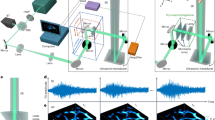
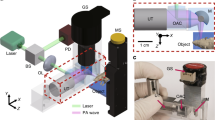
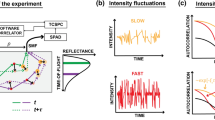
Abstract
Imaging deep haemodynamics non-invasively remains a quest. Although optical imaging techniques can be used to measure blood flow, they are generally limited to imaging within ∼1 mm below the skin’s surface. Here we show that such optical diffusion limit can be broken through by leveraging the spatial heterogeneity of blood and its photoacoustic contrast. Specifically, successive single-shot wide-field photoacoustic images of blood vessels can be used to visualize the frame-to-frame propagation of blood and to estimate blood flow speed and direction pixel-wise. The method, which we named photoacoustic vector tomography (PAVT), allows for the quantification of haemodynamics in veins more than 5 mm deep, as we show for regions in the hands and arms of healthy volunteers. PAVT may offer advantages for the diagnosis and monitoring of vascular diseases and for the mapping of the function of the circulatory system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are provided within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are available for research purposes from the corresponding author on reasonable request.
Code availability
The reconstruction codes based on the universal back-projection algorithm are proprietary and used in licensed technologies, yet they are available from the corresponding author on reasonable request.
References
Won, R. Mapping blood flow. Nat. Photonics 5, 393 (2011).
Rajan, V., Varghese, B., van Leeuwen, T. G. & Steenbergen, W. Review of methodological developments in laser Doppler flowmetry. Lasers Med. Sci. 24, 269–283 (2009).
Leitgeb, R. A., Werkmeister, R. M., Blatter, C. & Schmetterer, L. Doppler Optical Coherence Tomography. Prog. Retin. Eye Res. 41, 26–43 (2014).
Qureshi, M. M. et al. Quantitative blood flow estimation in vivo by optical speckle image velocimetry: publisher’s note. Optica 8, 1326 (2021).
Boas, D. A. & Dunn, A. K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 15, 011109 (2010).
Cinotti, E. et al. Quantification of capillary blood cell flow using reflectance confocal microscopy. Skin Res. Technol. 20, 373–378 (2014).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Wang, L. V. Tutorial on photoacoustic microscopy and computed tomography. IEEE J. Sel. Top. Quantum Electron. 14, 171–179 (2008).
Yao, J. et al. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 12, 407–410 (2015).
Kinnunen, M., Kauppila, A., Karmenyan, A. & Myllylä, R. Effect of the size and shape of a red blood cell on elastic light scattering properties at the single-cell level. Biomed. Opt. Express 2, 1803–1814 (2011).
Wang, L. V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 3, 503–509 (2009).
Brunker, J. & Beard, P. Velocity measurements in whole blood using acoustic resolution photoacoustic Doppler. Biomed. Opt. Express 7, 2789–2806 (2016).
Yao, J. & Wang, L. V. Photoacoustic brain imaging: from microscopic to macroscopic scales. Neurophotonics 1, 011003 (2014).
Guo, Z., Li, L. & Wang, L. V. On the speckle-free nature of photoacoustic tomography. Med. Phys. 36, 4084–4088 (2009).
Pakdaman Zangabad, R. et al. Photoacoustic flow velocity imaging based on complex field decorrelation. Photoacoustics 22, 100256 (2021).
Xu, M. & Wang, L. V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E 71, 016706 (2005).
Lurie, F., Kistner, R. L., Eklof, B. & Kessler, D. Mechanism of venous valve closure and role of the valve in circulation: a new concept. J. Vasc. Surg. 38, 955–961 (2003).
Szabo, T. L. Diagnostic Ultrasound Imaging: Inside Out (Academic Press, 2004).
Tanter, M. & Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 61, 102–119 (2014).
Errico, C. et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502 (2015).
Wang, L. V. & Wu, H. Biomedical Optics: Principles and Imaging (John Wiley & Sons, 2012).
American National Standards Institute. American National Standard for the Safe Use of Lasers ANSI z136.1–2014 (Laser Institute of America, 2014).
Cramer, H., & Grenader, U. The Nyquist frequency is that frequency whose period is two sampling intervals. Probability and Statistics: The Harald Cramer Volume p. 434 (Almqvist & Wiksell, 1959).
Lee, Y., Kang, J. & Yoo, Y. Automatic dynamic range adjustment for ultrasound B-mode imaging. Ultrasonics 56, 435–443 (2015).
Fernández-Colino, A. & Jockenhoevel, S. Advances in engineering venous valves: the pursuit of a definite solution for chronic venous disease. Tissue Eng. B Rev. 27, 253–265 (2021).
Petrila, T. & Trif, D. Basics of Fluid Mechanics and Introduction to Computational Fluid Dynamics (Springer Science & Business Media, 2004).
Murray, C. D. The physiological principle of minimum work. Proc. Natl Acad. Sci. USA 12, 207–214 (1926).
Wiedeman, M. P. Dimensions of blood vessels from distributing artery to collecting vein. Circ. Res. 12, 375–378 (1963).
Li, L. et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 1, 0071 (2017).
Keys, A. The oxygen saturation of the venous blood in normal human subjects. Am. J. Physiol. Leg. Content 124, 13–21 (1938).
Na, S., Zhang, Y. & Wang, L. V. Cross-ray ultrasound tomography and photoacoustic tomography of cerebral haemodynamics in rodents. Adv. Sci. 9, 2201104 (2022).
Yao, J., Maslov, K. I. & Wang, L. V. In vivo photoacoustic tomography of total blood flow and potential imaging of cancer angiogenesis and hypermetabolism. Technol. Cancer Res. Treat. 11, 301–307 (2012).
Beebe-Dimmer, J. L., Pfeifer, J. R., Engle, J. S. & Schottenfeld, D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 15, 175–184 (2005).
Lin, L. & Wang, L. V. The emerging role of photoacoustic imaging in clinical oncology. Nat. Rev. Clin. Oncol. 19, 365–384 (2022).
Na, S. et al. Massively parallel functional photoacoustic computed tomography of the human brain. Nat. Biomed. Eng. 6, 584–592 (2022).
Zhang, Y. et al. Transcranial photoacoustic computed tomography of human brain function. Preprint at https://arxiv.org/abs/2206.00248 (2022).
Yao, J., Maslov, K. I., Zhang, Y., Xia, Y. & Wang, L. V. Label-free oxygen-metabolic photoacoustic microscopy in vivo. J. Biomed. Opt. 16, 076003 (2011).
Montaldo, G., Tanter, M., Bercoff, J., Benech, N. & Fink, M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 56, 489–506 (2009).
Zhang, Y., Guo, Y. & Lee, W.-N. Ultrafast ultrasound imaging with cascaded dual-polarity waves. IEEE Trans. Med. Imaging 37, 906–917 (2018).
Yiu, B. Y., Lai, S. S. & Alfred, C. H. Vector projectile imaging: time-resolved dynamic visualization of complex flow patterns. Ultrasound Med. Biol. 40, 2295–2309 (2014).
Dong, J., Zhang, Y. & Lee, W.-N. Walled vessel-mimicking phantom for ultrasound imaging using 3D printing with a water-soluble filament: design principle, fluid-structure interaction (FSI) simulation, and experimental validation. Phys. Med. Biol. 65, 085006 (2020).
Demené, C. et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fultrasound sensitivity. IEEE Trans. Med. Imaging 34, 2271–2285 (2015).
Farnebäck, G. Two-frame motion estimation based on polynomial expansion. In Scandinavian Conference on Image Analysis 363–370 (Springer, 2003).
Leis, J. W. Digital Signal Processing Using MATLAB for Students and Researchers (John Wiley & Sons, 2011).
Acknowledgements
We thank K. Maslov, L. Li and R. Cao for discussions about the flow mechanism; S. L. Spitalnik and P. Buehler for discussions about blood physiology; S. Davis and B. Park for discussion on the potential improvement of flow visualization; and L. Li for the suggestion about fibre coupling and assistance in light alignment. This work was sponsored by the United States National Institutes of Health (NIH) grants U01 EB029823 (BRAIN Initiative) and R35 CA220436 (Outstanding Investigator Award).
Author information
Authors and Affiliations
Contributions
L.V.W., Y.Z. and J.O.-G. designed the study. Y.Z. and J.O.-G. built the system, analysed the data and wrote the paper with input from all authors. Y.Z., J.O.-G. and A.K. performed the experiments. L.V.W., Y.Z., J.O.-G. and A.K. interpreted the data. L.V.W. supervised the study and revised the paper.
Corresponding author
Ethics declarations
Competing interests
L.V.W. has a financial interest in Microphotoacoustics Inc., CalPACT LLC and Union Photoacoustic Technologies Ltd. These companies did not provide support for this work. All other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Jan Grimm, Ben McLarney, Chengbo Liu and Liming Nie for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Main Supplementary Information
Supplementary figures and video captions.
Supplementary Video 1
Visualization of blood flow in vivo.
Supplementary Video 2
Phantom validation.
Supplementary Video 3
In vivo blood flow of a vessel with a varying diameter.
Supplementary Video 4
In vivo blood flow of a vessel at an isosbestic wavelength.
Supplementary Video 5
In vivo blood flow at different wavelengths.
Supplementary Video 6
In vivo blood flow of a vessel at a depth of 3.5 mm.
Supplementary Video 7
In vivo blood flow of a vessel at a depth of 5.5 mm.
Supplementary Video 8
In vivo haemodynamics around a valve.
Supplementary Video 9
Measuring functional responses to a blood-pressure cuff.
Supplementary Video 10
Measuring blood flow in artery #1.
Supplementary Video 11
Measuring blood flow in artery #2.
Supplementary Video 12
Imaging time-variant blood flow.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Olick-Gibson, J., Khadria, A. et al. Photoacoustic vector tomography for deep haemodynamic imaging. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01148-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01148-5
This article is cited by
-
Photoacoustic method enables deep imaging of blood flow
Nature Reviews Cardiology (2024)
-
Fast capturing of deep blood flow
Nature Biomedical Engineering (2023)