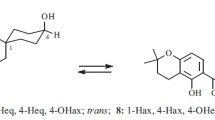
This research was designed to isolate pinostrobin from Kaempferia pandurata Roxb. rhizome using a chromatography method and to study its structural modification through ethylation and allylation reactions. Two compounds were afforded from the ethylation reaction that were 5-ethoxy-7-methoxyflavanone and 4′-ethoxy-6′-methoxychalcone and in the case of the allylation, 5-O-allylpinostrobin (5-allyloxy-7-methoxyflavanone) and 6-allylpinostrobin (6-allyl-5-hydroxy-7-methoxyflavanone) were obtained.
Similar content being viewed by others
References
N. K. Patel, G. Jaiswal, and K. K. Bhutani, Nat. Prod. Res., 30, 2017 (2016).
S. M. Adekenov and G. M. Baisarov, Chem. Nat. Compd., 57, 280 (2021).
G. K. Mukusheva, A. V. Lipeeva, P. Z. Zhanymkhanova, E. E. Shults, Y. V. Gatilov, M. M. Shakirov, and S. M. Adekenov, Chem. Heterocycl. Compd., 51, 146 (2015).
G. K. Mukusheva, P. Z. Zhanymkhanova, A. S. Turysbaeva, M. A. Pokrovskii, M. M. Shakirov, A. G. Pokrovskii, E. E. Shul′ts, and S. M. Adekenov, Chem. Nat. Compd., 51, 464 (2015).
S. D. Marliyana, D. Mujahidin, and Y. M. Syah, IOP Conf. Ser.: Mater. Sci. Eng., 349, 012057 (2018).
J. Kozlowska, E. Grela, D. Baczynska, A. Grabowiecka, and M. Aniol, Molecules, 24, 679 (2019).
H. Poerwono, S. Sasaki, Y. Hattori, and K. Higashiyama, Bioorg. Med. Chem. Lett., 20, 2086 (2010).
S. D. Marliyana, D. Mujahidin, and Y. M. Syah, IOP Conf. Ser.: Mater. Sci. Eng., 509, 012133 (2019).
M. Firdaus, N. Handayani, and L. T. Marfu′ah, Indones. J. Chem., 16, 229 (2016).
D. Fitriastuti, V. Alfiyah, Mustofa, Jumina, and M. I. D. Mardjan, Makara J. Sci., 25, 8 (2021).
M. Firdaus, T. Kusumaningsih, and A. Istiqomah, J. Chem. Technol. Metall., 57, 96 (2022).
G. Temel, N. Karaca, and N. Arsu, J. Polym. Sci. Part A Polym. Chem., 48, 5306 (2010).
N. Pahimanolis, P. Kilpelainen, E. Master, H. Ilvesniemi, and J. Seppala, Carbohydr. Polym., 131, 392 (2015).
M. Firdaus and M. A. R. Meier, Eur. Polym. J., 49, 156 (2013).
M. Firdaus, T. Kusumaningsih, A. H. Wibowo, and C. Hertiningtyas, Sains Malaysiana, 49, 2715 (2020).
A. Manvar and A. Shah, Org. Biomol. Chem., 12, 8112 (2014).
Y. Feng, Y. Hu, L. Man, T. Yuan, C. Zhang, and Z. Yang, Eur. Polym. J., 112, 619 (2019).
M. Yus, J. C. Gonzalez-Gómez, and F. Foubelo, Chem. Rev., 113, 5595 (2013).
G. C. Lloyd-Jones, A. J. Robinson, L. Lefort, and J. G. De Vries, Chem. Eur. J., 16, 9449 (2010).
A. Suryadi, S. Siswodihardjo, T. Widiandani, and R. Widyowati, Res. J. Pharm. Technol., 14, 2089 (2021).
L. Wiyono, B. C. Edina, R. A. Rahmawanti, N. N. Azizah, R. I. Paramita, E. H. Purwaningsih, and F. Fadilah, Res. J. Pharm. Technol., 13, 2797 (2020).
Acknowledgment
This research was funded by Universitas Sebelas Maret through the fundamental research scheme with contract No. 254/UN27.22/PT.01.03/2022.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2023, pp. 899–901
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marliyana, S.D., Firdaus, M., Wartono, M.W. et al. Ethylation And Allylation Reactions of Pinostrobin from the Rhizome of Kaempferia pandurata. Chem Nat Compd 59, 1063–1066 (2023). https://doi.org/10.1007/s10600-023-04197-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-04197-z