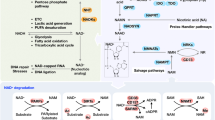
Abstract
Prolonged tachycardia—a risk factor for cardiovascular morbidity and mortality—can induce cardiomyopathy in the absence of structural disease in the heart. Here, by leveraging human patient data, a canine model of tachycardia and engineered heart tissue generated from human induced pluripotent stem cells, we show that metabolic rewiring during tachycardia drives contractile dysfunction by promoting tissue hypoxia, elevated glucose utilization and the suppression of oxidative phosphorylation. Mechanistically, a metabolic shift towards anaerobic glycolysis disrupts the redox balance of nicotinamide adenine dinucleotide (NAD), resulting in increased global protein acetylation (and in particular the acetylation of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase), a molecular signature of heart failure. Restoration of NAD redox by NAD+ supplementation reduced sarcoplasmic/endoplasmic reticulum Ca2+-ATPase acetylation and accelerated the functional recovery of the engineered heart tissue after tachycardia. Understanding how metabolic rewiring drives tachycardia-induced cardiomyopathy opens up opportunities for therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for the figures are available in figshare, with the identifier https://doi.org/10.6084/m9.figshare.24112587. RNA-seq data are available at the National Center for Biotechnology Information Gene Expression Omnibus repository, under accession number GSE242727. Publicly available data used in this study are available at the National Center for Biotechnology Information Gene Expression Omnibus repository, under accession numbers GSE116250 and GSE9794. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Fox, K. et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 372, 817–821 (2008).
Nanchen, D. et al. Resting heart rate and the risk of heart failure in healthy adults the rotterdam study. Circ. Heart Fail. 6, 403–410 (2013).
Böhm, M. et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 376, 886–894 (2010).
Hori, M. & Okamoto, H. Heart rate as a target of treatment of chronic heart failure. J. Cardiol. 60, 86–90 (2012).
Komajda, M. Heart rate in chronic heart failure: an overlooked risk factor. Eur. Heart J. 36, 648–649 (2015).
Watanabe, H. et al. Clinical characteristics, treatment, and outcome of tachycardia induced cardiomyopathy. Int. Heart J. 49, 39–47 (2008).
Ellis, E. R. & Josephson, M. E. What about tachycardia-induced cardiomyopathy? Arrhythmia Electrophysiol. Rev. 2, 82–90 (2013).
Inamori, T. et al. Inappropriate sinus tachycardia-induced cardiomyopathy with severe functional mitral regurgitation and successful treatment with ivabradine. J. Cardiol. Cases 25, 6–9 (2022).
Sağ, S., Çoşkun, H., Baran, İ., Güllülü, S. & Aydınlar, A. Inappropriate sinus tachycardia-induced cardiomyopathy during pregnancy and successful treatment with ivabradine. Anatol. J. Cardiol. 16, 212–213 (2016).
Kavanaugh, M. et al. Cardiomyopathy induced by sinus tachycardia in combat wounded: a case study. Mil. Med. 179, e1062–e1064 (2014).
Mayuga, K. A. et al. Sinus tachycardia: a multidisciplinary expert focused review. Circ. Arrhythmia Electrophysiol. 15, E007960 (2022).
Mueller, K. A. L. et al. Histopathological and immunological characteristics of tachycardia-induced cardiomyopathy. J. Am. Coll. Cardiol. 69, 2160–2172 (2017).
Nerheim, P., Birger-Botkin, S., Piracha, L. & Olshansky, B. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation 110, 247–252 (2004).
Montero, S., Ferrero-Gregori, A., Cinca, J. & Guerra, J. M. Long-term outcome of patients with tachycardia-induced cardiomyopathy after recovery of left ventricular function. Rev. Esp. Cardiol. 71, 681–683 (2018).
Huizar, J. F., Ellenbogen, K. A., Tan, A. Y. & Kaszala, K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 2328–2344 (2019).
Rodriguez, L. M. et al. Improvement in left ventricular function by ablation of atrioventricular nodal conduction in selected patients with lone atrial fibrillation. Am. J. Cardiol. 72, 1137–1141 (1993).
Redfield, M. M. et al. Tachycardia-related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin. Proc. 75, 790–795 (2000).
Hobai, I. A. & O’Rourke, B. Enhanced Ca2+-activated Na+–Ca2+ exchange activity in canine pacing-induced heart failure. Circ. Res. 87, 690–698 (2000).
Gabisonia, K. et al. Proteome dynamics and bioinformatics reveal major alterations in the turnover rate of functionally related cardiac and plasma proteins in a dog model of congestive heart failure. J. Card. Fail. 28, 588–600 (2022).
Seki, M. et al. Acute and chronic increases of circulating FSTL1 normalize energy substrate metabolism in pacing-induced heart failure. Circ. Heart Fail. 11, e004486 (2018).
Shen, W. et al. Progressive loss of myocardial ATP due to a loss of total purines during the development of heart failure in dogs. Circulation 100, 2113–2118 (1999).
O’Brien, P. J., Ianuzzo, C. D., Moe, G. W., Stopps, T. P. & Armstrong, P. W. Rapid ventricular pacing of dogs to heart failure: biochemical and physiological studies. Can. J. Physiol. Pharmacol. 68, 34–39 (2011).
Shite, J. et al. Antioxidant vitamins attenuate oxidative stress and cardiac dysfunction in tachycardia-induced cardiomyopathy. J. Am. Coll. Cardiol. 38, 1734–1740 (2001).
Nakamura, R. et al. Probucol attenuates left ventricular dysfunction and remodeling in tachycardia-induced heart failure: roles of oxidative stress and inflammation. Circulation 106, 362–367 (2002).
Wolff, M. R., Whitesell, L. F. & Moss, R. L. Calcium sensitivity of isometric tension Is increased in canine experimental heart failure. Circ. Res. 76, 781–789 (1995).
Perreault, C. L., Shannon, R. P., Komamura, K., Vatner, S. F. & Morgan, J. P. Abnormalities in intracellular calcium regulation and contractile function in myocardium from dogs with pacing-induced heart failure. J. Clin. Invest. 89, 932–938 (1992).
Qanud, K. et al. Reverse changes in cardiac substrate oxidation in dogs recovering from heart failure. Am. J. Physiol. Heart Circ. Physiol. 295, 2098–2105 (2008).
Schmitz, W. et al. Alterations in the myocardial capillary vasculature accompany tachycardia-induced cardiomyopathy. Basic Res. Cardiol. 87, 65–79 (1992).
Shannon, R. P., Komamura, K., Shen, Y. T., Bishop, S. P. & Vatner, S. F. Impaired regional subendocardial coronary flow reserve in conscious dogs with pacing-induced heart failure. Am. J. Physiol. 265, H801–H809 (1993).
Diguet, N. et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137, 2256–2273 (2018).
Tong, D. et al. NAD+ repletion reverses heart failure with preserved ejection fraction. Circ. Res. 128, 1629–1641 (2021).
Haywood, M. E. et al. Transcriptome signature of ventricular arrhythmia in dilated cardiomyopathy reveals increased fibrosis and activated TP53. J. Mol. Cell. Cardiol. 139, 124–134 (2020).
Gao, Z. et al. Key pathways associated with heart failure development revealed by gene networks correlated with cardiac remodeling. Physiol. Genomics 35, 222–230 (2008).
Mannhardt, I. et al. Human engineered heart tissue: analysis of contractile force. Stem Cell Rep. 7, 29–42 (2016).
Parikh, S. S. et al. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 121, 1323–1330 (2017).
Yang, X. et al. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 13, 657–668 (2019).
Gopinathannair, R. et al. Arrhythmia-induced cardiomyopathies: mechanisms, recognition, and management. J. Am. Coll. Cardiol. 66, 1714–1728 (2015).
Martin, C. A. & Lambiase, P. D. Pathophysiology, diagnosis and treatment of tachycardiomyopathy. Heart 103, 1543–1552 (2017).
Chen, Y. et al. Detailed characterization of microRNA changes in a canine heart failure model: relationship to arrhythmogenic structural remodeling. J. Mol. Cell. Cardiol. 77, 113–124 (2014).
De Lima, J. J. G. et al. Effects of FK506 in rat and human resistance arteries. Kidney Int. 55, 1518–1527 (1999).
Prystowsky, E. N. The history of atrial fibrillation: the last 100 years. J. Cardiovasc. Electrophysiol. 19, 575–582 (2008).
Slawik, J. et al. Irregular pacing of ventricular cardiomyocytes induces pro-fibrotic signalling involving paracrine effects of transforming growth factor beta and connective tissue growth factor. Eur. J. Heart Fail. 21, 482–491 (2019).
Ling, L. H. et al. Irregular rhythm adversely influences calcium handling in ventricular myocardium. Circ. Heart Fail. 5, 786–793 (2012).
Liu, Y., Chen, J., Fontes, S. K., Bautista, E. N. & Cheng, Z. Physiological and pathological roles of protein kinase A in the heart. Cardiovasc. Res. 118, 386–398 (2022).
Beckendorf, J., van den Hoogenhof, M. M. G. & Backs, J. Physiological and unappreciated roles of CaMKII in the heart. Basic Res. Cardiol. 113, 1–12 (2018).
Brixius, K., Wollmer, A., Bölck, B., Mehlhorn, U. & Schwinger, R. H. G. Ser16-, but not Thr17-phosphorylation of phospholamban influences frequency-dependent force generation in human myocardium. Pflugers Arch. Eur. J. Physiol. 447, 150–157 (2003).
Liu, R. et al. Tead1 is required for maintaining adult cardiomyocyte function, and its loss results in lethal dilated cardiomyopathy. JCI Insight 2, e93343 (2017).
Tanaka, N. et al. Heart-rate-proportional oxygen consumption for constant cardiac work in dog heart. Jpn J. Physiol. 40, 503–521 (1990).
Boerth, R. C., Corel, J. W., Pool, P. E. & Ross, J. Increased myocardial oxygen consumption and contractile state associated with increased heart rate in dogs. Circ. Res. 24, 725–734 (1969).
Gupte, S. A. et al. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J. Mol. Cell. Cardiol. 41, 340–349 (2006).
Daniels, L. J. et al. Elevated myocardial fructose and sorbitol levels are associated with diastolic dysfunction in diabetic patients, and cardiomyocyte lipid inclusions in vitro. Nutr. Diabetes 11, 1–7 (2021).
Zhang, J. et al. Accumulation of succinate in cardiac ischemia primarily occurs via canonical Krebs cycle activity. Cell Rep. 23, 2617–2628 (2018).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Cantó, C., Menzies, K. J. & Auwerx, J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53 (2015).
Garofalo, O., Cox, D. W. G. & Bachelard, H. S. Brain levels of NADH and NAD+ under hypoxic and hypoglycaemic conditions in vitro. J. Neurochem. 51, 172–176 (1988).
Eales, K. L., Hollinshead, K. E. R. & Tennant, D. A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 5, e190–e190 (2016).
Lee, C. F. et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 134, 883–894 (2016).
Gorski, P. A. et al. Role of SIRT1 in modulating acetylation of the sarco-endoplasmic reticulum Ca2+-ATPase in heart failure. Circ. Res. 124, e63–e80 (2019).
Xie, N. et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 5, 1–37 (2020).
Lemme, M. et al. Chronic intermittent tachypacing by an optogenetic approach induces arrhythmia vulnerability in human engineered heart tissue. Cardiovasc. Res. 116, 1487–1499 (2020).
Ronaldson-Bouchard, K. et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243 (2018).
Shen, S. et al. Physiological calcium combined with electrical pacing accelerates maturation of human engineered heart tissue. Stem Cell Rep. 17, 2037–2049 (2022).
Hirt, M. N. et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 74, 151–161 (2014).
Cui, C. et al. Structural and electrophysiological dysfunctions due to increased endoplasmic reticulum stress in a long-term pacing model using human induced pluripotent stem cell-derived ventricular cardiomyocytes. Stem Cell Res. Ther. 8, 109 (2017).
Geng, L. et al. Rapid electrical stimulation increased cardiac apoptosis through disturbance of calcium homeostasis and mitochondrial dysfunction in human induced pluripotent stem cell-derived cardiomyocytes. Cell. Physiol. Biochem. 47, 1167–1180 (2018).
Osorio, J. C. et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-α in pacing-induced heart failure. Circulation 106, 606–612 (2002).
Neglia, D. et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 293, 3270–3278 (2007).
Churko, J. M. et al. Defining human cardiac transcription factor hierarchies using integrated single-cell heterogeneity analysis. Nat. Commun. https://doi.org/10.1038/s41467-018-07333-4 (2018).
Zhang, J. Z. et al. A human iPSC double-reporter system enables purification of cardiac lineage subpopulations with distinct function and drug response profiles. Cell Stem Cell https://doi.org/10.1016/j.stem.2019.02.015 (2019).
Tiburcy, M. et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 135, 1832–1847 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 1–16 (2011).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21 (2014).
Mitacchione, G. et al. The gut hormone ghrelin partially reverses energy substrate metabolic alterations in the failing heart. Circ. Hear. Fail. 7, 643–651 (2014).
Acknowledgements
C.T. discloses support for the research described in this study from the American Heart Association (AHA) (20POST35080175) and the National Institutes of Health (NIH) (K99 HL164962). A.C. discloses support for the publication of this study from AHA (908136). H.Z. discloses support for the publication of this study from AHA (23CDA1050577). O.J.A. discloses support for the publication of this study from the NIH (K01 HL130608). F.A.R. discloses support for the publication of this study from NIH (R01 HL151345). J.C.W. discloses support for the publication of this study from the NIH (R01 HL163680, R01 HL141371, R01 HL113006, R01 HL150693 and P01 HL141084) and the National Aeronautics and Space Administration (80ARC022CA003).
Author information
Authors and Affiliations
Contributions
C.T. and J.C.W. initiated and oversaw the entire study. C.T. and A.C. performed and analysed the experiments. Y.L. performed the transcriptomic analysis. C.T. and Y.D. designed and performed the metabolomic experiments. A.W., O.J.A., L.M., M.R.G.T., H.Y., A.Z., M.A.W. and X.L. performed the data analysis. N.G. and F.A.R. provided critical experimental materials. C.K.L., H.Z., S.M.N., N.G. and F.A.R. provided critical input on the experimental design. C.T., A.C., C.K.L., S.M.N. and J.C.W. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.C.W. is a co-founder and scientific advisory board member of Greenstone Biosciences. All other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Thomas Eschenhagen, Zachary Laksman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Clinical characteristics of the patient cohorts.
a, Gender, age, and common co-morbidities of the patients. n = 14 patients without heart failure, 16 patients with heart failure, and 19 patients with heart failure and tachycardia. The number of patients with or without a co-morbidity is indicated by red or blue boxes respectively. b, Summary of medical therapies used on the patients. The number of patients using or not using a therapy is indicated by red or blue boxes respectively.
Extended Data Fig. 2 Improved iPSC-CM maturation by hormone and fatty acid supplementation.
Treatment of human iPSC-CMs (SCVI-273) with a maturation media containing triiodothyronine (10 nM), dexamethasone (1 μM), oleic acid (30 μM), and palmitic acid (80 μM) for 6 days promoted the maturation of iPSC-CMs. a, qPCR analysis of maturation markers in iPSC-CMs with or without the maturation treatment. n = 3 technical replicates. Unpaired Student’s t-test. b, Western blot analysis of OXPHOS proteins in EHTs with or without the maturation treatment, including NDUFB8, SDHB, ubiquinol-cytochrome c reductase core protein 2 (UQCRC2), MTCO2, and ATP5A. n = 4 EHTs per group. Unpaired Student’s t-test. c, Measurement of oxygen consumption rate (OCR) of iPSC-CMs with or without the maturation treatment. n = 17 wells. 10,000 cells/well. Two-tailed Mann–Whitney test. d, Analysis of calcium handling using Fluo-4 dye in iPSC-CMs with or without the maturation treatment. n = 31 immature cells and n = 30 mature cells. Two-tailed Mann–Whitney test. e, Contractility analysis of EHTs with or without the maturation treatment. n = 8 EHTs per group. Two-tailed Mann–Whitney test. For a, b, and e, data were normalized against the untreated cells or EHTs. Data are displayed as mean ± s.e.m.
Extended Data Fig. 3 Pacing at a physiological rate does not induce contractile dysfunction in EHTs.
EHTs (SCVI-273) were tachypaced or unpaced for 10 days and contractility measurements were performed on days 0, 2, 7, and 10. Functional parameters generated from the analysis include maximum contraction velocity (a), maximum relaxation velocity (b), spontaneous beating rate (c), and contractile force (d). n = 16 for unpaced EHTs and n = 14 for paced EHTs. Two-way ANOVA with Bonferroni’s multiple comparisons test. Data are displayed as mean ± s.e.m.
Extended Data Fig. 4 Pharmacological suppression of the beating rate increase blunts the deterioration of EHT contractility.
a, Experimental outline: EHTs (SCVI-273) were tachypaced for 5 days with or without drug treatment. Contractility was measured before and after tachypacing. b, Effect of carvedilol (250 ng/mL) on the beating rate (unpaced or paced) and the contractile force of tachypaced EHTs. n = 4 EHTs per group. c, Effect of FK506 (5 μM) on the beating rate (unpaced or paced) and the contractile force of tachypaced EHTs. n = 8 EHTs for the beating rate measurements and n = 5 EHTs for contractile force measurements. d, Effect of ivabradine (5 μM) on the beating rate (unpaced or paced) and the contractile force of tachypaced EHTs. n = 4 untreated EHTs and n = 8 ivabradine-treated EHTs. Unpaired Student’s t-test. Data are displayed as mean ± s.e.m.
Extended Data Fig. 5 Hypertrophic cardiomyopathy (HCM) EHTs have increased sensitivity to tachypacing-induced contractile dysfunction.
a, Experimental outline: HCM (MYBPC3 mutation) EHTs (SCVI-591) were tachypaced at 3 Hz for 5 days, then allowed to recover for 5 days. Contractile force (b), maximum contraction velocity (c), maximum relaxation velocity (d), and beating rate (e) were measured before tachypacing, 5 days after tachypacing, 1 day, 2 days, and 5 days after recovery. Data points of beating rates for EHTs that stopped beating were not shown. Data were normalized against the baseline values from day 0. n = 11 EHTs. One-way paired ANOVA with Tukey’s multiple comparison test. Only comparisons with day 0 are shown for statistical significance. *: P < 0.05; **: P < 0.01; ***: P < 0.001. Data are displayed as repeated measures of each EHT’s contractility over time.
Extended Data Fig. 6 Functional changes in the canine model of tachypacing-induced HF.
Functional parameters including: ejection fraction, heart rate, dp/dt max, systolic pressure, end-diastolic pressure and end-diastolic diameter were measured in healthy (NF) and tachypaced dogs (HF). n = 3 NF dogs and n = 4 HF dogs. Unpaired Student’s t-test. Data are displayed as mean ± s.e.m.
Extended Data Fig. 7 Reversible activation of glycolysis and hypoxia genes by tachypacing.
Tachypaced EHTs, unpaced control EHTs, and EHTs recovered from tachypacing were subjected to qPCR analysis for DEGs identified through RNA-Seq. Expression levels were normalized against the control EHTs. n = 3 technical replicates. One-way ANOVA with Bonferroni’s multiple comparisons test. EHTs were generated from line SCVI-273. Data are displayed as mean ± s.e.m.
Extended Data Fig. 8 Hypoxia downregulates oxidative phosphorylation genes.
iPSC-CMs (SCVI-273) were exposed to hypoxia (<1% O2) for 24 hours. Expression of complex I genes was quantified with qPCR analysis, including NFUFAB1, NDUFAF1, NDUFA2, NDUFA3, NDUFB9, NDUFV1, NDUFS5 and NDUFB11. Expression levels were normalized against hypoxia-treated cells. n = 6 wells of cells. Unpaired Student’s t-test. Data are displayed as mean ± s.e.m.
Extended Data Fig. 9 Effect of NAD+ supplementation on glucose metabolites.
Metabolomic analysis of EHTs (SCVI-273) treated with 1 mM NAD+ or vehicle (water) for 1 day after tachypacing. a, Quantification of glycolysis metabolites: glucose-6-phosphate, pyruvate, fructose-1-phosphate and dihydroxyacetone phosphate. b, Quantification of HBP pathway metabolites: glucosamine-6-phosphate and UDP‐GlcNAc. c, Quantification of PPP pathway metabolite: ribulose-5-phosphate. d, Quantification of TCA cycle metabolites: malic acid, aconitic acid, succinic acid, fumaric acid and citric acid. n = 7 untreated EHTs and n = 8 NAD+-treated EHTs. Two-tailed Mann–Whitney test. Data are displayed as mean ± s.e.m.
Extended Data Fig. 10 Upregulation of ECM remodelling genes in tachypaced EHTs after recovery.
The transcriptome of EHTs recovered from tachypacing was compared with the transcriptome of unpaced control EHTs (SCVI-273). a, Heatmap of the expression of DEGs. Gene ontology analysis for b) biological process, c) cellular component, and d) molecular function of the DEGs.
Supplementary information
Source data
Source Data for Figs. 2, 5–8 and Extended Data Fig. 2
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tu, C., Caudal, A., Liu, Y. et al. Tachycardia-induced metabolic rewiring as a driver of contractile dysfunction. Nat. Biomed. Eng (2023). https://doi.org/10.1038/s41551-023-01134-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-023-01134-x