0.8 Ma McKeen deposit and the analyzed vein sample from the 139.7 ± 2.1 Ma Washington mine in the Klamath Mountain orogenic gold province of northern California are both granite-hosted (Fig. 1; Taylor et al. 2022). The McKeen deposit produced nearly 25,000 oz Au (Hotz 1971), whereas total historic gold production from the Washington mine is estimated to be 300,000 oz Au (Shasta Gold Corp. 2014). The Craggy Peak composite pluton, host to the McKeen deposit, is calc-alkaline and peraluminous (Petersen 1993). Multiple rock types host the Washington mine, but the analyzed vein sample was hosted within a strongly altered quartz porphyry. Both deposits contain monazite in quartz-sericite-pyrite altered wall rock selvages and within gold, chalcopyrite, rutile, and/or titanite-bearing quartz veins. Xenotime was not identified from either deposit during this study.
The 127.5 ± 1.4 Ma Sixteen-to-One deposit in the Alleghany district of the Sierra Nevada foothills is hosted in greenschist-facies metasedimentary and mélange rocks with cross cutting serpentinite dikes (Snow et al. 2008). An estimated 1 Moz of Au has been produced, making it the most productive mine in the Alleghany district (Clark and Fuller 1968). A series of quartz veins are found within steep reverse faults that cut metasedimentary rocks and mélange of the Carboniferous and Permian Calaveras Formation (Bowen and Crippen 1997). No monazite was discovered in this study of the Sixteen-to-One deposit, but xenotime was found within the quartz veins in association with pyrite, white mica, carbonate, and other alteration phases.
The ~ 130 Ma Harvard deposit in the Mother Lode belt of the Sierra Nevada foothills is hosted in greenschist-facies graphitic slate, phyllite, chlorite schist, greenstone, and serpentinite (Marsh et al. 2008; Snow et al. 2008). The ore zone separates the graphitic slate, metavolcanic rocks, and metasedimentary rocks of the hanging wall from serpentinite and mafic rocks of the foot wall. Within the quartz veins, monazite and xenotime are found within and adjacent to quartz, white mica, and pyrite.
The Late Jurassic to Early Cretaceous Hickey (geologically constrained to be between ~ 170–165 Ma and 140 Ma), McKinley (155.00 ± 1.76 Ma), and Schroeder (159.21 ± 0.46 Ma) orogenic gold deposits are hosted within greenschist-facies rocks of the Klamath Mountains (Taylor et al. 2022). The Hickey deposit is part of the Liberty district that produced approximately 300,000 oz of lode gold (Ferrero 1990). The Hickey deposit is located within a shear zone that separates overlying siliceous schist from metavolcanic and metasedimentary rocks. Both the McKinley and Schroeder deposits are hosted by metabasalt. Monazite identified from the McKinley deposit (Fig. 2f) and the Schroeder deposit and both monazite and xenotime identified from the Hickey deposit are located in gold-bearing quartz veins associated with various hydrothermal and alteration phases.
Pogo, Alaska
The Pogo deposit contains ~ 7 Moz Au and is one of the largest deposits in the Tintina gold province, Alaska (Fig. 1; Goldfarb et al. 2007). The predominant host rock is amphibolite-grade paragneiss of the Yukon-Tanana terrane. In addition, altered granite and amphibolite are also host rocks for the veins. Multiple metamorphic events affecting the Yukon-Tanana terrane have been suggested, with the most recent being constrained between 130 and 115 Ma, but locally affecting areas as late as 107 Ma (Dilworth et al. 2007, and references within). The calc-alkaline late Early to Late Cretaceous (115–85 Ma) plutonic event that affected the Pogo area is post-collisional after terrane accretion (Mair et al. 2006; Dilworth et al. 2007). Emplacement of a suite of tonalite (107–103 Ma) is post-kinematic, weakly peraluminous, and has magnetic susceptibility that is overall low, but higher than older suites of granitoids in the region, and is thought to be broadly contemporaneous with gold mineralization although no causative pluton has been identified on the property to date (Dilworth et al. 2007). Fine-grained molybdenite within a fracture that crosses a quartz vein has been dated at 104 Ma (Selby et al. 2002), in contrast to 40Ar/39Ar plateau ages of hydrothermal mica ranging from 96.1 to 91.2 Ma (Smith et al. 2000; Rhys et al. 2003). A much younger 40Ar/39Ar age of sericite suggests an additional post-mineralization thermal event at Pogo at 61.5 ± 0.6 Ma (Selby et al. 2002).
Gold at Pogo is found within massive, shallowly dipping quartz veins as inclusions within quartz and arsenopyrite, as intergrowths with Bi-Te minerals and as invisible gold in loellingite and arsenopyrite. The veins are stacked and postdate metamorphic fabrics (Rhys et al. 2003). Microbreccia brittle textures are visible with a petrographic microscope but many veins appear massive in hand sample. Hydrothermal monazite and xenotime are found within the quartz veins and are associated with arsenopyrite, pyrite, galena, rutile, thorite, apatite, and carbonate and mica minerals (Fig. 2g–h). Xenotime can be found as inclusions in apatite whereas monazite grew on apatite. Xenotime and gold are found in fractures in quartz, indicating that these phases crystallized after the bulk of initial quartz deposition.
The genetic origin of Pogo remains controversial. Numerous researchers suggest that Pogo is an intrusion-related gold deposit (Smith et al. 2000; Rhys et al. 2003; Baker et al. 2006); mine geologists at Pogo currently subscribe to the intrusion-related model for fluids, metals, and heat derived from local calc-alkaline magmatic sources (Northern Star Resources Limited 2022). However, other researchers prefer the orogenic gold model to account for the geologic features of Pogo (Groves et al. 2003; Marsh et al. 2009; Goldfarb and Pitcairn 2022). Still, others decline to conclusively classify it one way or the other (Selby et al. 2002; Goldfarb et al. 2005; Hart 2007). Table 2 summarizes features that are used to argue for either genetic model.
Methods
The hand samples and thin sections from the Butte deposit (Mercer and Reed 2013); the Pebble deposit (e.g., Ayuso et al. 2013); the Shotgun deposit (Rombach and Newberry 2001); the Clear Creek deposits (Marsh et al. 2003); the Empire deposit (Taylor et al. 2015); the Hickey, McKinley, Schroeder, McKeen, and Washington deposits (Taylor et al. 2021); and the Harvard and Sixteen-to-One deposits (Marsh et al. 2008) were initially collected and prepared for previously published studies. Ore samples from the Pogo deposit were collected from underground mine faces for this current study and additional ongoing research.
Hydrothermal monazite and xenotime associated with oxidized porphyry Cu-Mo-Au, reduced intrusion-related, and orogenic gold deposits are typically < 20 µm in diameter (many are 5–10 µm; Fig. 2). Consequently, a scanning electron microscope (SEM) was utilized to locate the phosphates in thin sections through an automated search based upon a back-scattered electron (BSE)-brightness threshold characteristic of the phases using a JEOL JSM-5800LV and through X-ray intensity maps collected on a FEI Quanta 450 FEG at the U.S. Geological Survey in Denver, Colorado. Qualitative energy dispersive X-ray spectroscopy (EDS) chemical analyses of the crystals verified the mineralogy. Textural associations of the phosphates with surrounding minerals, potential mineral inclusions, and any mineral zonation were documented with BSE imagery.
Taylor et al. (2015) showed that both monazite and xenotime in ore from the granodiorite-hosted Empire orogenic gold deposit, Grass Valley, California, were hydrothermal in origin by utilizing textural criteria, the lack of xenotime within the host granodiorite, and distinct chemical differences such as Eu anomalies in monazite of primary magmatic origin found in the host rock and hydrothermal monazite within the ore. Samples from the Empire deposit were utilized in this study that used the above criteria, but textural characteristics were most important in identifying the origin of monazite and xenotime in the other analyzed ore samples. One feature that was searched for and would rule out hydrothermal origin was crystals that are broken, especially with broken edges that truncated growth banding that would suggest damage during transportation. However, the most important texture was intergrowth of the phosphate crystals with ore minerals and hydrothermal alteration phases (Fig. 2). If the phosphate crystals appear syn-formational or post-formational in relation to other known hydrothermal phases, then it can be safely deduced that the phosphates are not from the original host rock and are hydrothermal in origin.
Major and minor element chemistry was collected in situ using a JEOL 8900 electron microprobe (EMP) with five wavelength dispersive X-ray spectrometers and a JEOL 8530F Plus SuperProbe with five wavelength dispersive X-ray spectrometers at the U.S. Geological Survey in Denver, Colorado. Operating parameters specific to the microprobe sessions are found in Taylor and Adams (2018). Duplicate standards and unknowns from each session were cross checked for data consistency.
Although electron microprobe analyses have higher detection limits than other methods such as LA-ICP-MS, it was chosen as the analytical method due to its non-destructive nature which is particularly important for phases of the size encountered in this study. Both monazite and xenotime are known geochronometers (e.g., Vielreicher et al. 2003; Taylor et al. 2015), and their preservation is important for future geochronological U-Th-Pb investigations. Additionally, the small size of these phosphate crystals may not facilitate the use of a larger laser ablation beam. Monazite and xenotime are trace components of the ore and analyses have the potential to incorporate underlying or adjacent phases within the analytical beam due to their small size. Their potential destruction during an unsuccessful laser ablation analysis would decrease the population of crystals and lead to a less robust data set. The utilization of an electron microbeam allows for reanalysis of a crystal if the beam in the original analysis inadvertently overlaps with an adjacent phase and leads to tainted results.
Results
In situ geochemical data for monazite and xenotime from this study are presented in Figs. 3, 4, 5, 6, 7, 8, and 9 and found in Taylor and Adams (2018) and supplementary table S1. Due to the microscopic size of the crystals and imperfect certainty in EMP optics for beam location, some phosphate analyses were contaminated by adjacent minerals. Analyses with < 97% or > 103% weight totals, or elevated SiO2 or Al2O3 contents that indicate mixed mineral analyses, were excluded from further interpretation. A total of 247 monazite and 67 xenotime analyses were accepted. This includes 31 monazite and 13 xenotime from porphyry Cu-Mo-Au deposits, 130 monazite and 6 xenotime from reduced intrusion-related deposits, 50 monazite and 35 xenotime from orogenic gold deposits, and 36 monazite and 13 xenotime analyses from the controversially classified Pogo gold deposit.
Comparison of chondrite-normalized rare earth element (REE) patterns for hydrothermal monazite. Stray data points indicate that adjacent data were below detection limit. Data found below detection limit are not shown. Variously shaded lines represent individual analyses from a given deposit. A Porphyry Cu-Mo-Au hydrothermal monazite from Butte, Montana (blue) and Pebble, Alaska (red). B Orogenic gold hydrothermal monazite from deposits hosted in greenschist-facies metamorphic rocks (blue) and granitoids (red). C Reduced intrusion-related gold (RIRG) hydrothermal monazite from Clear Creek, Yukon (blue) and Shotgun, Alaska (red). D Pogo gold deposit hydrothermal monazite, Alaska. Chondrite values from McDonough and Sun (1995)
Box-and-whisker plot comparison of selected elemental compositions of hydrothermal monazite from porphyry Cu-Mo-Au deposits (shaded in red; Porphyry), reduced intrusion-related deposits (shaded in blue; RIRG), orogenic gold deposits (shaded in yellow; Orogenic), and the Pogo deposit (shaded in dark gray; Pogo). Box plots depict the minimum, first quartile, median, third quartile, and maximum, with outliers depicted as single points
Comparison of elemental characteristics of hydrothermal monazite formed from porphyry Cu-Mo-Au deposits (shaded in red) from Butte, Montana (red squares with no fill), and Pebble, Alaska (red circles); reduced intrusion-related deposits (shaded in blue) from Clear Creek, Yukon (light blue circles), and Shotgun Alaska (dark blue squares with no fill); orogenic gold deposits (shaded in yellow) hosted in granitoids (yellow circles) and metamorphic rocks (gold squares with no fill); and the Pogo deposit (shaded in dark gray with black diamonds). A Plot of Pr weight % versus Nd/Sm weight %. B Plot of Ce/Pr weight % versus Sm/Gd weight %. C Plot of Th weight % versus total LREE (Ce-Gd) weight %. D Y weight % versus Dy weight %. E U weight % versus Th weight %. F Y weight % verus La/Sm weight %
Comparison of chondrite-normalized REE patterns for hydrothermal xenotime. Stray data points indicate that adjacent data were below detection limit. Data found below detection limit are not shown. Variously shaded lines represent individual analyses from a given deposit. A Porphyry Cu-Mo-Au hydrothermal xenotime from Butte, Montana (blues) and Pebble, Alaska (reds). B Orogenic gold hydrothermal xenotime from deposits hosted in greenschist-facies metamorphic rocks (blues) and granitoids (reds). C Reduced intrusion-related gold (RIRG) hydrothermal xenotime from Clear Creek, Yukon (blues) and Shotgun, Alaska (reds). D Pogo gold deposit hydrothermal xenotime, Alaska. Chondrite values from McDonough and Sun (1995)
Box-and-whisker plot comparison of selected elemental compositions of hydrothermal xenotime from porphyry Cu-Mo-Au deposits (shaded in red; Porphyry), reduced intrusion-related deposits (shaded in blue; RIRG), orogenic gold deposits (shaded in yellow; Orogenic), and the Pogo deposit (shaded in dark gray; Pogo). Box plots depict the minimum, first quartile, median, third quartile, and maximum, with outliers depicted as single points
Comparison of elemental characteristics of hydrothermal xenotime formed from porphyry Cu-Mo-Au (shaded in red) from Butte, Montana (red squares with no fill), and Pebble, Alaska (red circles); reduced intrusion-related deposits (shaded in blue) from Clear Creek, Yukon (light blue circles), and Shotgun Alaska (dark blue squares with no fill); orogenic gold deposits (shaded in yellow) hosted in granitoids (yellow circles) and metamorphic rocks (gold squares with no fill); and the Pogo deposit (shaded in dark gray with black diamonds). A Th weight % versus Y weight %. B Gd/Tb weight % versus Yb/Sm weight %. C Ca weight % versus Sc weight %. D Ho weight % versus Y weight %
Chondrite-normalized REE patterns for hydrothermal monazite crystallized from calc-alkaline porphyry Cu-Mo-Au deposits compared with primary magmatic monazite found in calc-alkaline intrusions. Note the similarities between hydrothermal and magmatic monazite found in different calc-alkaline systems. Data for magmatic monazite from the Grass Valley granodiorite from Taylor et al. (2015). Chondrite values from McDonough and Sun (1995)
Monazite
Variations in REE concentrations and ratios in monazite demonstrate that there are some differences, albeit overlapping, among deposit classes in geochemical space. In chondrite-normalized REE profiles for monazite crystals, near linear profiles for both magmatic-hydrothermal deposit types contrast with the more sawtooth pattern for orogenic gold deposits and the Pogo deposit (Fig. 3). The spread in Sm and Gd chondrite-normalized values is large for orogenic gold deposits but narrow for magmatic-hydrothermal deposits. Beyond a few outliers, most analyses of magmatic-hydrothermal monazite have Eu anomalies ranging from strongly negative to absent, and negligible Ce anomalies. A large spread in negative to positive Eu anomalies is noted for orogenic gold deposits (Fig. 3). Both orogenic gold deposits and the Pogo deposit usually display slightly positive Ce anomalies.
The vast majority of the hydrothermal monazite analyzed tends to have As, Ho, Lu, Pb, Sc, Tm, and Yb concentrations below their respective detection limits (supplementary table S1; Taylor and Adams 2018). Low U concentrations (< 0.3 wt%; more than 50% of analyses below detection limits) characterize monazite crystals from reduced intrusion-related deposits (Figs. 4 and 5e). Terbium was most commonly detected in porphyry Cu-Mo-Au deposits. Erbium was detected mostly in monazite from orogenic gold deposits and the Pogo deposit (Fig. 4). Dysprosium concentrations were highly variable in orogenic gold deposits and the Pogo deposit, where determinations range from some of the higher values to numerous non-detects but were universally detected in all magmatic-hydrothermal monazite (Fig. 4). Both types of magmatic-hydrothermal systems have higher total LREE (La-Gd) in monazite than the majority of those from orogenic gold deposits or from the Pogo deposit (Fig. 5c). Praseodymium is particularly elevated and more restricted in concentration for both magmatic-hydrothermal systems than for orogenic gold deposits and the Pogo deposit (Figs. 4 and 5a).
In addition to restricted Pr values, magmatic-hydrothermal monazite also displays narrow Ce/Pr, Nd/Sm, and La/Sm ratios compared to orogenic gold and Pogo deposit monazite (Fig. 5a, b, f). A general positive trend between Dy and Y exists for magmatic-hydrothermal monazite, whereas orogenic gold deposits lack a systematic pattern; monazite from the Pogo deposit shows a positive correlation with a starkly different slope (Fig. 5d).
Xenotime
The use of REE content of xenotime as genetic discriminators is more ambiguous than it is for monazite, at least partially due to overall scarcity of hydrothermal xenotime compared to monazite. Xenotime from orogenic gold deposits has a fairly flat MREE-HREE profile, whereas porphyry Cu-Mo-Au deposits have a noticeable enrichment in Lu, and reduced intrusion-related deposits have a flat to declining MREE-HREE profile characterized by a minor sawtooth pattern (Fig. 6). Cerium anomalies are commonly not calculated for xenotime due to La, Ce, and/or Pr concentrations below detection limits and Eu anomalies for all analyses except three from the Pogo deposit were negative.
Although some distinctions in xenotime chemistry may be found between magmatic-hydrothermal and metamorphic-hydrothermal systems, they are not as divergent as those observed for monazite (Figs. 7 and 8). Lanthanum and Pr are typically found below detection limit in all classes of deposits, whereas Ce is commonly below detection limit except in granitoid-hosted orogenic gold deposits. Arsenic is usually below detection limit except in metamorphic rock-hosted orogenic gold deposits. Calcium and Pb concentrations in xenotime are typically below detection limit in reduced intrusion-related deposits but more elevated in other classes (Fig. 7), although Pb is also commonly found at very low concentrations for xenotime from orogenic gold deposits.
Discussion
Potential causes of chemical differences
The REEs are geochemically similar although their atomic radii systematically change with atomic number, which can lead to fractionation. The hydrothermal monazite and xenotime samples analyzed in this study exhibit geochemical differences in REE and other minor element substitutions that we attribute to the chemistry of mineralizing fluids. The chemistry of fluids at the time of mineralization, and particularly REE budget, is controlled by the complex interplay of many factors, including (1) the elements released/incorporated into precipitating alteration and ore minerals (including REE-incorporating minerals at/near the mineralizing site or along the fluid flow path), (2) changes in fluid composition due to reactions along the fluid flow path, and (3) the initial chemistry of fluids from their source.
Point (1) suggests that fluid:rock interaction during mineralization might be important, and our examination of orogenic vein samples hosted in different host rocks attempts to address this point. Fundamentally, these mineralizing fluids are interpreted to be broadly compositionally similar metamorphic-hydrothermal fluids that precipitated gold in veins in different rock types in the mineralizing environment. Therefore, we can investigate whether elements that are released during alteration of the local host rock may affect phosphate geochemistry. Since REE can substitute for Ca in many rock-forming minerals (e.g., feldspars, carbonates, apatite) due to similar ionic radii, the destruction or preservation of these rock-forming minerals during hydrothermal alteration may lead to fractionation of the REE budget available for crystallization of hydrothermal phases. The same would be true for other minor to trace element substitutions within the original rock-forming minerals. Despite diverse host rocks for the different orogenic gold deposits, the general chemical characteristics of hydrothermal monazite persist. However, there are some noted differences in xenotime chemistry between granite-hosted and metamorphic rock-hosted orogenic gold deposits, such as Sc and Ca concentrations, although they can still be distinguished in most respects from magmatic-hydrothermal xenotime (Fig. 8c). The same conclusion can be reached for the different magmatic-hydrothermal deposits since the host rocks for the Butte and Pebble porphyry Cu-Mo-Au deposits and the reduced intrusion-related Clear Creek and Shotgun deposits are of different ages, composition, and chemistry, yet both monazite and xenotime in each system share unique geochemical characteristics. This suggests that the composition of local host rock has a negligible effect on the chemistry of hydrothermal phosphates except in some instances for xenotime from orogenic gold deposits.
It is possible that differences in alteration products would affect the minor element content of these phosphates as some of these elements may preferentially fractionate into other phases. However, crystallization of other REE-compatible hydrothermal phases is also an insignificant contributor to geochemical differences noted in this study. Hydrothermal zircon and titanite, the other important REE-bearing phases identified within some of these deposits, are found in low concentrations and would therefore have a minimal potential to affect the availability of REE for phosphate crystallization. Additionally, differences between the proportions of these other phases does not demonstrably affect the chemistry of the phosphates when comparing those from a given mineral system. This is supported by the consistency of the phosphate chemistry within a given mineral system despite inevitable differences in minor mineral alteration assemblages.
Point (2) relating to potential changes in fluid chemistry caused by reactions along the hydrothermal fluid flow path are rendered mute for the magmatic-hydrothermal deposits in this study since the mineralizing pathways are spatially limited and only extend from the parental pluton into the immediately surrounding country rocks. Differences in these host rock types have been covered above regarding point (1). However, this could potentially play a role regarding fluid chemistry for orogenic gold deposits since the fluid pathway may traverse kilometers of rock. This factor can be considered negligible in this case, since a total of eight deposits were analyzed which are found within a diverse set of accreted terranes that are presumably composed of various protoliths at depth that the hydrothermal fluids traversed.
Of most interest are differences in the fluid chemistry that arise due to the different ways that fluids are generated (point 3). The greenschist- to amphibolite-facies transition that generates much of the metamorphic-hydrothermal fluids responsible for orogenic gold formation (Phillips and Powell 2010) is calculated to occur over a temperature range of 440 to 520 °C, partially influenced by the bulk-rock composition that is undergoing metamorphism and phase equilibria of volatile-bearing phases that are present (Elmer et al. 2006). This leads to a low-salinity H2O-CO2-H2S-bearing fluid due to the breakdown of the chlorite-calcite-albite-epidote-quartz-sulfide mineral assemblage, with orogenic veins typically crystallizing from that fluid at 250–400 °C (Goldfarb et al. 2005). Residual garnet and hornblende in the host rock acts as a sink for retaining REE and influences their availability to a hydrothermal fluid. Bisulfide complexes are the most important ligand for transporting gold within these systems (Seward 1973, 1991; Benning and Seward 1996; Loucks and Mavrogenes 1999) although chloride complexes are generally more important for transporting REE (Migdisov et al. 2016).
Magmatic-hydrothermal fluids are generated at higher temperatures and through different means. Within oxidized magmatic-hydrothermal porphyry Cu-Mo-Au systems, both hypersaline fluid and immiscible vapor are responsible for alteration and ore deposition at temperatures ranging from greater than 600 °C down to about 300 °C (Hedenquist and Lowenstern 1994). In contrast, reduced intrusion-related gold deposits are formed by low-salinity, H2O-CO2 fluids and may also have CH4-rich inclusions (Hart 2007). The metal chemistry of the magmatic-hydrothermal fluid phases is largely dependent upon the effects of crystal fractionation of the magma prior to fluid-magma separation and the source components of the magma. Feldspars, zircon, and apatite represent fractionated phases that will influence the REE chemistry of the magmatic-hydrothermal fluid. The temperature of the hydrothermal fluid will also affect fractionation of LREE and HREE since aqueous LREE chloride complexes are more stable and therefore more mobile compared to HREE as temperatures are increased (Migdisov et al. 2016). Overall, the REE pattern of magmatic-hydrothermal REE-bearing phosphates mimics the chemistry of the same igneous phases that were crystallized earlier from a calc-alkaline magma (Fig. 9), demonstrating the dependence of the hydrothermal fluid chemistry upon the chemistry of the source pluton as the system undergoes magmatic to hydrothermal evolution. This has additionally been demonstrated with hydrothermal zircon having indistinguishable REE patterns from late magmatic zircon (Pettke et al. 2005).
Deposit comparisons
Geochemical distinctions within hydrothermal monazite and xenotime can be used to discriminate between different types of gold deposits found within accreted terranes, reflective of the type of hydrothermal fluid responsible for gold mineralization (Figs. 3, 4, 5, 6, 7, and 8). Furthermore, analyses of different deposits within a single deposit class are remarkably similar.
As summarized in the “Results” section, chemical distinctions that can differentiate between different deposit classes are prevalent for monazite (Figs. 3, 4, and 5). Although Ce, La, Nd, and Th are common major constituents in monazite’s chemical structure, it is largely the more minor constituents that can discern the deposit class. For the actinides, U concentrations are much lower for the reduced intrusion-related deposits compared to the correspondingly reduced orogenic gold deposits (Fig. 4). The U and Th contents of the monazite studied are not influenced by radioactive decay as the ages of deposits within a deposit class are highly variable. The utilization of elemental ratios can clearly identify the deposit classes, partially due to the sawtooth nature of the REE profiles for orogenic gold deposits compared to the more linear trend for both magmatic-hydrothermal deposit classes. This is acutely evident for Nd/Sm, Sm/Gd, Ce/Pr, and La/Sm (Fig. 5). Of those ratios, Sm and Pr feature prominently in the discrimination figures because both classes of magmatic-hydrothermal gold deposits have fairly restricted Sm and Pr compared to a wider range found within orogenic gold deposits.
Migdisov et al. (2016) noted differences in mobility of LREE versus HREE in aqueous chloride complexes in response to changing temperatures, with greater LREE mobility at higher temperatures. The potentially higher temperatures of formation and higher chloride activity within magmatic-hydrothermal fluids could be a reason that LREE-bearing monazite is more common than HREE-bearing xenotime within these systems. This is consistent with the higher total LREE content with less variation in monazite from magmatic-hydrothermal systems compared to orogenic gold deposits (Fig. 5c). Distinctive differences in the occurrence of monazite to xenotime within these deposit classes are clearly noted, but this metric should not be taken as a definitive discriminator and instead should be viewed as supplementary to the geochemical data. The paucity of magmatic-hydrothermal xenotime compared to those of metamorphic-hydrothermal origin leads to more difficulty in effectively using this phase for geochemical discrimination compared to monazite. However, non-REE elemental substitutions such as Ca, Sc, and Th in xenotime may be better chemical discriminators, although some distinctions can be found in Gd/Tb and Yb/Sm ratios. Additionally, the chemistry of hydrothermal xenotime from orogenic gold deposits is more sensitive to host rock type, whereas the chemistry of monazite appears to be independent of host rock.
Pogo gold deposit classification
Our proposed discrimination scheme as presented above suggests that phosphate (particularly monazite) compositions vary based on the deposit type, and thus source of mineralizing fluids, as shown in Figs. 4, 5, 7, and 8. Using this framework, we compared monazite and xenotime crystal chemistries from the Pogo gold deposit to see whether compositions are consistent with one or the other of the competing genetic models—an intrusion-related magmatic-hydrothermal origin (as proposed by some for the deposit; e.g., Smith et al. 1999; Rhys et al. 2003) or a metamorphic-hydrothermal fluid derived orogenic gold deposit (e.g., Groves et al. 2003; Marsh et al. 2009; Goldfarb and Pitcairn 2022).
The monazite REE patterns of Pogo display a subtle yet jagged sawtooth pattern in the LREE range that resembles that of orogenic gold deposits and is distinct from the smoother LREE pattern of both magmatic-hydrothermal gold system types studied. Furthermore, the slope of the Dy-Y join is much flatter than the magmatic-hydrothermal-related monazite crystals (Fig. 3, also reflected in Fig. 5d). The consistently low Tb contents found below detection limits of Pogo and orogenic gold deposits are unlike the more elevated concentrations associated with porphyry Cu-Mo-Au deposits (Fig. 3).
Element relationships demonstrated on plots of Fig. 5 indicate that the Pogo monazite chemistry (gray field) is very similar to orogenic monazite chemistry (yellow field). Furthermore, there is little to no overlap in plotted parameters between Pogo and both porphyry Cu-Mo-Au and reduced intrusion-related deposits (Fig. 5a–d). Pogo (and many orogenic gold) samples have lower Pr concentrations (~ 1.5–2.5 wt%; Fig. 5a) and higher Ce/Pr ratios (Ce/Pr = 11–16; Fig. 5b) than magmatic-hydrothermal deposits. Total LREE concentrations are also slightly lower and more like orogenic Th-bearing monazite (Fig. 5c). As a population, the Sm/Gd ratio is lower than the reduced intrusion-related deposits. Pogo monazite also has distinctly lower Dy concentrations at a given Y concentration than the magmatic-hydrothermal samples, but which overlaps with some orogenic gold monazite (Fig. 5d).
The chemistry of hydrothermal xenotime is more ambiguous, especially regarding most REE. This may in part be a consequence of limited abundance of xenotime, particularly in the magmatic-hydrothermal systems. However, our reconnaissance data suggest the use of Y, Sc, and Ca, along with occurrence of xenotime can complement the monazite data. In particular, in the Ca vs. Sc plot, the xenotime data from Pogo overlap with and follow a similar trend (elevated Ca) to metamorphic rock-hosted orogenic gold deposits, distinct from data for reduced intrusion-related systems (Sc > detection) and porphyry Cu-Mo-Au systems (Ca > 0.2 wt%; Fig. 8c). Additionally, xenotime geochemical data for orogenic gold deposits and the Pogo deposit for Y, Ho, Gd/Tb, and Yb/Sm tend to cluster, whereas a large spread in data for components is observed for magmatic-hydrothermal systems despite the limited number of xenotime crystals available for analyses. The relative proportion of monazite:xenotime for the Pogo deposit (2.8) is nearly identical to that for the studied metamorphic rock-hosted orogenic gold deposits (2.5).
The compositional data for monazite and xenotime formed as alteration products at Pogo show strong similarities to the ranges of compositions from known orogenic gold deposits and dissimilarities with magmatic-hydrothermal (and particularly reduced intrusion-related) deposits based on several distinctive parameters examined in this study. The in situ major and minor element geochemistry associated with these hydrothermal phases are evidence suggesting that the Pogo gold deposit is in fact an orogenic gold deposit, as is also suggested by some geologic evidence such as the lack of identification of a causative pluton.
Conclusions
Geochemically distinguishing between different types of gold deposits, particularly orogenic versus reduced intrusion-related, has proven problematic. This in turn has led to controversial classifications that are utilized in regional exploration and mine development. This study has established a viable method of discriminating between orogenic gold, reduced intrusion-related gold, and porphyry Cu-Mo-Au deposits using the chemistry and occurrence of hydrothermal monazite and xenotime. The observed chemical variations in these hydrothermal phosphate crystals are inferred to reflect different sources for the mineralizing fluids. Although there is overlap in compositions, hydrothermal monazite from orogenic gold deposits displays distinctly different compositions in parameters such as REE profiles, Dy, Er, Pr, Y, Nd/Sm, and La/Sm. The potential of hydrothermal xenotime as a chemical discriminator is less robust. However, differences in some HREEs, Ca, and Sc have been noted for xenotime between the different classes of deposits studied. The abundance of xenotime relative to monazite may be an indicator if the fluids are sourced from exsolved magmatic fluids or from metamorphic dehydration reactions, especially if used in conjunction with monazite chemistry.
When this geochemical discrimination approach is applied to the Pogo deposit, monazite chemistry, and to a lesser extent xenotime chemistry, is consistent with Pogo being an orogenic gold deposit. This classification concurs with that suggested by some researchers (e.g., Groves et al. 2003; Marsh et al. 2009) and contrasts with the current mine model and what other researchers have advocated (e.g., Smith et al. 2000; Rhys et al. 2003; Baker et al. 2006). This geochemical discrimination tool may prove significant for exploration in regions that contain both orogenic and magmatic-hydrothermal gold deposits. Understanding what type of gold deposits are found in an area will help establish what geologic criteria geologists should focus on during exploration and mine development.
Data availability
All geochemical data are publicly available in Taylor and Adams (2018) and in supplementary table 1.
Code availability
Not applicable.
References
Allen TL, Hart CJR, Marsh EE (1998) Placer gold and associated heavy minerals of the Clear Creek drainage, central Yukon: past to present. In: Roots CF, Emonds DS (eds) Yukon Exploration and Geology 1998, Exploration and Geological Services Division, Yukon, Indian and Northern Affairs Canada, pp 197–214
Ayuso RA, Kelley KD, Eppinger RG, Forni F (2013) Pb-Sr-Nd isotopes of surficial materials at the Pebble porphyry Cu-Au-Mo deposit, southwestern Alaska: can the mineralizing fingerprint be detected through cover? Econ Geol 108:543–562
Baker T, Ebert S, Rombach C, Ryan CG (2006) Chemical compositions of fluid inclusions in intrusion-related gold systems, Alaska and Yukon, using PIXE microanalysis. Econ Geol 101:311–327
Benning LG, Seward TM (1996) Hydrosulphide complexing of Au (I) in hydrothermal solutions from 150–400° C and 500–1500 bar. Geochim Cosmochim Acta 60:1849–1871
Berry AL, Dalrymple GB, Lanphere MA, Van Essen JC (1979) Summary of miscellaneous potassium-argon age measurements, USGS, Menlo Park, California for years 1972–1974. U.S. Geological Survey Circular 727
Blevins PL, Chappell BW (1992) The role of magma sources, oxidation states and fractionation in determining the granite metallogeny of eastern Australia. Earth Environ Sci Trans R Soc Edinb 83:305–316
Bowen OE Jr, Crippen RA Jr (1997) California’s Mother Lode, north San Juan to Sattley, El Dorado, Placer, and Nevada Counties. Calif Geol 6:178–186
Clark WB, Fuller WP Jr (1968) The original sixteen to one mine. Calif Division of Mines Geol (Min Inf Serv) 21:71–75
Dilles JH, Martin MW, Stein H, Rusk B (2003) Re-Os and U-Pb ages for the Butte copper district, Montana—a short- or long-lived hydrothermal system? [abs.]. Geological Society of America Abstracts with Programs 35 (6): pp 400
Dilworth KM, Mortensen JK, Ebert S, Tosdal RM, Smith MT, Roberts P (2007) Cretaceous reduced granitoids in the Goodpaster Mining District, east central Alaska. Canadian J of Earth Science 44:1347–1373
Elmer FL, White RW, Powell R (2006) Devolatilization of metabasic rocks during greenschist-amphibolite facies metamorphism. J of Metamorphic Geol 24:497–513
Ferrero T (1990) The Liberty gold mining district, Siskiyou County, California. Calif Geol 43:123–133
Goldfarb RJ, Pitcairn I (2022) Orogenic gold: is a genetic association with magmatism realistic? Miner Deposita. https://doi.org/10.1007/s00126-022-01146-8
Goldfarb RJ, Leach DL, Pickthorn WJ, Paterson CJ (1988) Origin of lode-gold deposits of the Juneau gold belt, southeastern Alaska. Geology 16:440–443
Goldfarb RJ, Baker T, Dubé B, Groves DI, Hart CJR, Gosselin P (2005) Distribution, character, and genesis of gold deposits in metamorphic terranes. Econ Geol 100th Anniv Volume pp 407–450
Goldfarb RJ, Marsh EE, Hart CJR, Mair JL, Miller ML, Johnson C (2007) Geology and origin of epigenetic lode gold deposits, Tintina Gold Province, Alaska and Yukon. In: Gough LP, Day WC (eds) Recent U.S. geological survey studies in the Tintina gold province, Alaska, United States, and Yukon, Canada—results of a 5-year project. U.S. Geological Survey Scientific Investigations Report 2007–5289-A
Goldfarb RJ, Hart CJR, Marsh EE (2008) Orogenic gold and evolution of the Cordilleran orogeny. In: Spencer JE, Titley SR (eds) Ores and orogenesis: Circum-Pacific tectonics, geologic evolution, and ore deposits. Arizona Geol Soc Digest 22:311–323
Groves DI, Goldfarb RJ, Gebre-Mariam M, Hagemann SG, Robert F (1998) Orogenic gold deposits: a proposed classification in the context of their crustal distribution and relationship to other gold deposit types. Ore Geol Rev 13:7–27
Groves DI, Goldfarb RJ, Robert F, Hart CJR (2003) Gold deposits in metamorphic belts: overview of current understanding, outstanding problems, future research, and exploration significance. Econ Geol 98:1–29
Hart CJR (2005) Classifying, distinguishing and exploring for intrusion-related gold systems. The Gangue 87:1–9
Hart CJR (2007) Reduced intrusion-related gold systems. In: Goodfellow WD (ed) Mineral deposits of Canada: a synthesis of major deposit types, district metallogeny, the evolution of geological provinces, and exploration methods. Geological Association of Canada, Mineral Deposits Division, Special Publication No. 5, pp 95–112
Hedenquist JW, Lowenstern JB (1994) The role of magmas in the formation of hydrothermal ore deposits. Nature 370:519–527
Hofstra AH, Kreiner DC (2020) Systems-deposits-commodities-critical minerals table for the earth mapping resources initiative. U.S. Geological Survey Open-File Report 2020–1042
Hotz PE (1971) Geology of lode gold districts in the Klamath Mountains, California and Oregon. U.S. Geological Survey Bulletin 1290
Houston RA, Dilles JH (2013) Structural geologic evolution of the Butte district, Montana. Econ Geol 108:1397–1424
Ishihara S (1981) The granitoid series and mineralization. Econ Geol 75th Anniv Vol pp 458–484
John DA, Ayuso RA, Barton MD, Blakely RJ, Bodnar RJ, Dilles JH, Gray F, Graybeal FT, Mars JC, McPhee DK, Seal RR, Taylor RD, Vikre PG (2010) Porphyry copper deposit model, chap. B of Mineral deposit models for resource assessment. U.S. Geological Survey Scientific Investigations Report 2010–5070–B
Kelley KD, Lang JR, Eppinger RG (2013) The giant Pebble Cu-Au-Mo deposit and surrounding region, southwest Alaska: introduction. Econ Geol 108:397–404
Kositcin N, McNaughton NJ, Griffin BJ, Fletcher IR, Groves DI, Rasmussen B (2003) Textural and geochemical discrimination between xenotime of different origin in the Archaean Witwatersrand Basin, South Africa. Geochim Cosmochim Acta 67:709–731
Lang JR, Baker T (2001) Intrusion-related gold systems: the present level of understanding. Miner Deposita 36:477–489
Lang JR, Gregory MJ, Rebagliati CM, Payne JG, Oliver JL, Roberts K (2013) Geology and magmatic-hydrothermal evolution of the giant Pebble porphyry copper-gold-molybdenum deposit, southwest Alaska. Econ Geol 108:437–462
Li N, Chen YJ, Fletcher IR, Zeng QT (2011) Triassic mineralization with Cretaceous overprint in the Dahu Au-Mo deposit, Xiaoqinling gold province: constraints from SHRIMP monazite U-Th-Pb geochronology. Gondwana Res 20:543–552
Loucks RR (2021) Deep entrapment of buoyant magmas by orogenic tectonic stress: its role in producing continental crust, adakites, and porphyry copper deposits. Earth Sci Rev 220:103744
Loucks RR, Mavrogenes JA (1999) Gold solubility in supercritical hydrothermal brines measured in synthetic fluid inclusions. Science 284:2159–2163
Lund K, Aleinikoff JN, Kunk MJ, Unruh DM, Hodges WC, du Bray EA, O’Neill JM, Zeihen G (2002) SHRIMP U-Pb and 40Ar/39Ar age constraints for timing of mineralization in the Boulder batholith, Montana. Econ Geol 97:241–267
Lund K, McAleer RJ, Aleinikoff JN, Cosca MA, Kunk MJ (2018) Two-event lode-ore deposition at Butte, USA: 40Ar/39Ar and U-Pb documentation of Ag-Au-polymetallic lodes overprinted by younger stockwork Cu-Mo ores and penecontemporaneous Cu lodes. Ore Geol Rev 102:666–700
Mair JL, Hart CJR, Stephens JR (2006) Deformation history of the western Selwyn Basin, Canada: implications for orogen evolution and the setting of mid-Cretaceous magmatism. Geol Soc Am Bull 118:304–323
Marsh EE, Goldfarb RJ, Hart CJR, Johnson CA (2003) Geology and geochemistry of the Clear Creek intrusion-related gold occurrences, Tintina gold province, Yukon, Canada. Canadian J of Earth Sciences 40:681–699
Marsh EE, Goldfarb RJ, Kunk MJ, Groves DI, Bierlein FP, Creaser RA (2008) New constraints on the timing of gold formation in the Sierra Foothills province, central California. Arizona Geol Soc Digest 22:369–388
Marsh EE, Landis GP, Emsbo P, Todorov TI, Goldfarb RJ (2009) Geochemistry of fluid inclusions from orogenic and intrusion-related gold deposits in the Tintina gold province, Alaska and the Yukon Territory [abs.]. Geol Soc America Abstracts with Programs 256(7) pp 256
McDonough WF, Sun S-s (1995) The composition of the Earth. Chem Geol 120:223–253
Mercer CN, Reed MH (2013) Porphyry CU-Mo stockwork formation by dynamic, transient hydrothermal pulses: mineralogic insights from the deposit at Butte, Montana. Econ Geol 108:1347–1377
Migdisov A, Williams-Jones AE, Brugger J, Caporuscio FA (2016) Hydrothermal transport, deposition, and fractionation of the REE: experimental data and thermodynamic calculations. Chem Geol 439:13–42
Murphy DC (1997) Geology of the McQuesten River region, northern McQuesten and Mayo Map area, Yukon Territory. Exploration and Geological Services Division, Yukon, Indian and Northern Affairs Canada, Bulletin 6
Northern Star Resources Limited, 2022, https://www.nsrltd.com/our-assets/pogo-production-centre. Accessed 25 October 2022
Pease RC (2009) Idaho-Maryland mine project, Grass Valley CA, technical report. Prepared for Emgold Mining Corporation
Petersen SW (1993) Geology and geochemistry of the Craggy Peak pluton, Deadman Peak pluton, and the Billy’s Peak mafic complex, Klamath Mountains, California. Dissertation, Texas Tech University
Pettke T, Audétat A, Schaltegger U, Heinrich CA (2005) Magmatic-to-hydrothermal crystallization in the W-Sn mineralized Mole Granite (NSW, Australia) Part II: evolving zircon and thorite trace element chemistry. Chem Geol 220:191–213
Phillips GN, Powell R (2010) Formation of gold deposits: a metamorphic devolatilization model. J Metamorphic Geol 28:689–718
Pitcairn IK, Teagle DAH, Craw D, Olivo GR, Kerrich R, Brewer TW (2006) Sources of metals and fluids in orogenic gold deposits: insights from the Otago and Alpine Schists, New Zealand. Econ Geol 101:1525–1546
Qiu K, Yu H, Wu M, Geng J, Ge X, Gou Z, Taylor RD (2019) Discret Zr and REE mineralization of the Baerzhe rare-metal deposit, China. Am Miner 104:1487–1502
Rombach CS, Newberry RJ (2001) Shotgun deposit: granite porphyry-hosted gold-arsenic mineralization in southwestern Alaska, USA. Miner Deposita 36:607–621
Rhys D, DiMarchi J, Smith M, Friesen R, Rombach C (2003) Structural setting, style and timing of vein-hosted gold mineralization at the Pogo deposit, east central Alaska. Miner Deposita 38:863–875
Rusk BG, Reed MH, Dilles JH, Klemm LM, Heinrich CA (2004) Compositions of magmatic hydrothermal fluids determined by LA-ICP-MS of fluid inclusions from the porphyry copper-molybdenum deposit at Butte, MT. Chem Geol 210:173–199
Schandl ES, Gorton MP (2004) A textural and geochemical guide to the identification of hydrothermal monazite: criteria for selection of samples for dating epigenetic hydrothermal ore deposits. Econ Geol 99:1027–1035
Selby D, Creaser RA, Hart CJR, Rombach CS, Thompson JFH, Smith MT, Bakke AA, Goldfarb RJ (2002) Absolute timing of sulfide and gold mineralization: a comparison of Re-Os molybdenite and Ar-Ar mica methods from the Tintina Gold Belt, Alaska. Geology 30:791–794
Selby D, Creaser RA, Heaman LM, Hart CJR (2003) Re-Os and U-Pb geochronology of the Clear Creek, Dublin Gulch, and Mactung deposits, Tombstone Gold Belt, Yukon, Canada: absolute timing relationships between plutonism and mineralization. Canadian J Earth Sci 40:1839–1852
Seward TM (1973) Thio complexes of gold and the transport of gold in hydrothermal ore solutions. Geochim Cosmochim Acta 37:379–399
Seward TM (1991) The hydrothermal geochemistry of gold. In: Foster RP (ed) Gold Metallogeny and Exploration pp 37–62
Shasta Gold Corp. (2014). http://www.shastagoldcorp.com/?page_id=16. Accessed September 2014
Sibson RH (1990) Conditions for fault-valve behavior. Geol Soc London Special Publication 54:15–28
Sibson RH, Robert F, Poulsen KH (1988) High-angle reverse faults, fluid-pressure cycling, and mesothermal gold-quartz deposits. Geology 16:551–555
Sillitoe RH (1993) Gold-rich porphyry copper deposits: geological model and exploration implications. In: Kirkham RV, Sinclair WD, Thorpe RI, Duke JM (eds) Mineral deposit modeling: Geol Assoc Canada Special Paper 40 pp 465–478
Sillitoe RH (2000) Gold-rich porphyry deposits: descriptive and genetic models and their role in exploration and discovery. SEG Rev 13:315–345
Singer DA, Berger VI, Moring BC (2008) Porphyry copper deposits of the world—database and grade and tonnage models, 2008. U.S. Geological Survey Open-File Report 2008–1155
Smith M, Thompson JFH, Bressler J, Layer P, Mortensen JK, Abe I, Takaoka H (1999) Geology of the Liese Zone, Pogo property, east-central Alaska. SEG Discovery 38:1–21
Smith CM, Canil D, Rowins SM, Friedman R (2012) Reduced granitic magmas in an arc setting: the Catface porphyry Cu-Mo deposit of the Paleogene Cascade Arc. Lithos 154:361–373
Smith MT, Thompson JFH, Moore KH, Bressler JR, Layer P, Mortensen JK, Abe I, Takaoka H (2000) The Liese zone, Pogo property: a new high-grade gold deposit in Alaska. In: Tucker TL, Smith MT (eds) The Tintina Gold Belt: Concepts, exploration and discoveries. British Columbia-Yukon Chamber of Mines, Cordilleran Round-Up Special Volume 2 pp 131–144
Smithson DM, Rowins SM (2005) Reduced I-type magmatism and porphyry Cu-Au mineralization in the west Central Cascades, WA: the ca. 37 Ma North Fork deposit. Goldschmidt Conference Abstracts, p. A566
Snow CA, Bird DK, Metcalf J, McWilliams M (2008) Chronology of gold mineralization in the Sierra Nevada foothills from 40Ar/39Ar dating of mariposite. Int Geol Rev 50:503–518
Takagi T, Tsukimura K (1997) Genesis of oxidized- and reduced-type granites. Econ Geol 92:81–86
Taylor RD, Goldfarb RJ, Monecke T, Fletcher IR, Cosca MA, Kelly NM (2015) Application of U-Th-Pb phosphate geochronology to young orogenic gold deposits: new age constraints on the formation of the Grass Valley gold district, Sierra Nevada foothills province, California. Econ Geol 110:1313–1337
Taylor RD, Adams DT (2018) Electron microprobe data for monazite and xenotime used in consideration of gold deposit formation models (ver. 2.0, May, 2023). U.S. Geological Survey Data Release. https://doi.org/10.5066/F70Z72G1
Taylor RD, Monecke T, Reynold TJ, Monecke J (2021) Paragenesis of an orogenic gold deposit: new insights on mineralizing processes at the Grass Valley district, California. Econ Geol 116:323–356
Taylor RD, Morgan LE, Jourdan F, Monecke T, Marsh EE, Goldfarb RJ (2022) Late Jurassic-Early Cretaceous orogenic gold mineralization in the Klamath Mountains, California: constraints from 40Ar/39Ar dating of hydrothermal muscovite. Ore Geol Rev 141:104661
Thompson JFH, Sillitoe RH, Baker T, Lang JR, Mortensen JK (1999) Intrusion-related gold deposits associated with tungsten-tin provinces. Miner Deposita 34:323–334
Van Wyck N, Armitage A (2013) Technical report on the Shotgun gold project Southwest Alaska. Technical Report prepared for TNR Gold Corp
Vielreicher NM, Groves DI, Fletcher IR, McNaughton NM, Rasmussen B (2003) Hydrothermal monazite and xenotime geochronology: a new direction for precise dating of orogenic gold mineralization. SEG Newsletter 53(1):10–16
Acknowledgements
Samples for Pebble were provided by Erin Marsh and Karen Kelley, and samples for Butte were provided by Celestine Mercer. Samples of orogenic gold veins from California were collected by Ryan Taylor and Erin Marsh. Sumitomo Metal Mining Co., Ltd. graciously provided access and permission to collect samples at Pogo. David Adams helped provide technical and analytical assistance on the scanning electron microscope and electron microprobe. Reviews by George Case, Iain Pitcairn, and Editor-in-Chief Bernd Lehmann greatly improved the quality of this manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US government.
Funding
This study was funded by the U.S. Geological Survey Mineral Resources Program.
Author information
Authors and Affiliations
Contributions
Fieldwork: RT, GG; analytical work: RT, HL; interpretive model: RT, GG; writing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editorial handling: B. Lehmann
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taylor, R.D., Graham, G.E. & Lowers, H.A. Hydrothermal monazite and xenotime chemistry as genetic discriminators for intrusion-related and orogenic gold deposits: implications for an orogenic origin of the Pogo gold deposit, Alaska. Miner Deposita (2023). https://doi.org/10.1007/s00126-023-01240-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00126-023-01240-5