Abstract
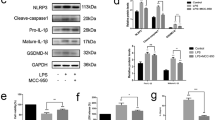
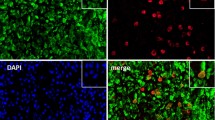
Rheumatoid arthritis (RA) is an enduring, progressive autoimmune disorder. Abnormal activation of fibroblast-like synoviocytes (FLSs) has been proposed as the initiating factor for inflammation of the synovium and bone destruction. Neutrophil extracellular traps (NETs), which are web-like structures composed of DNA, histones, and granule proteins, are involved in the development of RA in multiple aspects. Pyroptosis, gasdermin-mediated inflammatory programmed cell death, plays a vital function in the etiopathogenesis of RA. However, the exact mechanism underlying NETs-induced pyroptosis in FLSs of RA and its impact on cellular pathogenic behavior remain undefined. In this study, we demonstrated that gasdermin E (GSDME) expression was upregulated in RA plasma and synoviums, which was positively correlated with the elevated cell-free DNA (cfDNA) and citrullinated histone 3 (Cit H3) levels in the plasma. Additionally, in vitro experiments have shown that NETs triggered caspase 3/GSDME-mediated pyroptosis in RA-FLSs, characterized by decreased cell viability, cell membrane blebbing, and rupture, as well as increased levels of pyroptosis-related proteins and pro-inflammatory cytokines. Again, silencing GSDME significantly inhibited pyroptosis and suppressed the migration, invasion, and secretion of pro-inflammatory cytokines in RA-FLSs. Furthermore, we also found that the nuclear factor-kappa B (NF-κB) pathway, serving as an upstream mechanism, was involved in FLS pyroptosis. In conclusion, our investigation indicated that NETs could induce RA-FLS pyroptosis and facilitate phenotypic transformation through targeting the NF-κB/caspase 3/GSDME axis. This is the first to explore the crucial role of NETs-induced FLS pyroptosis in the progression of RA, providing novel targets for the clinical management of refractory RA.








Similar content being viewed by others
Availability of Data and Materials
The data and materials used in this study are available from the corresponding authors upon reasonable request.
References
Nygaard, G., and G.S. Firestein. 2020. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nature Reviews Rheumatology 16 (6): 316–333. https://doi.org/10.1038/s41584-020-0413-5.
Lillegraven, S., F.H. Prince, N.A. Shadick, V.P. Bykerk, B. Lu, M.L. Frits, et al. 2012. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Annals of the Rheumatic Diseases 71 (5): 681–686. https://doi.org/10.1136/ard.2011.154625.
Komatsu, N., and H. Takayanagi. 2022. Mechanisms of joint destruction in rheumatoid arthritis-immune cell-fibroblast-bone interactions. Nature Reviews Rheumatology 18 (7): 415–429. https://doi.org/10.1038/s41584-022-00793-5.
Bartok, B., and G.S. Firestein. 2010. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunological Reviews 233 (1): 233–255. https://doi.org/10.1111/j.0105-2896.2009.00859.x.
Wu, Z., D. Ma, H. Yang, J. Gao, G. Zhang, K. Xu, et al. 2021. Fibroblast-like synoviocytes in rheumatoid arthritis: surface markers and phenotypes. International Immunopharmacology 93: 107392. https://doi.org/10.1016/j.intimp.2021.107392.
Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D.S. Weiss, et al. 2004. Neutrophil extracellular traps kill bacteria. Science 303 (5663): 1532–1535. https://doi.org/10.1126/science.1092385.
Apel, F., A. Zychlinsky, and E.F. Kenny. 2018. The role of neutrophil extracellular traps in rheumatic diseases. Nature Reviews Rheumatology 14 (8): 467–475. https://doi.org/10.1038/s41584-018-0039-z.
Chen, W., Q. Wang, Y. Ke, and J. Lin. 2018. Neutrophil function in an inflammatory milieu of rheumatoid arthritis. Journal of Immunology Research 2018: 8549329. https://doi.org/10.1155/2018/8549329.
Khandpur, R., C. Carmona-Rivera, A. Vivekanandan-Giri, A. Gizinski, S. Yalavarthi, J.S. Knight, et al. 2013. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Science Translational Medicine 5 (178): 178ra40. https://doi.org/10.1126/scitranslmed.3005580.
Carmona-Rivera, C., P.M. Carlucci, R.R. Goel, E. James, S.R. Brooks, C. Rims, et al. 2020. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight 5 (13): e139388. https://doi.org/10.1172/jci.insight.139388.
Carmona-Rivera, C., P.M. Carlucci, E. Moore, N. Lingampalli, H. Uchtenhagen, E. James, et al. 2017. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Science Immunology 2 (10): eaag3358. https://doi.org/10.1126/sciimmunol.aag3358.
Wigerblad, G., and M.J. Kaplan. 2023. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nature Reviews Immunology 23 (5): 274–288. https://doi.org/10.1038/s41577-022-00787-0.
Liu, X., S. Xia, Z. Zhang, H. Wu, and J. Lieberman. 2021. Channelling inflammation: gasdermins in physiology and disease. Nature Reviews Drug Discovery 20 (5): 384–405. https://doi.org/10.1038/s41573-021-00154-z.
Yu, P., X. Zhang, N. Liu, L. Tang, C. Peng, and X. Chen. 2021. Pyroptosis: mechanisms and diseases. Signal Transduction and Targeted Therapy 6 (1): 128. https://doi.org/10.1038/s41392-021-00507-5.
Yang, P., W. Feng, C. Li, Y. Kou, D. Li, S. Liu, et al. 2021. LPS induces fibroblast-like synoviocytes RSC-364 cells to pyroptosis through NF-κB mediated dual signalling pathway. Journal of Molecular Histology 52 (4): 661–669. https://doi.org/10.1007/s10735-021-09988-8.
Wu, T., X. Zhang, Q. Zhang, Y. Zou, J. Ma, L. Chen, et al. 2022. Gasdermin-E mediated pyroptosis-a novel mechanism regulating migration, invasion and release of inflammatory cytokines in rheumatoid arthritis fibroblast-like synoviocytes. Frontiers in Cell and Developmental Biology 9: 810635. https://doi.org/10.3389/fcell.2021.810635.
Zhai, Z., F. Yang, W. Xu, J. Han, G. Luo, Y. Li, et al. 2022. Attenuation of rheumatoid arthritis through the inhibition of tumor necrosis factor-Induced caspase 3/gasdermin E-mediated pyroptosis. Arthritis & Rheumatology 74 (3): 427–440. https://doi.org/10.1002/art.41963.
Wu, X., K. Li, H. Yang, B. Yang, X. Lu, L. Zhao, et al. 2020. Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. Journal of Autoimmunity 106: 102336. https://doi.org/10.1016/j.jaut.2019.102336.
Aletaha, D., T. Neogi, A.J. Silman, J. Funovits, D.T. Felson, C.O. Bingham, et al. 2010. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases 69 (9): 1580–1588. https://doi.org/10.1136/ard.2010.138461.
Brinkmann, V., B. Laube, U. Abu Abed, C. Goosmann, and A. Zychlinsky. 2010. Neutrophil extracellular traps: how to generate and visualize them. Journal of Visualized Experiments (36): e1724. https://doi.org/10.3791/1724.
Berthelot, J., B. Le Goff, A. Neel, Y. Maugars, and M. Hamidou. 2017. NETosis: at the crossroads of rheumatoid arthritis, lupus, and vasculitis. Joint, Bone, Spine 84 (3): 255–262. https://doi.org/10.1016/j.jbspin.2016.05.013.
Barnado, A., L.J. Crofford, and J.C. Oates. 2016. At the bedside: neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. Journal of Leukocyte Biology 99 (2): 265–278. https://doi.org/10.1189/jlb.5BT0615-234R.
Bach, M., J. Moon, R. Moore, T. Pan, J.L. Nelson, and C. Lood. 2020. A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis & Rheumatology 72 (1): 47–56. https://doi.org/10.1002/art.41062.
Pérez-Sánchez, C., P. Ruiz-Limón, M.A. Aguirre, Y. Jiménez-Gómez, I. La Rosa, M.C. Ábalos-Aguilera, et al. 2017. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. Journal of Autoimmunity 82: 31–40. https://doi.org/10.1016/j.jaut.2017.04.007.
Wang, W., W. Peng, and X. Ning. 2018. Increased levels of neutrophil extracellular trap remnants in the serum of patients with rheumatoid arthritis. International Journal of Rheumatic Diseases 21 (2): 415–421. https://doi.org/10.1111/1756-185X.13226.
Fousert, E., R. Toes, and J. Desai. 2020. Neutrophil extracellular traps (NETs) take the central stage in driving autoimmune responses. Cells 9 (4): 915. https://doi.org/10.3390/cells9040915.
Wu, D., Y. Li, and R. Xu. 2023. Can pyroptosis be a new target in rheumatoid arthritis treatment? Frontiers in Immunology 14: 1155606. https://doi.org/10.3389/fimmu.2023.1155606.
Chen, L., Y. Zhao, D. Lai, P. Zhang, Y. Yang, Y. Li, et al. 2018. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death & Disease 9 (6): 597. https://doi.org/10.1038/s41419-018-0538-5.
Li, H., Y. Li, C. Song, Y. Hu, M. Dai, B. Liu, et al. 2021. Neutrophil extracellular traps augmented alveolar macrophage pyroptosis via AIM2 inflammasome activation in LPS-induced ALI/ARDS. Journal of Inflammation Research 14: 4839–4858. https://doi.org/10.2147/JIR.S321513.
Cui, Y., Y. Yang, W. Tao, W. Peng, D. Luo, N. Zhao, et al. 2023. Neutrophil extracellular traps induce alveolar macrophage pyroptosis by regulating NLRP3 deubiquitination, aggravating the development of septic lung injury. Journal of Inflammation Research 16: 861–877. https://doi.org/10.2147/JIR.S366436.
Zheng, F., L. Ma, X. Li, Z. Wang, R. Gao, C. Peng, et al. 2022. Neutrophil extracellular traps induce glomerular endothelial cell dysfunction and pyroptosis in diabetic kidney disease. Diabetes 71 (12): 2739–2750. https://doi.org/10.2337/db22-0153.
Nemeth, T., G. Nagy, and T. Pap. 2022. Synovial fibroblasts as potential drug targets in rheumatoid arthritis, where do we stand and where shall we go? Annals of the Rheumatic Diseases 81 (8): 1055–1064. https://doi.org/10.1136/annrheumdis-2021-222021.
Capece, D., D. Verzella, I. Flati, P. Arboretto, J. Cornice, and G. Franzoso. 2022. NF-κB: blending metabolism, immunity, and inflammation. Trends in Immunology 43 (9): 757–775. https://doi.org/10.1016/j.it.2022.07.004.
Tan, Y., R. Sun, L. Liu, D. Yang, Q. Xiang, L. Li, et al. 2021. Tumor suppressor DRD2 facilitates M1 macrophages and restricts NF-κB signaling to trigger pyroptosis in breast cancer. Theranostics 11 (11): 5214–5231. https://doi.org/10.7150/thno.58322.
Liu, Z., X. Yao, W. Jiang, W. Li, S. Zhu, C. Liao, et al. 2020. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Journal of Neuroinflammation 17 (1): 90. https://doi.org/10.1186/s12974-020-01751-2.
Ge, G., J. Bai, Q. Wang, X. Liang, H. Tao, H. Chen, et al. 2022. Punicalagin ameliorates collagen induced arthritis by downregulating M1 macrophage and pyroptosis via NF-κB signaling pathway. Science China Life Sciences 65 (3): 588–603. https://doi.org/10.1007/s11427-020-1939-1.
Zhang, Y., X. Cai, B. Wang, B. Zhang, and Y. Xu. 2023. Exploring the molecular mechanisms of the involvement of GZMB-caspase-3-GSDME pathway in the progression of rheumatoid arthritis. Molecular Immunology 161: 82–90. https://doi.org/10.1016/j.molimm.2023.07.013.
Acknowledgements
We gratefully thank all the patients and the staff members who generously collaborated with this research.
Funding
Grant support was provided by the National Natural Science Foundation of China (No. 81960302), Natural Science Foundation of Gansu Province (No. 22JR5RA975), and Gansu Clinical Medical Research Center (No. 21JR7RA437).
Author information
Authors and Affiliations
Contributions
Conception and design: JM and HLS; Collection and acquisition of surgical synovium samples and clinical data: MT, JL, CHL and JYH; Performed research: JM, MT; Acquisition of data: JM; Analysis and interpretation of data: JM, MT and JXZ. Drafting the manuscript: JM. All authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Lan Zhou University Second Hospital (protocol no: 2023A-533). The patients/participants provided their written informed consent to participate in this study.
Consent for Publication
The manuscript has been approved by all authors for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, J., Tan, M., Li, J. et al. Neutrophil Extracellular Traps Induce Pyroptosis of Rheumatoid Arthritis Fibroblast-Like Synoviocytes via the NF-κB/Caspase 3/GSDME Pathway. Inflammation (2023). https://doi.org/10.1007/s10753-023-01951-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-023-01951-x