Abstract
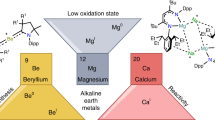
More than a century old, magnesium Grignard reagents remain essential to the toolbox of organic chemists. Although similar reagents with the neighbouring group 2 metal Ca have been explored, the considerably higher polarity and reactivity of the Ca–C bond result in undesired decomposition pathways. Ca Grignard reagents have found academic interest but have never fully developed into an established synthetic tool. Recent research activities, however, provide facile access to these highly reactive organocalcium species, including in situ preparation and ball milling approaches to tackle the challenge of controlling their extreme sensitivity. Heavier Grignard reagents are not just more reactive but profit from unique chemical transformations. Insight into the transition metal-like properties of Ca, Sr and Ba is only just emerging. Considering the rapidly developing field of alkaline-earth metal-mediated catalysis, heavy Grignard reagents will probably have a bright future.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Frankland, E. Ueber die Isolirung der organischen Radicale. Justus Liebigs Ann. Chem. 71, 171–213 (1849).
Hallwachs, W. & Schafarik, A. Ueber die Verbindungen der Erdmetalle mit organischen Radicalen. Justus Liebigs Ann. Chem. 109, 206–209 (1859).
Grignard, V. Sur quelques nouvelles combinaisons organométalliques du magnèsium et leur application à des synthèses d’alcools et d’hydrocarbures. C. R. Hebd. Séances Acad. Sci. 130, 1322–1324 (1900).
Cahours, A. Recherches sur les radicaux organométalliques. Ann. Chim. Phys. 58, 5–82 (1860).
Durand, J.-F. Préparation directe des composés organogluciques mixtes. C. R. Hebd. Séances Acad. Sci. 182, 1162–1164 (1926).
Gilman, H. & Schulze, F. Organoberyllium halides. J. Am. Chem. Soc. 49, 2904–2908 (1927).
Graedel, T. E., Harper, E. M., Nassar, N. T. & Reck, B. K. Criticality of metals and metalloids. Proc. Natl Acad. Sci. USA 112, 4257–4262 (2015).
Sun, X. Supply chain risks of critical metals: sources, propagation, and responses. Front. Energy Res. 10, 957884 (2022).
Asako, S., Takahashi, I., Nakajima, H., Ilies, L. & Takai, K. Halogen–sodium exchange enables efficient access to organosodium compounds. Commun. Chem. 4, 76 (2021).
Tortajada, A., Anderson, D. E. & Hevia, E. Gram-scale synthesis, isolation and characterisation of sodium organometallics: nBuNa and NaTMP. Helv. Chim. Acta 105, e202200060 (2022).
Wilson, A. S. S., Hill, M. S., Mahon, M. F., Dinoi, C. & Maron, L. Organocalcium-mediated nucleophilic alkylation of benzene. Science 358, 1168–1171 (2017).
Harder, S. in Early Main Group Metal Catalysis: Concepts and Reactions (Wiley-VCH, 2019).
Kim, S. B. et al. Synthesis of calcium(II) amidinate precursors for atomic layer deposition through a redox reaction between calcium and amidines. Angew. Chem. Int. Ed. 55, 10228–10233 (2016).
Buchanan, W. D., Allisa, D. G. & Ruhlandt-Senge, K. Synthesis and stabilization — advances in organoalkaline earth metal chemistry. Chem. Commun. 46, 4449–4465 (2010).
Hanusa, T. P. New developments in the organometallic chemistry of calcium, strontium and barium. Polyhedron 9, 1345–1362 (1990).
Johns, A. M., Chmely, S. C. & Hanusa, T. P. Solution interaction of potassium and calcium bis(trimethylsilyl)amides; preparation of Ca[N(SiMe3)2]2 from dibenzylcalcium. Inorg. Chem. 48, 1380–1384 (2009).
Gentner, T. X. & Mulvey, R. E. Alkali-metal mediation: diversity of applications in main-group organometallic chemistry. Angew. Chem. Int. Ed. 60, 9247–9262 (2021).
Michel, O. et al. Diene dissolution of the heavier alkaline earth metals. Eur. J. Inorg. Chem. 2012, 998–1003 (2012).
Mashima, K. et al. Diene complexes of calcium and strontium: first crystal structures of calcium- and strontium-diene complexes, M(2,3-dimethyl-1,4-diphenyl-1,3-butadiene)(THF)4 (M = Ca and Sr). J. Am. Chem. Soc. 116, 6977–6978 (1994).
Beckmann, E. Einige Anwendungen von metallischem Calcium. Ber. Dtsch. Chem. Ges. 38, 904–906 (1905).
Gilman, H. & Schulze, F. Organocalcium iodides. J. Am. Chem. Soc. 48, 2463–2467 (1926).
Bryce-Smith, D. & Skinner, A. C. Organometallic compounds of group II. Part IV. Preparation and reactions of organocalcium halides. J. Chem. Soc. 1963, 577–585 (1963).
Maercker, A. Ether cleavage with organo-alkali-metal compounds and alkali metals. Angew. Chem. Int. Ed. 26, 972–989 (1987).
Kawabata, N., Matsumura, A. & Yamashita, S. Preparation of organocalcium halides. Tetrahedron 29, 1069–1071 (1973).
Masthoff, R. & Krieg, G. Über Organometallverbindungen der II. Hauptgruppe; Triphenylmethylcalciumchlorid. Z. Chem. 6, 433–434 (1966).
Fischer, R., Gärtner, M., Görls, H. & Westerhausen, M. Synthesis of 2,4,6-trimethylphenylcalcium iodide and degradation in THF solution. Angew. Chem. Int. Ed. 45, 609–612 (2006).
Westerhausen, M., Koch, A., Görls, H. & Krieck, S. Heavy Grignard reagents: synthesis, physical and structural properties, chemical behavior, and reactivity. Chem. Eur. J. 23, 1456–1483 (2017).
Fischer, R., Görls, H. & Westerhausen, M. Reinvestigation of the synthesis of phenylcalcium iodide and the first structural characterization of a heavy Grignard reagent as [((THF)2CaPhI)3·(THF)CaO] with a central Ca4 tetrahedron. Inorg. Chem. Commun. 8, 1159–1161 (2005).
Ruspic, C. & Harder, S. Synthesis and structure of an arylcalcium compound with an unusual calcium tetrahedron containing an encapsulated oxide. Organometallics 24, 5506–5508 (2005).
Mulvey, R. E. et al. Cleave and capture chemistry illustrated through bimetallic-induced fragmentation of tetrahydrofuran. Nat. Chem. 2, 588–591 (2010).
Westerhausen, M. et al. Heavier group 2 Grignard reagents of the type aryl-Ae(L)n-X (post-Grignard reagents). Top. Organomet. Chem. 45, 29–72 (2013).
Domenicano, A., Vaciago, A. & Coulson, C. A. Molecular geometry of substituted benzene derivatives. II. A bond angle versus electronegativity correlation for the phenyl derivatives of second-row elements. Acta Crystallogr. B31, 1630–1641 (1975).
Köhler, M., Langer, J., Görls, H. & Westerhausen, M. Solution stability of organocalcium compounds in ethereal media. Organometallics 33, 6381–6388 (2014).
Schlenk, W. & Schlenk, W. Jr. Über die Konstitution der Grignardschen Magnesiumverbindungen. Ber. Dtsch. Chem. Ges. 62B, 920–924 (1929).
Ohtaki, H. & Radnai, T. Structure and dynamics of hydrated ions. Chem. Rev. 93, 1157–1204 (1993).
Gentner, T. X. et al. Heteroleptic heavier alkaline earth metal amide complexes stabilized by a superbulky β-diketiminate ligand. Organometallics 38, 2485–2493 (2019).
Fischer, R. et al. THF solvates of extremely soluble bis(2,4,6-trimethylphenyl)calcium and tris(2,6-dimethoxyphenyl)dicalcium iodide. Angew. Chem. Int. Ed. 46, 1618–1623 (2007).
Langer, J., Krieck, S., Görls, H. & Westerhausen, M. An efficient general synthesis of halide-free diarylcalcium. Angew. Chem. Int. Ed. 48, 5741–5744 (2009).
Gowenlock, B. G., Lindsell, W. E. & Singh, B. Preparation and isolation of alkyliodostrontium and alkyliodobarium compounds. J. Organomet. Chem. 101, C37–C39 (1975).
Langer, J., Görls, H. & Westerhausen, M. Stability and reactivity of phenylstrontium compounds in solution. Organometallics 29, 2034–2039 (2010).
Langer, J., Gärtner, M., Fischer, R., Görls, H. & Westerhausen, M. Reinvestigation of the reaction of strontium and barium with iodobenzene and molecular structure of the heavy Grignard reagent [((THF)2BaPh2)4·(THF)BaO] with an oxygen-centered square Ba5 pyramid. Inorg. Chem. Commun. 10, 1001–1004 (2007).
Gowenlock, B. G., Lindsell, W. E. & Singh, B. The organometallic chemistry of the alkaline-earth metals. Part 3. Preparation and properties of alkylhalogenometal compounds and related species of calcium, strontium, and barium. J. Chem. Soc. Dalton Trans. 1978, 657–664 (1978).
Yanagisawa, A., Habaue, S., Yasue, K. & Yamamoto, H. Allylbarium reagents: unprecedented regio- and stereoselective allylation reactions of carbonyl compounds. J. Am. Chem. Soc. 116, 6130–6141 (1994).
Köhler, M., Görls, H., Langer, J. & Westerhausen, M. 1-Alkenylcalcium iodide: synthesis and stability. Chem. Eur. J. 20, 5237–5239 (2014).
Lappert, M. F. & Liu, D.-S. Recent studies on metal and metalloid bis(trimethylsilyl)methyls and the transformation of the bis(trimethylsilyl)methyl into the azaallyl and β-diketinimate ligands. J. Organomet. Chem. 500, 203–217 (1995).
Cloke, F. G. N., Hitchcock, P. B., Lappert, M. F., Lawless, G. A. & Royo, B. Lipophilic strontium and calcium alkyls, amides and phenoxides; X-ray structures of the crystalline square-planar [{trans-Sr(NR′2)2(µ-1,4-dioxane)}∞] and tetrahedral [CaR2(1,4-dioxane)2];R′ = SiMe3, R = CH(SiMe3)2]. J. Chem. Soc. Chem. Commun. 1991, 724–726 (1991).
Crimmin, M. R. et al. Bis(trimethylsilyl)methyl derivatives of calcium, strontium and barium: potentially useful dialkyls of the heavy alkaline earth elements. Chem. Eur. J. 14, 11292–11295 (2008).
Köhler, M., Koch, A., Görls, H. & Westerhausen, M. Trimethylsilylmethylcalcium iodide, an easily accessible Grignard-type reagent of a heavy alkaline earth metal. Organometallics 35, 242–248 (2016).
Causero, A., Elsen, H., Pahl, J. & Harder, S. Calcium hydride reactivity: formation of an anionic N-heterocyclic olefin ligand. Angew. Chem. Int. Ed. 56, 6906–6910 (2017).
Wolf, B. M., Stuhl, C., Maichle-Mössmer, C. & Anwander, R. Dimethylcalcium. J. Am. Chem. Soc. 140, 2373–2383 (2018).
Krieck, S., Schüler, P., Peschel, J. M. & Westerhausen, M. Straightforward one-pot syntheses of silylamides of magnesium and calcium via an in situ Grignard Metalation Method. Synthesis 51, 1115–1122 (2019).
Schüler, P. et al. In situ Grignard Metalation Method, part II: scope of the one-pot synthesis of organocalcium compounds. Chem. Eur. J. 28, e202201897 (2022).
de Bruin-Dickason, C. N., Deacon, G. B., Jones, C., Junk, P. C. & Wiecko, M. Functionalised alkaline earth iodides from Grignard synthons “PhAeI(THF)n” (Ae = Mg-Ba). Eur. J. Inorg. Chem. 2019, 1030–1038 (2019).
Schüler, P., Sengupta, S., Krieck, S. & Westerhausen, M. In situ-generation of magnesium- and calcium-based Grignard reagents for amide synthesis. Chem. Eur. J. 29, e202300833 (2023).
Fraser, R. R., Mansour, T. S. & Savard, S. Acidity measurements on pyridines in tetrahydrofuran using lithiated silylamines. J. Org. Chem. 50, 3232–3234 (1985).
Sengupta, S. et al. Synthesis of sterically encumbered alkaline-earth metal amides applying the in situ Grignard reagent formation. Chem. Eur. J. 29, e202201359 (2022).
Barbier, P. Synthèse du diéthylhepténol. C. R. Hebd. Séances Acad. Sci. 128, 110–111 (1899).
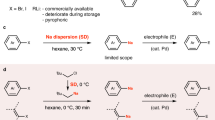
Gao, P., Jiang, J., Maeda, S., Kubota, K. & Ito, H. Mechanochemically generated calcium-based heavy Grignard reagents and their application to carbon–carbon bond-forming reactions. Angew. Chem. Int. Ed. 61, e202207118 (2022).
Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 42, 7649–7659 (2013).
Kaupp, G. Mechanochemistry: the varied applications of mechanical bond-breaking. CrystEngComm 11, 388–403 (2009).
Howard, J. L., Cao, Q. & Browne, D. L. Mechanochemistry as an emerging tool for molecular synthesis: what can it offer? Chem. Sci. 9, 3080–3094 (2018).
Fiss, B. G. et al. A. Mechanochemical methods for the transfer of electrons and exchange of ions: inorganic reactivity from nanoparticles to organometallics. Chem. Soc. Rev. 50, 8279–8318 (2021).
Green, S. P., Jones, C. & Stasch, A. Stable magnesium(I) compounds with Mg-Mg bonds. Science 318, 1754–1757 (2007).
Jędrzkiewicz, D. et al. Access to a labile monomeric magnesium radical by ball-milling. Angew. Chem. Int. Ed. 61, e202200511 (2022).
Jędrzkiewicz, D., Langer, J. & Harder, S. Low-valent Mg(I) complexes by ball-milling. Z. Anorg. Allg. Chem. 648, e202200138 (2022).
Schorigin, P., Issaguljanz, W., Gussewa, A., Ossipova, V. & Poljakowa, C. Über die Darstellung und Verarbeitung von Organomagnesiumverbindungen ohne Anwendung von Äther, i. Mitteil.: über die Darstellung von β-Phenyl-äthylalkohol. Ber. Dtsch. Chem. Ges. 64, 2584–2590 (1931).
Speight, I. R. & Hanusa, T. P. Exploration of mechanochemical activation in solid-state fluoro-Grignard reactions. Molecules 25, 570 (2020).
Takahashi, R. et al. Mechanochemical synthesis of magnesium-based carbon nucleophiles in air and their use in organic synthesis. Nat. Commun. 12, 6691 (2021).
Pfennig, V. S., Villella, R. C., Nikodemus, J. & Bolm, C. Mechanochemical Grignard reactions with gaseous CO2 and sodium methyl carbonate. Angew. Chem. Int. Ed. 61, e202116514 (2022).
Harrowfield, J. M., Hart, R. J. & Whitaker, C. R. Magnesium and aromatics: mechanically-induced Grignard and McMurry reactions. Aust. J. Chem. 54, 423–425 (2001).
Zaluska, A., Zaluski, L. & Strom-Olsen, J. O. Lithium–beryllium hydrides: the lightest reversible metal hydrides. J. Alloy. Compd. 307, 157–166 (2000).
Waddell, D. C., Clark, T. D. & Mack, J. Conducting moisture sensitive reactions under mechanochemical conditions. Tetrahedron Lett. 53, 4510–4513 (2012).
Snieckus, V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem. Rev. 90, 879–933 (1990).
Markies, P. R., Nomoto, T., Schat, G., Akkerman, O. S. & Bickelhaupt, F. Unusual metalation and halogen-metal exchange reactions between 1,3-xylyl crown ethers and organomagnesium reagents. X-ray structure of 2-[(p-tert-butylphenyl)magnesio]-1,3-xylylene-18-crown-5. Organometallics 10, 3826–3837 (1991).
Pahl, J., Brand, S., Elsen, H. & Harder, S. Highly Lewis acidic cationic alkaline earth metal complexes. Chem. Commun. 54, 8685–8688 (2018).
Garcia, L., Anker, M. D., Mahon, M. F., Maron, L. & Hill, M. S. Coordination of arenes and phosphines by charge separated alkaline earth cations. Dalton Trans. 47, 12684–12693 (2018).
Pahl, J., Friedrich, A., Elsen, H. & Harder, S. Cationic magnesium π–arene complexes. Organometallics 37, 2901–2909 (2018).
Thum, K. et al. Lewis acidic cationic strontium and barium complexes. Eur. J. Inorg. Chem. 2021, 2643–2653 (2021).
Thum, K. et al. Unsupported Mg–alkene bonding. Chem. Eur. J. 27, 2513–2522 (2021).
Barrett, A. G. M. et al. Catalytic 2,3,4-hexatriene formation by terminal alkyne coupling at calcium. Chem. Commun. 2009, 2299–2301 (2009).
Rösch, B. et al. Nucleophilic aromatic substitution at benzene with powerful strontium hydride and alkyl complexes. Angew. Chem. Int. Ed. 58, 5396–5401 (2019).
Martin, J. et al. Highly active superbulky alkaline earth metal amide catalysts for hydrogenation of challenging alkenes and aromatic rings. Angew. Chem. Int. Ed. 59, 9102–9112 (2020).
Stegner, P. et al. Metallic barium: a versatile and efficient hydrogenation catalyst. Angew. Chem. Int. Ed. 60, 4252–4258 (2021).
Färber, C. et al. Teaming up main group metals with metallic iron to boost hydrogenation catalysis. Nat. Commun. 13, 3210 (2022).
Martin, J., Eyselein, J., Grams, S. & Harder, S. Hydrogen isotope exchange with superbulky alkaline earth metal amide catalysts. ACS Catal. 10, 7792–7799 (2020).
Mai, J. et al. Alkaline-earth metal mediated benzene-to-biphenyl coupling. Angew. Chem. Int. Ed. 62, e202212463 (2023).
Wilson, A. S. S., Hill, M. S., Mahon, M. F., Dinoi, C. & Maron, L. Dehydrohalogenation of halobenzenes and C(sp3)-X (X = F, OPh) bond activation by a molecular calcium hydride. Tetrahedron 82, 131931 (2021).
Wiesinger, M. et al. Carbon-halogen bond activation with powerful heavy alkaline earth metal hydrides. Eur. J. Inorg. Chem. 2021, 3731–3741 (2021).
Li, H. et al. Intramolecular C–F and C–H bond cleavage promoted by butadienyl heavy Grignard reagents. Nat. Commun. 5, 4508 (2014).
Ong, D. Y., Tejo, C., Xu, K., Hirao, H. & Chiba, S. Hydrodehalogenation of haloarenes by a sodium hydride–iodide composite. Angew. Chem. Int. Ed. 56, 1840–1844 (2017).
Cui, B., Jia, S., Tokunaga, E. & Shibata, N. Defluorosilylation of fluoroarenes and fluoroalkanes. Nat. Commun. 9, 4393 (2018).
Maitland, B. et al. A simple route to calcium and strontium hydride clusters. Angew. Chem. Int. Ed. 56, 11880–11884 (2017).
Wiesinger, M. et al. Simple access to the heaviest alkaline earth metal hydride: a strongly reducing hydrocarbon-soluble barium hydride cluster. Angew. Chem. Int. Ed. 56, 16654–16659 (2017).
Kwan, E. E., Zeng, Y., Besser, H. A. & Jacobsen, E. N. Concerted nucleophilic aromatic substitutions. Nat. Chem. 10, 917–923 (2018).
Kaupp, M. “Non-VSEPR” structures and bonding in d0 systems. Angew. Chem. Int. Ed. 40, 3534–3565 (2001).
Kaupp, M., Schleyer, Pv. R., Dolg, M. & Stoll, H. The equilibrium structures of monomeric group 2 and lanthanide(II) metallocenes MCp2 (M = calcium, strontium, barium, samarium, europium, ytterbium) studied by ab initio calculations. J. Am. Chem. Soc. 114, 8202–8208 (1992).
Pal, R. et al. Linear MgCp*2 vs bent CaCp*2: London dispersion, ligand-induced charge localizations, and pseudo-pregostic C–H···Ca interactions. Inorg. Chem. 57, 4906–4920 (2018).
Kaupp, M., Schleyer, Pv. R., Stoll, H. & Preuss, H. The question of bending of the alkaline earth dihalides MX2(M = Be, Mg, Ca, Sr, Ba; X = F, Cl, Br, I). An ab initio pseudopotential study. J. Am. Chem. Soc. 113, 6012–6020 (1991).
Gagliardi, L. & Pyykkö, P. Cesium and barium as honorary d elements: CsN7Ba as an example. Theor. Chem. Acc. 110, 205–210 (2003).
Zhou, M. & Frenking, G. Transition-metal chemistry of the heavier alkaline earth atoms Ca, Sr, and Ba. Acc. Chem. Res. 54, 3071–3082 (2021).
Manning, M. F. & Krutter, H. M. Electronic energy bands in metallic calcium. Phys. Rev. 51, 761–764 (1937).
Wright, L. & Weller, S. The catalytic activity of barium and calcium hydrides. III. Hydrogen exchange with some C4 hydrocarbons. J. Am. Chem. Soc. 76, 5948–5950 (1954).
Trapnell, B. M. W. The activities of evaporated metal films in gas chemisorption. Proc. R. Soc. A Math. Phys. Eng. Sci. 218, 566–577 (1953).
Turnbull, J. C. Barium, strontium, and calcium as getter in electron tubes. J. Vac. Sci. Technol. 14, 636–639 (1977).
Wu, X. et al. Observation of alkaline earth complexes M(CO)8(M = Ca, Sr, or Ba) that mimic transition metals. Science 361, 912–916 (2018).
Wang, Q. et al. Transition-metal chemistry of alkaline-earth elements: the trisbenzene complexes M(Bz)3 (M=Sr, Ba). Angew. Chem. Int. Ed. 58, 17365–17374 (2019).
Wang, Q. et al. Octa-coordinated alkaline earth metal–dinitrogen complexes M(N2)8 (M=Ca, Sr, Ba). Nat. Commun. 10, 3375 (2019).
Landis, C. R., Hughes, R. P. & Weinhold, F. Comment on “Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals”. Science 365, eaay2355 (2019).
Zhao, L., Pan, S., Zhou, M. & Frenking, G. Response to comment on “Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals”. Science 365, eaay5021 (2019).
Jeung, G., Daudey, J. P. & Malrieu, J. P. Theoretical study of the electronic states of calcium and calcium hydride. Chem. Phys. Lett. 98, 433–438 (1983).
Rösch, B. et al. Dinitrogen complexation and reduction at low-valent calcium. Science 371, 1125–1128 (2021).
Stegner, P. et al. d–d dative bonding between iron and the alkaline-earth metals calcium, strontium, and barium. Angew. Chem. Int. Ed. 59, 14615–14620 (2020).
Holleman, A. F., Wiberg, E. & Wiberg N. in Lehrbuch der Anorganischen Chemie 102nd edn. 2146–2147 (de Gruyter, 2007).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 5, 751–767 (1976).
Acknowledgements
The Friedrich-Alexander University Erlangen-Nürnberg is acknowledged for support.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, contributed substantially to the discussion of the content and reviewed and/or edited the manuscript before submission. S.H. wrote the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Matthias Westerhausen, Robert Mulvey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harder, S., Langer, J. Opportunities with calcium Grignard reagents and other heavy alkaline-earth organometallics. Nat Rev Chem 7, 843–853 (2023). https://doi.org/10.1038/s41570-023-00548-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00548-0