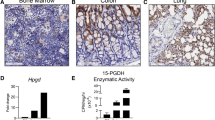
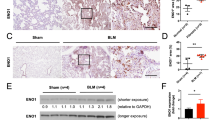
Abstract
Purpose
Acute rejection is a frequent complication among lung transplant recipients and poses substantial therapeutic challenges. 15-hydroxyprostaglandin dehydrogenase (15-PGDH), an enzyme responsible for the inactivation of prostaglandin E2 (PGE2), has recently been implicated in inflammatory lung diseases. However, the role of 15-PGDH in lung transplantation rejection remains elusive. The present study was undertaken to examine the expression of 15-PGDH in rejected lung allografts and whether inhibition of 15-PGDH ameliorates acute lung allograft rejection.
Methods
Orthotopic mouse lung transplantations were performed between donor and recipient mice of the same strain or allogeneic mismatched pairs. The expression of 15-PGDH in mouse lung grafts was measured. The efficacy of a selective 15-PGDH inhibitor (SW033291) in ameliorating acute rejection was assessed through histopathological examination, micro-CT imaging, and pulmonary function tests. Additionally, the mechanism underlying the effects of SW033291 treatment was explored using CD8+ T cells isolated from mouse lung allografts.
Results
Increased 15-PGDH expression was observed in rejected allografts and allogeneic CD8+ T cells. Treatment with SW033291 led to an accumulation of PGE2, modulation of CD8+ T-cell responses and mitochondrial activity, and improved allograft function and survival.
Conclusion
Our study provides new insights into the role of 15-PGDH in acute lung rejection and highlights the therapeutic potential of inhibiting 15-PGDH for enhancing graft survival. The accumulation of PGE2 and modulation of CD8+ T-cell responses represent potential mechanisms underlying the benefits of 15-PGDH inhibition in this model. Our findings provide impetus for further exploring 15-PGDH as a target for improving lung transplantation outcomes.





Similar content being viewed by others
References
van der Mark SC, Hoek RAS, Hellemons ME (2020) Developments in lung transplantation over the past decade. Eur Respir Rev. https://doi.org/10.1183/16000617.0132-2019
Trachuk P, Bartash R, Abbasi M, Keene A (2020) Infectious complications in lung transplant recipients. Lung 198:879–887. https://doi.org/10.1007/s00408-020-00403-9
Graham CN, Watson C, Barlev A, Stevenson M, Dharnidharka VR (2022) Mean lifetime survival estimates following solid organ transplantation in the US and UK. J Med Econ 25:230–237. https://doi.org/10.1080/13696998.2022.2033050
Assadiasl S, Nicknam MH (2022) Cytokines in Lung Transplantation. Lung 200:793–806. https://doi.org/10.1007/s00408-022-00588-1
Todd JL, Neely ML, Kopetskie H, Sever ML, Kirchner J, Frankel CW, Snyder LD, Pavlisko EN, Martinu T, Tsuang W, Shino MY, Williams N, Robien MA, Singer LG, Budev M, Shah PD, Reynolds JM, Palmer SM, Belperio JA, Weigt SS (2020) Risk factors for acute rejection in the first year after lung transplant. A multicenter study. Am J Respir Crit Care Med 202:576–585. https://doi.org/10.1164/rccm.201910-1915OC
Swaminathan AC, Todd JL, Palmer SM (2021) Advances in human lung transplantation. Annu Rev Med 72:135–149. https://doi.org/10.1146/annurev-med-080119-103200
Armati M, Cattelan S, Guerrieri M, Messina M, Perea B, Genovese M, d’Alessandro M, Gangi S, Cameli P, Perillo F, Bennett D, Fossi A, Bargagli E, Bergantini L, Tuscany Transplant G (2023) Collagen type IV alpha 5 chain in bronchiolitis obliterans syndrome after lung transplant: the first evidence. Lung. https://doi.org/10.1007/s00408-023-00632-8
d’Alessandro M, Bergantini L, Fossi A, De Vita E, Perillo F, Luzzi L, Paladini P, Sestini P, Rottoli P, Bargagli E, Bennett D (2021) The role of galectins in chronic lung allograft dysfunction. Lung 199:281–288. https://doi.org/10.1007/s00408-021-00449-3
Maher SA, Belvisi MG (2010) Prostanoids and the cough reflex. Lung 188:9–12. https://doi.org/10.1007/s00408-009-9190-2
Wanders A, Tufveson G, Gerdin B (1992) Effects of prostaglandin E2 (PGE2) and drugs affecting PGE2 degradation on acute rejection of rat cardiac allografts. Scand J Thorac Cardiovasc Surg 26:33–37. https://doi.org/10.3109/14017439209099050
Fujimoto Y, Iwagaki H, Ozaki M, Ogino T, Murata H, Sun DS, Sadamori H, Takahashi HK, Tanaka N, Yagi T (2005) Involvement of prostaglandin receptors (EPR2-4) in in vivo immunosuppression of PGE2 in rat skin transplant model. Int Immunopharmacol 5:1131–1139. https://doi.org/10.1016/j.intimp.2005.01.014
Ogawa M, Suzuki J, Kosuge H, Takayama K, Nagai R, Isobe M (2009) The mechanism of anti-inflammatory effects of prostaglandin E2 receptor 4 activation in murine cardiac transplantation. Transplantation 87:1645–1653. https://doi.org/10.1097/TP.0b013e3181a5c84c
Cheng H, Huang H, Guo Z, Chang Y, Li Z (2021) Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 11:8836–8854. https://doi.org/10.7150/thno.63396
Huang W, Li H, Kiselar J, Fink SP, Regmi S, Day A, Yuan Y, Chance M, Ready JM, Markowitz SD, Taylor DJ (2023) Small molecule inhibitors of 15-PGDH exploit a physiologic induced-fit closing system. Nat Commun 14:784. https://doi.org/10.1038/s41467-023-36463-7
Zhang Y, Desai A, Yang SY, Bae KB, Antczak MI, Fink SP, Tiwari S, Willis JE, Williams NS, Dawson DM, Wald D, Chen WD, Wang Z, Kasturi L, Larusch GA, He L, Cominelli F, Di Martino L, Djuric Z, Milne GL, Chance M, Sanabria J, Dealwis C, Mikkola D, Naidoo J, Wei S, Tai HH, Gerson SL, Ready JM, Posner B, Willson JK, Markowitz SD (2015) Tissue regeneration. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 348:aaa2340. https://doi.org/10.1126/science.aaa2340
Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P, Holbrook CA, Schurch CM, Ho ATV, Blau HM (2021) Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science. https://doi.org/10.1126/science.abc8059
Smith JN, Dawson DM, Christo KF, Jogasuria AP, Cameron MJ, Antczak MI, Ready JM, Gerson SL, Markowitz SD, Desai AB (2021) 15-PGDH inhibition activates the splenic niche to promote hematopoietic regeneration. JCI Insight. https://doi.org/10.1172/jci.insight.143658
Smith JNP, Witkin MD, Jogasuria AP, Christo KF, Raffay TM, Markowitz SD, Desai AB (2020) Therapeutic targeting of 15-PGDH in murine pulmonary fibrosis. Sci Rep 10:11657. https://doi.org/10.1038/s41598-020-68336-0
Barnthaler T, Theiler A, Zabini D, Trautmann S, Stacher-Priehse E, Lanz I, Klepetko W, Sinn K, Flick H, Scheidl S, Thomas D, Olschewski H, Kwapiszewska G, Schuligoi R, Heinemann A (2020) Inhibiting eicosanoid degradation exerts antifibrotic effects in a pulmonary fibrosis mouse model and human tissue. J Allergy Clin Immunol 145(818–833):e811. https://doi.org/10.1016/j.jaci.2019.11.032
Rubino M, Travers JG, Headrick AL, Enyart BT, Lemieux ME, Cavasin MA, Schwisow JA, Hardy EJ, Kaltenbacher KJ, Felisbino MB, Jonas E, Ambardekar AV, Bristow MR, Koch KA, McKinsey TA (2023) Inhibition of eicosanoid degradation mitigates fibrosis of the heart. Circ Res 132:10–29. https://doi.org/10.1161/CIRCRESAHA.122.321475
Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, George G, Toprak D, Abdelnour E, D’Agostino E, Goldberg HJ, Perrella MA, Forteza RM, Rosas IO, Visner G, El-Chemaly S (2015) Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest 125:4255–4268. https://doi.org/10.1172/JCI79693
Imani J, Liu K, Cui Y, Assaker JP, Han J, Ghosh AJ, Ng J, Shrestha S, Lamattina AM, Louis PH, Hentschel A, Esposito AJ, Rosas IO, Liu X, Perrella MA, Azzi J, Visner G, El-Chemaly S (2021) Blocking hyaluronan synthesis alleviates acute lung allograft rejection. JCI Insight. https://doi.org/10.1172/jci.insight.142217
Maeyashiki T, Jang J-H, Janker F, Yamada Y, Inci I, Weder W, Piegeler T, Jungraithmayr W (2019) The amide local anesthetic ropivacaine attenuates acute rejection after allogeneic mouse lung transplantation. Lung 197:217–226. https://doi.org/10.1007/s00408-019-00197-5
Issa F, Schiopu A, Wood KJ (2010) Role of T cells in graft rejection and transplantation tolerance. Expert Rev Clin Immunol 6:155–169. https://doi.org/10.1586/eci.09.64
Liu H, Liu L, Liu K, Bizargity P, Hancock WW, Visner GA (2009) Reduced cytotoxic function of effector CD8+ T cells is responsible for indoleamine 2,3-dioxygenase-dependent immune suppression. J Immunol 183:1022–1031. https://doi.org/10.4049/jimmunol.0900408
Varanasi SK, Ma S, Kaech SM (2019) T cell metabolism in a state of flux. Immunity 51:783–785. https://doi.org/10.1016/j.immuni.2019.10.012
Yap M, Brouard S, Pecqueur C, Degauque N (2015) Targeting CD8 T-cell metabolism in transplantation. Front Immunol 6:547. https://doi.org/10.3389/fimmu.2015.00547
Desdin-Mico G, Soto-Heredero G, Mittelbrunn M (2018) Mitochondrial activity in T cells. Mitochondrion 41:51–57. https://doi.org/10.1016/j.mito.2017.10.006
Lisci M, Griffiths GM (2023) Arming a killer: mitochondrial regulation of CD8(+) T cell cytotoxicity. Trends Cell Biol 33:138–147. https://doi.org/10.1016/j.tcb.2022.05.007
Cui Y, Chen G, Yang Z (2020) Mitochondrial superoxide mediates PM(2.5)-induced cytotoxicity in human pulmonary lymphatic endothelial cells. Environ Pollut 263:114423. https://doi.org/10.1016/j.envpol.2020.114423
Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, Pretlow TP, Newman RA, Willis J, Dawson D, Markowitz SD (2006) 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci USA 103:12098–12102. https://doi.org/10.1073/pnas.0603235103
Walker NM, Badri LN, Wadhwa A, Wettlaufer S, Peters-Golden M, Lama VN (2012) Prostaglandin E2 as an inhibitory modulator of fibrogenesis in human lung allografts. Am J Respir Crit Care Med 185:77–84. https://doi.org/10.1164/rccm.201105-0834OC
Okamoto T, Okamoto S, Fujimoto Y, Tabata Y, Uemoto S (2013) Suppression of acute rejection by administration of prostaglandin E2 receptor subtype 4 agonist in rat organ transplantation models. J Surg Res 183:852–859. https://doi.org/10.1016/j.jss.2013.01.039
Birrell MA, Maher SA, Dekkak B, Jones V, Wong S, Brook P, Belvisi MG (2015) Anti-inflammatory effects of PGE2 in the lung: role of the EP4 receptor subtype. Thorax 70:740–747. https://doi.org/10.1136/thoraxjnl-2014-206592
Vancheri C, Mastruzzo C, Sortino MA, Crimi N (2004) The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol 25:40–46. https://doi.org/10.1016/j.it.2003.11.001
Hamberg M, Samuelsson B (1971) On the metabolism of prostaglandins E 1 and E 2 in man. J Biol Chem 246:6713–6721
Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN (2008) Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 181:4389–4396. https://doi.org/10.4049/jimmunol.181.6.4389
Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188:21–28. https://doi.org/10.4049/jimmunol.1101029
Yang X, Ma N, Szabolcs MJ, Zhong J, Athan E, Sciacca RR, Michler RE, Anderson GD, Wiese JF, Leahy KM, Gregory S, Cannon PJ (2000) Upregulation of COX-2 during cardiac allograft rejection. Circulation 101:430–438. https://doi.org/10.1161/01.cir.101.4.430
Rangel EB, Moura LA, Franco MF, Pacheco-Silva A (2007) Up-regulation of cyclooxygenase-2 in different grades of acute human renal allograft rejection. Prostaglandins Leukot Essent Fatty Acids 76:235–243. https://doi.org/10.1016/j.plefa.2007.01.005
Osma-Garcia IC, Punzon C, Fresno M, Diaz-Munoz MD (2016) Dose-dependent effects of prostaglandin E2 in macrophage adhesion and migration. Eur J Immunol 46:677–688. https://doi.org/10.1002/eji.201545629
Rangel Moreno J, Estrada Garcia I, La Luz De, Garcia Hernandez M, Aguilar Leon D, Marquez R, Hernandez Pando R (2002) The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology 106:257–266. https://doi.org/10.1046/j.1365-2567.2002.01403.x
Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, Sugimoto S, Tietjens JR, Patterson GA, Krupnick AS, Kreisel D (2008) CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol 180:4754–4762. https://doi.org/10.4049/jimmunol.180.7.4754
Harper SJ, Ali JM, Wlodek E, Negus MC, Harper IG, Chhabra M, Qureshi MS, Mallik M, Bolton E, Bradley JA, Pettigrew GJ (2015) CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection. Proc Natl Acad Sci USA 112:12788–12793. https://doi.org/10.1073/pnas.1513533112
Burkett JB, Doran AC, Gannon M (2023) Harnessing prostaglandin E(2) signaling to ameliorate autoimmunity. Trends Immunol 44:162–171. https://doi.org/10.1016/j.it.2023.01.004
Ledderose C, Bao Y, Lidicky M, Zipperle J, Li L, Strasser K, Shapiro NI, Junger WG (2014) Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J Biol Chem 289:25936–25945. https://doi.org/10.1074/jbc.M114.575308
Kumar A, Chamoto K, Chowdhury PS, Honjo T (2020) Tumors attenuating the mitochondrial activity in T cells escape from PD-1 blockade therapy. elife. https://doi.org/10.7554/eLife.52330
Bueno V, Pestana JO (2002) The role of CD8+ T cells during allograft rejection. Braz J Med Biol Res 35:1247–1258. https://doi.org/10.1590/s0100-879x2002001100001
Martinu T, Chen DF, Palmer SM (2009) Acute rejection and humoral sensitization in lung transplant recipients. Proc Am Thorac Soc 6:54–65. https://doi.org/10.1513/pats.200808-080GO
Tong M, Tai HH (2005) 15-Hydroxyprostaglandin dehydrogenase can be induced by dexamethasone and other glucocorticoids at the therapeutic level in A549 human lung adenocarcinoma cells. Arch Biochem Biophys 435:50–55. https://doi.org/10.1016/j.abb.2004.11.031
Snyder LD, Palmer SM (2006) Immune mechanisms of lung allograft rejection. Semin Respir Crit Care Med 27:534–543. https://doi.org/10.1055/s-2006-954610
Hsiao HM, Scozzi D, Gauthier JM, Kreisel D (2017) Mechanisms of graft rejection after lung transplantation. Curr Opin Organ Transpl 22:29–35. https://doi.org/10.1097/MOT.0000000000000371
Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, Cummings OW, Heidler KM, Keller MR, Burlingham WJ, Wilkes DS (2011) Neutralizing IL-17 prevents obliterative bronchiolitis in Murine orthotopic lung transplantation. Am J Transpl 11:911–922. https://doi.org/10.1111/j.1600-6143.2011.03482.x
Martinu T, Oishi H, Juvet SC, Cypel M, Liu M, Berry GJ, Hwang DM, Keshavjee S (2019) Spectrum of chronic lung allograft pathology in a mouse minor-mismatched orthotopic lung transplant model. Am J Transpl 19:247–258. https://doi.org/10.1111/ajt.15167
Acknowledgements
The illustration was partly generated using Figdraw (https://www.figdraw.com/static/index.html#/) and Servier Medical Art (provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license).
Funding
This work was supported by National Natural Science Foundation of China [Grant Number 81974050]; Scientific Research Common Program of Beijing Municipal Commission of Education [Grant Number KM202010025003]; and Clinical Research Fund from Wu Jieping Medical Foundation [Grant Number 320.6750.2021-12-4].
Author information
Authors and Affiliations
Contributions
YC contributed to conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing—original draft, and writing—review & editing. ZL contributed to conceptualization, data curation, formal analysis, investigation; methodology, and writing—review & editing. ZY contributed to data curation, formal analysis, funding acquisition, investigation, methodology, and writing—review & editing. JL contributed to data curation, formal analysis, investigation, methodology, and writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, Y., Lv, Z., Yang, Z. et al. Inhibition of Prostaglandin-Degrading Enzyme 15-PGDH Mitigates Acute Murine Lung Allograft Rejection. Lung 201, 591–601 (2023). https://doi.org/10.1007/s00408-023-00651-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-023-00651-5