Abstract
The Santa María and Antares Zn-Pb(-Ag) skarn deposits in the Velardeña Mining District are located in central–NW Mexico. They lie 470 m apart along the contact between Oligocene felsic intrusions and Cretaceous limestones, and were developed during prograde, retrograde, post-ore (Santa María), and late stages. Firstly, the prograde stage was formed by fluids at ~ 600 °C and 15 wt% NaCl equiv., and consists of garnet + wollastonite ± clinopyroxene and biotite ± K-feldspar assemblages. Secondly, the retrograde/ore stage was formed by fluids at 300–500 °C with salinities of 20–30 wt% CaCl2 (Santa María) and > 40 wt% NaCl equiv. (Antares). It comprises assemblages of chlorite, amphibole, epidote, calcite, scapolite, quartz, sericite, adularia, fluorite, and muscovite associated with sphalerite, pyrite, galena, pyrrhotite, arsenopyrite, chalcopyrite, and Pb-Bi-Sb sulfosalts. Thirdly, the post-ore stage was formed by fluids at ~ 400 °C and 20–30 wt.% CaCl2 and comprises poorly mineralized calcite veins. Fourthly, the late stage was formed by fluids at < 300 °C and 20–30 wt.% CaCl2 (Santa María) and ~ 15 wt% NaCl equiv. (Antares), and crystallized tetrahedrite-group minerals and pyrite + marcasite. δ18Ofluid between ~ 14‰ and 23‰ at Santa María and between ~ 12‰ and 17‰ at Antares show a less-modified magmatic affinity for mineralizing fluids at Antares; δ13Cfluid between 0‰ and –6‰ register recycling of sedimentary C. Moreover, sulfides with δ34SVCDT between –3‰ and 2‰ reveal a magmatic source for S. Altogether, these data suggest that, at Santa María, magmatic-derived fluids actively interacted with the wall rocks, whereas at Antares the fluid-rock interaction was milder. In both deposits, metal deposition was triggered by the cooling and neutralization of ore-bearing fluids with carbonate rocks. Our 40Ar/39Ar dates for adularia of ca. 37.5 Ma place the deposits within the Eocene–early Miocene metallogenetic epoch of central–NW Mexico, during which other world-class skarn-epithermal systems were emplaced (e.g., Concepción del Oro and Mazapil-Peñasquito).
Similar content being viewed by others
Introduction
Skarn deposits are developed in carbonate successions intruded by granitic–granodioritic bodies in orogenic belts (Einaudi and Burt 1982; Baker et al. 2004; Meinert et al. 2005). These deposits are governed by variables that determine the evolution and type of mineralization in terms of metal distribution, mineralogy, and geometry. These variables include temperature and depth of formation, structural arrangement, nature of wall rocks (i.e., Mg-rich or Ca-rich), fO2 and fS2 in fluids, and proximity to the heat and/or fluids sources (Meinert et al. 2005; Kouzmanov and Pokrovski 2012; Chang et al. 2019). Many economically important sulfide-rich skarn deposits occur worldwide (Meinert et al. 2005; Chang et al. 2019). In Mexico, these deposits are common along the Mexican Orogen, where silicic magmatism during the Cenozoic intruded carbonate rocks (Megaw et al. 1988; Staude and Barton 2001; Camprubí 2013; Ferrari et al. 2018). Several Eocene–Oligocene skarn and epithermal deposits occur in the “Sector Transversal de Parras” of the Mexican Orogen, including Velardeña, Mazapil, Concepción del Oro, Mapimí, and Dinamita (Fig. 1c; Buseck 1966; Camprubí 2009, 2013). Sulfide-rich skarns in the Zn-Pb(-Ag) Velardeña Mining District, which is located in NE Durango state in NW Mexico, occur in the Santa María and San Lorenzo ranges, the most relevant deposits being Santa María, Antares, La Industria, La Esperanza, and San Mateo (Fig. 1b). Combined, the Santa María and Antares skarn deposits have indicated resources of 14.6 Mt, grading at 5% Zn, 0.4% Pb, 0.2% Cu, 20 g/t Ag, and 0.3 g/t Au (Industrias Peñoles 2010).
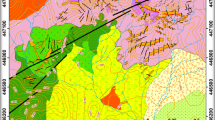
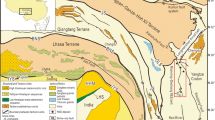
Location and geology of the Santa María and Antares ore deposits. a Stratigraphic column. b Geological map showing the location of the Santa María and Antares ore deposits, along with other deposits in the vicinity (SGM 1997; Industrias Peñoles 2010). c Locator map showing the Santa María range within Mexico, the geologic provinces (the Sierra Madre Occidental, the Sector Transversal de Parras [STP], Mexican Orogen, and Mesa Central; Ortega-Gutiérrez et al. 1992), and polymetallic skarn deposits along the STP. CDO = Concepción del Oro, DIN = Dinamita, MAP = Mapimí, MAZ-PEÑ = Mazapil-Peñasquito
Previous studies on metal occurrences in the Santa María and San Lorenzo ranges relied on field descriptions rather than detailed genetic models (e.g., Spurr and Garrey 1908; Cruz-Pérez and López-Escalona 1981; Gilmer et al. 1988; Hernández 1991; Hoffmann 2003; Pinet and Tremblay 2009; SGM 2013; Jiménez-Franco et al. 2020). In addition, Gilmer et al. (1988) and Jiménez-Franco et al. (2020) proposed mechanisms of skarn evolution based on composition of fluid inclusions, sulfur isotopes, and elemental data. However, the relationship between the Santa María and Antares skarn bodies remains unclear. The spatial proximity of these deposits represents a natural laboratory to study the influence exerted by different variables during skarn formation (e.g., quantity/nature of fluids and degree of water–rock interaction), provided that the ages of the deposits are the same. Further, geochronological estimates have relied solely on the ages of intrusive rocks, and the genetic models oversimplify the evolution of Santa María and Antares, in part due to scant isotope data.
In the present study, we analyzed representative drill-core sample suites from the Antares and Santa María skarn deposits using petrography, fluid inclusion microthermometry, electron probe microanalyses (EPMA), stable isotopes (C, O, and S), and 40Ar/39Ar geochronology. This allows us to characterize mineralogical assemblages, constrain the composition of mineralizing fluids, evaluate the sources of chemical components, and determine the age of mineralization, which ultimately underpin the first comprehensive genetic model for both deposits. Additionally, the Antares ore deposit is associated with the main stock and limited geochemical contributions from host limestones, while the Santa Maria ore deposit is associated with a smaller intrusion and a greater potential for geochemical contributions from host limestones. By comparing these two skarn deposits, we assess the diversity of ore-bearing fluids and ore-forming processes at a district scale. Furthermore, this conceptual approach reveals some features of ore-bearing fluids that do not correspond to those “typical” of skarn deposits (e.g., moderate-to-high temperatures), thus elevating the research potential that polymetallic skarns have yet to offer.
Geological background
The Velardeña Mining District lies in the westernmost termination of the Sector Transversal de Parras within the Mexican Orogen, ~ 50 km north of the E–W boundary with the Mesa Central, and ~ 100 km east of the Sierra Madre Occidental (Ortega-Gutiérrez et al. 1992; Fig. 1c). It is part of the Basin and Range Province, which is characterized by extensional tectonics that were active from ca. 13 to 5.5 Ma (Henry and Aranda-Gomez 2000). The Mexican Orogen or Mexican fold-and-thrust belt extends from the Isthmus of Tehuantepec to northwestern Sonora (Fig. 1c). It comprises thick (> 3 km) deformed late Mesozoic carbonate and clastic successions that overlie Lower to Middle Triassic rocks that, in turn, overlie Proterozoic high-grade metamorphic rocks of the Oaxaquia subcontinental block (Eguiluz y de Antuñano et al. 2000; Fitz-Díaz et al. 2018; Ortega-Gutiérrez et al. 2018). The Mesa Central is a plateau located in north–central Mexico (Fig. 1c) that consists of marine sedimentary strata deposited during two regional regressive-transgressive cycles: Upper Triassic–Lower Jurassic and Upper Jurassic–Upper Cretaceous (Nieto-Samaniego et al. 2005, 2007). The Sierra Madre Occidental is a major silicic large igneous province (Bryan 2007) that runs from the USA–Mexico border to western Mexico, with a NW general trend (Fig. 1c). The province records three main magmatic events: (1) Laramide acid volcanism (100–50 Ma), (2) syn-extensional silicic pulse (45.0–12.5 Ma), and (3) bimodal volcanism associated with the opening of the Gulf of California (12.5 Ma– present; Ferrari et al. 2005, 2007, 2018). The second event included large felsic flare-ups comprising thick packages of ignimbrites, rhyolitic domes, and intrusive bodies, with which polymetallic skarns along the Sector Transversal de Parras are associated (Camprubí 2013).
The oldest unit that crops out in the Santa María range is the Aurora Formation, which includes reefal limestones with abundant Albian cephalopods and rudists (Fig. 1a–b). The Aurora Formation is overlain by the Albian-Cenomanian Cuesta del Cura Formation, which consists of monotonous limestone strata interbedded with black flint, siltstones, and carbonaceous mudstones (Imlay 1937; Ángeles-Villeda et al. 2005). Mudstone and muddy limestone of the Cenomanian–Turonian Indidura Formation overlie the Cuesta del Cura Formation (Imlay 1937). These units, along with the terrigenous-volcanic Caracol Formation (Santonian–Coniacian), which is not exposed in the Santa María range, mark a progressive shallowing of the basin produced by a regional regressive event that ended marine conditions that had prevailed since the Upper Jurassic (Nieto-Samaniego et al. 2005, 2007). This succession is overlain by molassic deposits of the Ahuichila Formation (Eguiluz y de Antuñano et al. 2022) and capped by rhyolitic tuffs, basalts, and andesites associated with the volcanic events of the Sierra Madre Occidental province (SGM 1997; Ferrari et al. 2005, 2007, 2018). These rocks are crosscut by the Velardeña Intrusive Complex (Ramírez-Peña 2014), which encompasses felsic rocks that have been dated at 33.4 ± 1.7 (rhyolitic dike) and 33.1 ± 1.4 Ma (quartz-latite) via K/Ar on magmatic biotite (Felder 1979). These igneous rocks were initially divided by Gilmer et al. (1988) into quartz-latite porphyries, rhyolitic dikes, and quartz-sanidine porphyries. They exhibit metaluminous and calc-alkaline character (Jiménez-Franco 2012) and belong to the magnetite series of Ishihara (1977), given the presence of magnetite as an accessory phase.
The Velardeña Intrusive Complex is linked to the formation of several skarn and epithermal deposits in the Velardeña Mining District, including the Santa María and Antares skarn deposits (Spurr and Garrey 1908; Hernández 1991; Industrias Peñoles 2010; Jiménez-Franco et al. 2020). These skarn systems occur along the contact between the Cuesta del Cura limestones and intrusive rocks (Figs. 1a–b and 2) and generally strike NW–SE. According to Industrias Peñoles (2010), the Santa María ore bodies are associated with the N60W-striking Santa María aplitic dike, whereas the Antares ore bodies are linked to a rhyolite stock and other aplitic dikes (Fig. 2). The prograde skarn exhibits replacement textures and is dominated by calcic clinopyroxene and garnet, whereas the retrograde skarn consists mostly of actinolite, epidote, and chlorite (Jiménez-Franco et al. 2020). Ore assemblages consist of sphalerite/marmatite, galena, pyrite, pyrrhotite, chalcopyrite, and arsenopyrite, with minor amounts of boulangerite, marcasite, tetrahedrite-group minerals, Bi-sulfosalts, and scheelite (Cruz-Pérez and López-Escalona 1981; Hernández 1991; Industrias Peñoles 2010). These deposits were produced by intermediate-temperature (~ 220–450 °C) magmatic-derived hydrothermal fluids at hypabyssal depths around 3–4 km (Gilmer et al. 1988; Jiménez-Franco et al. 2020).
Cross section SE-60 from Fig. 1b depicting North and South Antares ore bodies and the drill cores. Cross section SE-400 is projected on SE-60 to show drill cores across the Santa María ore bodies
Materials and methods
A total of 13 drill cores across the Santa María and Antares skarn deposits were provided by Industrias Peñoles (Fig. 2). The samples are representative of the lithotypes and variations in ore and gangue assemblages found in both skarn deposits. Paragenetic relationships were studied in hand samples, thick polished sections, and thin sections at the Instituto de Geología, Universidad Nacional Autónoma de México (UNAM), and brief descriptions per sample are provided in Table S1 (Supplementary Material). We use mineral abbreviations from Warr (2021).
EPMA analyses were performed on a JEOL JXA-8900R electron probe micro-analyzer at the Laboratorio Nacional de Geoquímica y Mineralogía (LANGEM-UNAM, Mexico City, Mexico) at 20 keV, 20 nA beam current, 40 s acquisition time, and 1 µm spot. Detailed descriptions of standards and results are provided in Tables S2–S3 (Supplementary Material). Geothermometric and fS2 estimations were carried out on arsenopyrite and tetrahedrite-group minerals. In arsenopyrite, geothermometric and fS2 estimations are based on Kretschmar and Scott (1976) and Sharp et al. (1985), employing average values of at% As ± 1σ error per sample (n = 22 samples) and considering the paragenesis with pyrrhotite and/or pyrite. In tetrahedrite-group minerals, tetrahedrite geothermometry is based on the Zn/(Zn + Fe) versus Ag/(Ag + Cu) diagram with isotherms proposed by Sack (2005).
Fluid inclusion studies were carried out on 23 samples that represent each stage of each skarn deposit. Petrographic description of fluid inclusion assemblages (FIA), classification (primary, secondary, or pseudo-secondary), and trapping mechanism follows criteria by Roedder (1984), Goldstein and Reynolds (1994), and Van Den Kerkhof and Hein (2001). Microthermometric analyses were performed on a Linkam THMSG 600 stage at the LANGEM-UNAM, which operates in the range between –200 and 600 °C. The accuracy is ± 0.2 °C for low-temperature tests and ± 2 °C for high-temperature tests. For Santa María, data processing was completed using BULK software (Bakker 2003), by using the state equations for the H2O-NaCl-CaCl2 system, the ionic interaction model by Bakker (1999), and a NaCl/(NaCl + CaCl2) ratio of 0.05. The NaCl/(NaCl + CaCl2) ratio in the fluids was estimated using the equation:
postulated by Chi and Ni (2007), where y is the NaCl fraction, x is the hydrohalite solubilization temperature, and a (0.33124402), b (–0.031518028), and c (0.2293273) constants were provided by the authors. For Antares, microthermometric data was processed with HokieFlincs_H2O-NaCl (Steele-MacInnis et al. (2012)) considering the H2O-NaCl system and ignoring the effects of additional solutes. Salinity calculations were done by using halite solubilization temperatures for fluid inclusions with daughter halite crystals, and ice melting temperature for liquid + vapor inclusions. To characterize volatile species in fluid inclusions, ATR-FT-IR spectroscopy analyses were carried out on one fluid inclusion-rich sample of fluorite from Santa María (sample SM-58–11). This analysis was conducted with a Perkin Elmer FT-IR (Fourier transform infrared) spectrophotometer Spectrum 100, coupled with an ATR (attenuated total reflection) device, available at the Faculty of Chemistry of the UNAM (Mexico City, Mexico).
Carbon and oxygen stable isotopes of calcite were analyzed in 82 samples from a range of lithologies from Santa María and Antares (Table S4 in the Supplementary Material). These samples were carefully extracted with a microdrill, which was rinsed with acetone between samples to avoid contamination. Measurements were carried out at the LANGEM-UNAM, following the procedure of McCrea (1950). The CO2 released during the calcite-orthophosphoric (H3PO4) acid reaction for 50 h at 25 °C was measured on a Thermo Finnigan MAT 253 mass spectrometer coupled with a Gas Bench II, following Révész et al. (2001) and Révész and Landwehr (2002). δ13C is reported as per mil deviations (‰) from the standard VPDB (Vienna Pee Dee Belemnite), normalized to –46.6‰ for LSVEC (LiCO3), δ18O is normalized to 1.95‰ for NBS19 (Coplen et al. 2006). δ18O is presented as per mil deviations from the VSMOW (Vienna Standard Mean Ocean Water) standard and normalized as indicated by Coplen (1988).
Sulfur isotope analyses were carried out on 61 pure sulfide separates that were handpicked using a stereoscopic microscope (Table S4 in the Supplementary Material). Analyses were performed at the Centres Científics i Tecnològics (Universitat de Barcelona, Barcelona, Spain) on a Thermo Fisher Delta Plus XP mass spectrometer coupled with a TC-EA pyrolizer and a Carlo Erba 1108 elemental analyzer. Sulfides were placed in Sn capsules with V2O5 to release SO2 which was subsequently measured using the spectrometer. Results are expressed in delta notation (δ34S) as per mil deviations with respect to VCDT (Vienna Canyon Diablo Troilite). The precision of the analysis is better than ± 0.2‰. Data were calibrated with international reference materials: IAEA NBS-127, IAEA SO-5, IAEA SO-6, IAEA S-1, IAEA S-2, IAEA S-3, IAEA S-4, and YCEM.
Age dating using 40Ar/39Ar was performed on two samples of adularia (SM-43–12 and SM-58–12) from Santa María (Table S5 in the Supplementary Material). Adularia grains were prepared using conventional techniques, including crushing, sieving, washing, ultrasonic bathing, and separating using a Frantz model LB-1. Age determinations on the adularia separate (size fraction of 180–150 µm) were obtained at the Oregon State University (OSU) Argon Laboratory (Corvallis, Oregon, USA). Adularia mineral separates, with weights between 1.52 and 1.96 mg, as well as sanidine flux monitors (FCT 28.201 ± 0.023 Ma, 1σ; Kuiper et al. 2008), were placed in irradiation package 20-OSU-01 and irradiated for 6 h at the CLICIT position of the TRIGA nuclear reactor at OSU. Irradiated samples were loaded onto Cu-planchets in an ultra-high vacuum sample chamber and incrementally heated by scanning a defocused Synrad 30W CO2 laser beam, at increasing laser powers across the sample, to release the argon. After each heating step, and before analysis, reactive gases were cleaned for ~ 6 min using a set of AP10 Zr-Al getters; two hot getters operated at 450 °C and two at 21 °C. Argon isotopic measurements were performed using a Thermo Scientific ARGUS-VI multi-collector noble gas mass spectrometer (spectrometer “F” at OSU lab). All dates were calculated using the corrected value for the original constant for total 40 K decay to 40Ar by Steiger and Jäger (1977) with a new value of 5.530 ± 0.097 × 10–10/yr (2σ) as reported by Min et al. (2000). For all other constants used in the age calculations, we refer to Table 2 in Koppers et al. (2003). Individual J-values for each sample were calculated by parabolic extrapolation of the measured flux gradient against irradiation height and typically give 0.1–0.3% uncertainties (1σ). Incremental heating plateau ages and isochron ages were calculated as weighted means with 1/σ2 as the weighting factor (Taylor 1997) and as YORK2 least-square fits with correlated errors (York 1968) using the ArArCALC v2.6.2 software from Koppers (2002).
Ore and gangue assemblages
Santa María
The Santa María skarn deposit occurs along the contact between the Santa María dike and limestones or marbles (Fig. 2). The Santa María dike consists of pervasively altered aplites with scarce relicts of tabular plagioclase phenocrysts. Unaltered limestones are mud-supported and contain less than 10% grains plus bioclasts (i.e., mudstone; Dunham 1962), while marbles vary from fine- to coarse-grained depending on the degree of recrystallization (Fig. 3a). A detailed analysis of replacement textures and crosscutting relationships suggests that the skarn system developed during four main stages: prograde, retrograde, post-ore, and late (Fig. 4). Drill core and hand sample pictures and photomicrographs are shown in Figs. 3a–d and 5. Additional photomicrographs are provided in Fig. S1 in the Supplementary Material.
Lithotypes in the Santa María (a–d) and Antares (e–h) deposits. a Recrystallized limestone hosting a calcite vein with pyrite and arsenopyrite clusters. Crystal size coarsens towards the vein. b Contact between banded garnet + wollastonite exoskarn and a sphalerite + pyrite + calcite manto, parallel to banding. c Aplite replaced by massive adularia, embedding rounded patches of fluorite and sphalerite, and irregular clusters of arsenopyrite. The primary texture was obliterated by hydrothermal alteration minerals. d Massive pyrite and Fe-rich sphalerite partly replacing aplite, crosscut by a calcite vein. e Endoskarn composed of garnet, sphalerite, and pyrrhotite in contact with porphyritic rhyolite with feldspar phenocrysts. f Massive garnet + wollastonite exoskarn. g Fine-grained aplite altered to biotite + amphibole. Notice aplite clasts cemented by calcite in breccia on the right. h Massive sulfide that consists of pyrrhotite, galena, and sphalerite associated with massive quartz. Key: Adl = adularia, Amp = amphibole, Apy = arsenopyrite, Bt = biotite, Cal = calcite, Fl = fluorite, Fsp = feldspar, Gn = galena, Grt = garnet, Po = pyrrhotite, Py = pyrite, Qz = quartz, Sp = sphalerite, Wol = wollastonite
Photomicrographs illustrating the main ore and gangue assemblages of the Santa María skarn deposit. Thin sections: a Garnet porphyroblast replaced by epidote and surrounded by massive calcite (sample SM-43–19). b Garnet poikiloblast with sphalerite and tremolite (?) inclusions, embedded in calcite (sample SM-43–21). c Chlorite + calcite pseudomorphs after garnet surrounded by interstitial pyrite (sample SM-43–14). Fe-oxyhydroxides also occur as yellowish patches. d Actinolite finely intergrown with sphalerite, both filling spaces between prograde garnet (sample SM-43–21). e Fluorite and quartz coexisting with sphalerite (sample SM-42–12). f Pseudo-rhombohedral adularia intergrown with or encapsulated in sphalerite, arsenopyrite, and pyrite (same sample as in Fig. 3c, SM-58–12). Thick polished sections: g Massive pyrrhotite and sphalerite embedding euhedral arsenopyrite (sample SM-43–13). Observe, lillianite (Pb3-2xAgxBi2+xS6)-gustavite (AgPbBi3S6) and bird’s-eye-textured pyrite + marcasite replacing pyrrhotite, as well as pyrrhotite inclusions in sphalerite. h Euhedral pyrite crystals surrounded by pyrite + marcasite aggregates and sphalerite (sample SM-43–14). i Massive galena embedding pyrite and rhombic arsenopyrite (sample SM-42–12). j Pyrite, arsenopyrite, chalcopyrite, and sphalerite surrounded by anhedral tetrahedrite-group minerals (group 1) in a carbonate vein (sample SM-43–17). Tetrahedrite-group minerals occupy interstices between pyrite grains and fill voids in pyrite masses. Mineral abbreviations: Adl = adularia, Act = actinolite, Apy = arsenopyrite, Cal = calcite, Chl = chlorite, Ccp = chalcopyrite, Ep = epidote, Ttr = tetrahedrite-group minerals, Flr = fluorite, Gn = galena, Grt = garnet, Lil = lillianite, Gus = gustavite, Mrc = marcasite, Po = pyrrhotite, Py = pyrite, Sp = sphalerite. Image type: PPL = plane-polarized light, RL = reflected light, XPL = cross-polarized light
Prograde stage
In the exoskarn zone, the prograde stage is poorly preserved due to the overprinting of retrograde alterations. Exoskarns display medium- to coarse-grained granoblastic texture, locally banded; consist of garnet and wollastonite (Figs. 3b and 5a); and replace limestones. Idiomorphic garnet crystals can show zonation, and wollastonite forms feathery aggregates. The endoskarn zone was not observed at Santa María. Instead, in igneous rocks, the prograde stage is limited to local biotite alteration.
Retrograde stage
In the exoskarn zone, the retrograde stage largely obliterated prograde minerals and crystallized the following assemblages during four stages (Fig. 4): (1) scarce poikiloblastic fine-grained garnet encapsulating tremolite and sphalerite crystals, all embedded in calcite and quartz (Fig. 5b); (2) sericite, epidote, chlorite, calcite, quartz, tremolite, and lesser green biotite, epidote, and muscovite (Fig. 5c); (3) actinolite, sphalerite, quartz, calcite, and pyrite (Fig. 5d); and (4) chlorite, fluorite, quartz, calcite, epidote, tremolite, muscovite, and lesser tourmaline and actinolite accompanied by or intergrown with massive bodies, bands, veins, and patches of sulfides and sulfosalts (Fig. 5e). In contrast, the igneous rocks contain retrograde assemblages of (1) actinolite, quartz, pyrite, and lesser scapolite (stage 3 in Fig. 4) in irregular patches on top of biotite-altered aplites, overprinted by (2) adularia, fluorite, quartz, very fine-grained muscovite (referred hereafter as sericite), and calcite (stage 4 in Fig. 4) associated with the massive ore (Fig. 5f). Fine-grained adularia is the dominant alteration mineral in the aplites, occurring as distinctively beige massive replacements (Fig. 3c). Frequently, pseudo-rhombohedral adularia crystals are intergrown with the sulfides (Fig. 5f).
The retrograde stage at Santa María skarn deposits is divided into four stages: stage 1 through stage 4 (Fig. 4). Stage 4 is the main ore stage, consisting of Zn-Pb-Cu ± Ag massive ore, and overprints both the exoskarn and aplites. normally, sulfides and sulfosalts form massive replacement zones that obliterate any remaining sedimentary or igneous textures (e.g., Fig. 3d), thus precluding the identification of the protoliths. In places, the ore occurs as (1) carbonate replacement ore bodies in marble and limestone, (2) mantos in prograde exoskarns (Fig. 3b), and (3) small patches (< 1 cm) in aplites (Fig. 3c). Pyrite and sphalerite, commonly showing chalcopyrite disease, form centimeter-scale clusters in close association with galena, arsenopyrite, pyrrhotite, and chalcopyrite (Fig. 5g–i). Sphalerite varies in color from deep red to light orange (Fig. 5e). Arsenopyrite is euhedral and can be replaced by pyrrhotite, while pyrrhotite is normally encapsulated in pyrite and sphalerite (Fig. 5g). Arsenopyrite also forms clusters and irregular masses with lesser pyrite and seldom displays concentric zoning. Galena forms isolated crystals and inter-crystalline masses (Fig. 5i). Locally, Pb-Bi ± Sb sulfosalts replace pyrrhotite or are intergrown with galena and/or sphalerite (Fig. 5g). Scant magnetite is restricted to inclusions in sphalerite.
Post-ore stage
The post-ore stage comprises blocky calcite veins and veinlets (< 5 cm wide), which crosscut massive sulfide assemblages. Massive calcite is accompanied by minor amounts of quartz and barite (Fig. 3a, d). In one sample (SM-43–17), these veins contain small clusters (< 3 cm) of arsenopyrite, pyrite, and lesser galena, chalcopyrite, and sphalerite (Figs. 3a and 5j). Late tetrahedrite-group minerals (group 1 in Fig. 4) fill vugs between sulfides and/or replace them (Fig. 5j). Moreover, the post-ore stage locally has breccias cemented by coarse-grained blocky calcite, in which angular clasts of aplite, marble, or massive sulfide ore are slightly rotated.
Late stage
Late-stage alteration assemblages comprise chlorite, calcite, sericite, and epidote. This stage also crystallized widespread bird’s-eye-textured pyrite + marcasite replacing pyrrhotite (Fig. 5g–h) as well as tetrahedrite-group minerals (group 2 in Fig. 4) replacing chalcopyrite, galena, pyrrhotite, and/or sphalerite. Epidote in this stage is fine-grained and poorly crystallized, in contrast with well-developed larger crystals from stage 4 (Fig. 5a).
Antares
North and South Antares occur along the contact between a porphyritic rhyolite stock or aplite dikes and the Cuesta del Cura Formation (Fig. 2). Porphyritic rhyolites consist of quartz, plagioclase, and sanidine phenocrysts surrounded by fine-grained quartz-feldspathic groundmass (Figs. 3e and 6e). Based on paragenetic relationships observed petrographically, we found that ore bodies at Antares formed during prograde, retrograde, and late stages (Fig. 4). Drill core/hand sample pictures and photomicrographs are shown in Figs. 3e–h and 6. Additional photomicrographs of gangue assemblages are provided in Fig. S2 in the Supplementary Material.
Photomicrographs illustrating the main ore and gangue assemblages of the Antares skarn deposit. Thin sections. Exoskarn: (a–b) Calcite fillings within garnet, clinopyroxene, and wollastonite (samples AS-122–02 and AS-155–8). Note that calcite replaces garnet and wollastonite in b. c Sulfides intergrown with quartz and tremolite, the latter overprinted by prehnite crystals (sample AS-122–06). Endoskarn: d Biotite-rich alteration replaced by tremolite + actinolite + pyrrhotite assemblages (sample SM-58–2). e Sanidine phenocryst surrounded by a cryptocrystalline groundmass replaced by fine-grained tremolite (sample AS-166–8). Tremolite is associated with sphalerite and quartz, and crosscut by epidote grains. f Coarse scapolite crystals replaced by tremolite (AS-166–7). Thick polished sections: g Endoskarn mineralization of pyrrhotite, molybdenite, and chalcopyrite. Pyrrhotite is altered to pyrite and marcasite (sample AS-125–11). h Massive sphalerite and pyrrhotite from the exoskarn zone (sample AS-125–2). Pyrrhotite embeds euhedral arsenopyrite. i Sphalerite and pyrrhotite replacing outer growth bands of garnet (sample AN-179–10). j Massive sulfide assemblages consisting of pyrrhotite, pyrite, chalcopyrite, and jamesonite, as well as pyrite + marcasite replacing pyrrhotite (sample AS-125–4). Mineral abbreviations: Act = actinolite, Bt = biotite, Ccp = chalcopyrite, Di = diopside, Ep = epidote, Grt = garnet, Ja = jamesonite, Mol = molybdenite, Mrc = marcasite, Po = pyrrhotite, Qz = quartz, Sa = sanidine, Scp = scapolite, Sp = sphalerite, Tr = tremolite, Wol = wollastonite. Image type: PPL = plane-polarized light, RL = reflected light, XPL = cross-polarized light
Prograde stage
Unlike Santa María, the prograde skarn at Antares displays well-developed exoskarn and endoskarn zones. The exoskarn zone is up to 20 m wide and is dominated by beige and green garnet, clinopyroxene, and wollastonite (Figs. 3f and 6a–b). Similarly, the endoskarn zone is ~ 10–15 m wide and is dominated by garnet and clinopyroxene (Fig. 3e). Coarse garnet (~ 5 mm) is frequently zoned and may form monomineralic bands. Clinopyroxene forms aggregates that usually overprints garnet (Fig. 6a). Wollastonite occurs as radial aggregates of tabular crystals that overprint garnet and clinopyroxene (Fig. 6b).
Retrograde stage
In the retrograde stage, the exoskarn is replaced by retrograde actinolite, tremolite, quartz, calcite, epidote, chlorite, prehnite, and lesser scapolite (Fig. 6c). The endoskarn is replaced by (1) biotite ± K-feldspar or tremolite-actinolite, fluorite, scapolite, quartz, and calcite assemblages (stage 1 in Fig. 4; Fig. 6d–f), overprinted by (2) muscovite, sericite, epidote, quartz, and prehnite assemblages (stage 2 in Fig. 4; Fig. 6e). The retrograde minerals accompany the economically most important mineralization in the deposit (Fig. 6g–j), which consists of pyrrhotite, arsenopyrite, sphalerite, chalcopyrite, and molybdenite (stage 1) overprinted by arsenopyrite, pyrite, galena, and minor pyrrhotite, molybdenite, Pb-Sb ± Bi sulfosalts, and marcasite (stage 2). Most ore associations are found as disseminations, veinlets, and massive bodies. Pyrrhotite is abundant and forms massive aggregates and veins with calcite and quartz. Traces of tabular molybdenite were recognized in the endoskarn (Fig. 6g). Euhedral arsenopyrite crystals are embedded in pyrrhotite and sphalerite masses (Fig. 6h). Massive sphalerite and chalcopyrite tend to concentrate in the proximal exoskarn zone. In some cases, pyrrhotite and sphalerite replace garnet outer rims or occupy vugs between garnet crystals (Fig. 6i). Galena occurs as fillings and may host exsolution lamellae of Pb-Sb ± Bi sulfosalts (Fig. 6j).
Late stage
The late stage comprises chlorite, epidote, calcite, and sericite assemblages that are associated with fine-grained pyrite + marcasite (bird’s-eye textured) replacing pyrrhotite (Fig. 6g). Late calcite and fluorite veins crosscut the endoskarn and are haloed by epidote and Fe-oxyhydroxides.
Mineral composition
EPMA analyses were performed on (1) clinopyroxene from the prograde stage at Antares, (2) garnet from the prograde stage at Santa María and Antares, (3) arsenopyrite and Pb-Bi-Sb sulfosalts from the ore and post-ore stages at Santa María and Antares, and (4) tetrahedrite-group minerals from the post-ore and late stages at Santa María. The results and additional calculations are provided in Table S3 (Supplementary Material).
Prograde stage
Clinopyroxene
Only pyroxene crystals from the exoskarn zone at Antares were analyzed (sample AS-155–8), which are mostly diopside (Di84–92Hd7–15Jhn0–3; Fig. 7a). The johannsenite component is low, as MnO does not exceed 1 wt%.
Classification diagrams for ore and gangue minerals from Santa María (SMB) and Antares (AMB) skarn deposits. a Garnet and clinopyroxene compositional plots after Einaudi and Burt (1982). b Composition of arsenopyrite from the ore and post-ore stages at Antares and Santa María, according to its association with pyrrhotite and/or pyrite. c Bi2S3-Sb2S3-PbS (left) and (Ag + Cu)-Pb-(Bi + Sb) (right) compositional diagrams for ore-stage Pb-Bi-Sb sulfosalts, modified from Buzatu et al. (2015) and Gvozdev et al. (2020). In the (Ag + Cu)-Pb-(Bi + Sb) plot, dotted gray lines indicate the number of galena-like slabs (N), and abbreviations in bold font are Sb-dominated phases whereas those in italics are Bi-dominated. Reflected light photomicrographs of each sulfosalt are also provided. d Classification scheme for tetrahedrite-group minerals from Santa María, modified from Sack (2005) and Sack et al. (2005). Key: Bnj = benjaminite, Bou = boulangerite, Cos = cosalite, Ekm = eskimoite, Gn = galena, Gbit = galenobismutite, Gus = gustavite, Hyv = heirovskýite, Ja = jamesonite, Lil = lillianite, Lö = löllingite, Mtd = matildite, Our = ourayite, Po = pyrrhotite, Py = pyrite, Tnt = tennantite, Tsur = treasurite, Ttr = tetrahedrite, Vkg = vikingite
Calcic garnet
Grains from the exoskarn in both deposits have similar compositions within the grossular-andradite solid solution (Antares: Grs49–83Adr12–49 and Santa María: Grs52–72Adr27–47), with pyralspitic components normally lower than 2.0 mol% (Fig. 7a). Some spots show minor MnO enrichments up to 1.1 wt%. Stoichiometric calculations were made following Hawthorne (2021). Garnet zonation observed petrographically in sample SM-43–20 from Santa María results from Al-rich and Fe-poor (Grs61–71Adr29–39) cores versus Al-poor and Fe-rich (Grs52–68Adr48–32) rims. In contrast, linear compositional profiles performed on garnet from Antares (samples AS-155–8 and AS-122–2) revealed oscillatory zonation of varying thickness (Table S3 in the Supplementary Material).
Retrograde-ore stage
Arsenopyrite
At Santa María, ore-stage arsenopyrite displays constant compositions regardless of its association with pyrrhotite and/or pyrite, averaging with error at 1σ: (a) 45.4 ± 1.4 wt% As, 19.9 ± 0.8 wt% S, and 34.4 ± 0.5 wt% Fe (arsenopyrite + pyrite assemblage; n = 77); (b) 44.7 ± 0.9 wt% As, 20.6 ± 0.4 wt% S, and 34.8 ± 0.2 wt% Fe (arsenopyrite + pyrite + pyrrhotite assemblage; n = 23); and (c) 45.0 ± 1.1 wt% As, 19.9 ± 0.4 wt% S, and 33.8 ± 0.6 wt% Fe (arsenopyrite + pyrrhotite assemblage; n = 19). Likewise, at Antares, ore-stage arsenopyrite yielded (a) 43.3 ± 0.9 wt%, 21.7 ± 0.5 wt% S, and 35.1 ± 0.3 wt% Fe (arsenopyrite + pyrite assemblage; n = 5); (b) 44.5 ± 1.1 wt% As, 20.6 ± 0.9 wt% S, and 34.7 ± 0.5 wt% Fe (arsenopyrite + pyrite + pyrrhotite assemblage; n = 51); and (c) 44.7 ± 1.3 wt% As, 20.2 ± 1.0 wt% S, and 34.6 ± 0.6 wt% Fe (arsenopyrite + pyrrhotite assemblage; n = 51). In all spots, trace elements, including Bi (< 0.3 wt%), Ni (< 0.2 wt%), Sb (< 0.9 wt%), and Co (< 0.6 wt%), do not exceed 1.0 wt%. Noteworthy, arsenopyrite + pyrite assemblages are conspicuous at Santa María, whereas arsenopyrite + pyrrhotite ± pyrite assemblages dominate at Antares (Fig. 7b).
Pb-Bi-Sb sulfosalts
The compositional constraints presented herein must be considered tentative, since most spot analyses totaled in the unreliable 90.0–98.5 wt% range, perhaps due to an underestimation of Bi by the EPMA. That is not the case of Se-bearing galena (Santa María) and boulangerite (Antares), which lie within the permissive interval of 100.0 ± 1.5 wt%. The main difference between Pb-sulfosalts from Santa María and those from Antares is the Bi/Sb ratio, which averages 0.2 at Santa María and 1.5 at Antares (Fig. 7c). At Santa María, these sulfosalts occur in two samples (SM-43–13 and SM-43–21), are Bi-dominated (16.4–21.8 wt% Bi and < 10.4 wt% Sb), and grade up to 6.2 wt% Se. In the Pb-(Bi + Sb)-(Ag + Cu) compositional diagram (Buzatu et al. 2015), sulfosalts show variable contents of Pb and (Bi + Sb) at constant (Ag + Cu) ~ 2.5 wt%, and plot close to the lillianite-gustavite and heyrovskýite-eskimoite compositional trends, with 5 and 9 galena-like slabs respectively (Fig. 7c). Galena-like slabs are used to characterize this type of sulfosalts (i.e., lillianite homologues) and refers to octahedral chains that extend in a zig-zag pattern throughout the mineral structure (Buzatu et al. 2015). At Antares, Pb-sulfosalts from sample AN-179–10 are enriched in Sb (17.7–26.3 wt%) compared to Bi (< 4.6 wt%) and arrange linearly between Pb and (Bi + Sb) in Fig. 7c, at low quantities of Ag + Cu (~ 0.8 wt%). Therefore, these crystals were classified as Bi-bearing jamesonite, boulangerite, and a lillianite homologue with 6 galena-like slabs (Fig. 7c).
Post-ore stage
Arsenopyrite
Grains from sulfide clusters in calcite veins average 43.5 ± 0.5 wt% As, 21.4 ± 0.2 wt% S, and 34.3 ± 0.3 wt% Fe (n = 10), with total trace element contents lower than 1.0 wt% (Bi < 0.2 wt%, Ni < 0.3 wt%, Sb < 0.5 wt%, and Co < 0.1 wt%).
Tetrahedrite group
These sulfosalts, which occupy interstices between sulfide grains in post-ore calcite veins (Fig. 8), belong to group 1 in Fig. 7d and yield Ag/(Ag + Cu) between 0.01 and 0.05, Zn/(Zn + Fe) between 0.35 and 0.70, and As/(As + Sb) between 0.11 and 0.82 (n = 29). This composition range coincides with that of the tetrahedrite Fe-tetrahedrite Zn and tennantite Fe-tennantite Zn series (Fig. 7d).
Tetrahedrite geothermometry after Sack (2005). The grouping of samples is the same as in Fig. 7c and reflected light photomicrographs of each group are provided as insets. Key: Ccp = chalcopyrite, Gn = galena, Lö = löllingite, May = miargyrite, Po = pyrrhotite, Py = pyrite, Pyg = pyrargyrite, Sp = sphalerite, Ttr = Tetrahedrite-group minerals
Late stage
Tetrahedrite group
These sulfosalts cluster into groups 2A and 2B in Fig. 7d. Sulfosalts from group 2A replace ore-stage chalcopyrite, galena, sphalerite, and/or pyrrhotite (Fig. 8), and yield Ag/(Ag + Cu) between 0.33 and 0.56, Zn/(Zn + Fe) between 0.07 and 0.38, and As/(As + Sb) < 0.10 (tetrahedrite Fe-freibergite; n = 23). Sulfosalts from group 2B only replace ore-stage chalcopyrite (Fig. 8) and yield Ag/(Ag + Cu) of 0.28, Zn/(Zn + Fe) between 0.73 and 0.89, and As/(As + Sb) between 0.10 and 0.16 (tetrahedrite Fe-tetrahedrite Zn; n = 5).
Geothermometric and fS2 estimations
Retrograde-ore stage
Arsenopyrite
At Santa María, temperature and sulfur fugacity estimations for the ore stage range from (a) 359° to 590 °C and –8.7 to –4.5 log fS2 (arsenopyrite + pyrite assemblage), (b) 325° to 500 °C and –11.0 to –4.5 log fS2 (arsenopyrite + pyrrhotite assemblage), and (c) 300° to 460 °C and –10.7 to –5.4 log fS2 (arsenopyrite + pyrite + pyrrhotite assemblage; Table 1). At Antares, temperature and sulfur fugacity estimations for the ore stage range from (a) 320° to 520 °C and –9.8 to –4.5 log fS2 (arsenopyrite + pyrite + pyrrhotite assemblage), (b) 310° to 420 °C and –8.8 to –6.6 log fS2 (arsenopyrite + pyrite assemblage), and (c) 300° to 510 °C and –12.4 to –4.4 log fS2 (arsenopyrite + pyrrhotite assemblage; Table 1). Therefore, arsenopyrite crystallized at similar temperatures and fS2 ranges in both skarn deposits. However, these data should be treated with caution, as this method often overestimates crystallization temperatures compared to other techniques (e.g., fluid inclusions; Voudouris et al. 2013).
Post-ore stage
Arsenopyrite
We obtained temperature from 345° to 515 °C and sulfur fugacity from –8.7 to –6.4 log fS2 in arsenopyrite associated with pyrite from post-ore veins (Table 1).
Tetrahedrite group
Compositional data of sulfosalts from group 1 cluster beneath the 170 °C isotherm in the Zn/(Zn + Fe) versus Ag/(Ag + Cu) plot (Sack 2005; Fig. 8), suggesting crystallization temperatures at or below this value.
Late stage
Tetrahedrite group
Compositional data obtained in sulfosalts from group 2A are equivalent to crystallization temperatures between 170° and 300 °C (Fig. 8), whereas those obtained in group 2B indicate temperatures around 170 °C (Sack 2005).
Fluid inclusion studies
Fluid inclusion studies were performed on samples from Santa María and Antares that are representative of the prograde, ore, post-ore, and late stages. Fluids from Santa María are calcic brines and hence the salinity data is reported as wt% CaCl2, whereas those from Antares are sodic ± potassic brines and salinities are reported as wt% NaCl equiv. Summarized microthermometric data are provided in Table 2 and are shown in Fig. 9. The errors presented throughout this section are 1σ.
Salinity versus temperature of homogenization (Th) plot for fluid inclusion assemblages (FIA) hosted by garnet, quartz, sphalerite, fluorite, and calcite from the prograde (Prog), ore, post-ore (PO), and late (L) stages at Santa María and Antares. Error bars are 1σ. A summarized paragenetic sequence for FIAs from both deposits is depicted in the lower-right corner of each plot. Data from previous fluid inclusion studies by Gilmer et al. (1988) and Jiménez-Franco et al. (2020) are also portrayed. Key: Apy = arsenopyrite, Ttr = tetrahedrite-group minerals, GT = geothermometry
Santa María
Fluid inclusion analyses were carried out on samples from the exoskarn zone, massive sulfides, and late calcite veins. We studied 8 individual fluid inclusions from the prograde stage (2 FIAs), 269 from the ore stage (35 FIAs), 104 from the post-ore stage (10 FIAs), and 18 from the late stage (3 FIAs) (Fig. 9a). Photomicrographs of the analyzed FIAs are provided in Fig. S3 in the Supplementary Material.
Prograde stage
Primary fluid inclusions in garnet are up to 5 µm in diameter, have a degree of filling (F: proportion of the liquid phase) of 0.80–0.90; appear isolated in growth zones; and display rhombic, irregular, and square shapes. Given their small size, phase changes at low temperatures could not be determined with precision. During heating tests, these inclusions did not exhibit signs of shrinkage of the vapor bubble or sudden movements, even at 600 °C (the upper limit of the equipment). Thus, we consider 600 °C as their minimum temperature of homogenization (Th). Secondary fluid inclusions in garnet have F ~ 0.90, are up to 7 µm in size, and arrange across intragranular trends. Eutectic temperatures (Te) average –90.7 °C, thus indicating the occurrence of volatiles (e.g., CH4); ice melting temperatures (Tmi) average –28.9 °C (24.5 wt% CaCl2); and Th average 437 °C. Overall, these data resemble those of the ore stage (see next section).
Ore stage
Primary, pseudosecondary, and secondary FIAs in sphalerite, calcite, quartz, and fluorite from the ore stage were analyzed. Primary FIAs comprise isolated individuals or clusters of randomly distributed fluid inclusions, whereas pseudosecondary FIAs encompass fluid inclusions along sealed fractures. In all cases, fluid inclusions do not exceed 30 μm in diameter; are liquid + vapor (L + V) with constant F at ~ 0.80; and display polygonal, cuneiform, round, and irregular shapes. Fluid inclusions hosted by sphalerite and calcite usually show negative crystal morphologies. Trapped solids of rounded opaque minerals and halite are sparse, and occasional large (> 50 μm) and irregular fluid inclusions in fluorite may contain daughter solids. Additionally, one FIA hosted by quartz (associated with adularia + fluorite alteration) contains L-rich, V-rich, and L + V + halite fluid inclusions, which indicates heterogeneous trapping (Figs. 3c and S3e; i.e., boiling).
The evolutionary trajectory of a representative primary fluid inclusion in ore-stage sphalerite is shown in Fig. S4 in the Supplementary Material. Most ore-stage primary and pseudosecondary fluid inclusions freeze completely at around –100 °C, although some never achieve solidus even at about –200 °C. Such behavior is attributed to metastability phenomena that are common in H2O-NaCl-CaCl2 systems (Chu et al. 2016). Te vary between –120° and –90 °C, suggesting the occurrence of methane. Antarcticite-hydrohalite crystals melt between –41.1° and –60.7 °C (–50.2 °C on average), thus encompassing the ternary eutectic point of the H2O-NaCl-CaCl2 system (–51.0 °C; Steele-MacInnis et al. 2016). The variations in the antarcticite-hydrohalite melting point can result from the occurrence of additional solutes (e.g., MgCl2, FeCl2, FeCl3, etc.; Xu 2000; Steele-MacInnis et al. 2016). Tmi average –24.4 ± 4.0 °C (n = 2) in FIAs enclosed in calcite, –26.4 ± 5.3 °C (n = 17) in sphalerite, –22.8 ± 5.8 °C (n = 13) in fluorite, and –40.7 ± 10.2 °C (n = 3) in quartz, equivalent to salinities of 22.6 ± 1.8, 23.4 ± 1.9, 21.7 ± 2.6, and 27.8 ± 2.9 wt% CaCl2, respectively. Homogenization to liquid occurs at 374 ± 7 °C in FIAs encapsulated in calcite, 392 ± 23 °C in sphalerite, 399 ± 36 °C in fluorite, and 377 ± 34 °C in quartz. No clathrates were observed.
Post-ore stage
Calcite from late veins contains L + V primary and pseudosecondary FIAs. Primary FIAs consist of isolated individuals or clusters of fluid inclusions in growth zones, and pseudosecondary FIAs are trapped along sealed fractures. These inclusions are normally square or round, attain sizes up to 30 μm in diameter, and have F between 0.80 and 0.90. Also, they locally contain subtabular and round opaque or translucent trapped solids that are too small for microscopic identification. Some isolated FIAs comprise V-rich, L-rich, and L + V + daughter solid inclusions (Fig. S3i), which suggests heterogeneous trapping. The microthermometric behavior of these FIAs and those of the ore stage is similar. Te range between –120.0° and –100.0 °C and antarcticite-hydrohalite melting temperatures average –55.0 °C. These features indicate that methane is present and CaCl2 is the main solute, followed by NaCl and, probably, additional other bivalent cation chlorides (e.g., MgCl2 or FeCl2; Steele-MacInnis et al. 2016). Excluding the boiling FIA, which shows Tmi averaging –9.7 °C (13.9 wt% CaCl2), Tmi vary considerably in these FIAs (–45.5° to –17.9 °C) and average –30.1 ± 11.0 °C (24.4 ± 3.7 wt% CaCl2; n = 10). Homogenization into liquid occurs at 375 ± 51 °C.
Late stage
Secondary FIAs in ore-stage quartz and fluorite were analyzed. In quartz, they line up along discontinuous intragranular trails, have F ~ 0.90, display oval shapes, and are up to 10 μm in size. Only one Te at –100.2 °C was observed. These FIAs show Tmi at –25.7 ± 4.5 °C (23.1 ± 1.9 wt% CaCl2 equiv.; n = 2) and Th at 213 ± 43 °C. In fluorite, secondary fluid inclusions with F ~ 0.90 are up to 15 μm in diameter and average Tmi at –7.6 °C (12.0 wt% CaCl2) and Th at 162 °C.
Antares
At Antares, fluid inclusion analyses were carried out on samples from the exoskarn zone, rhyolite stock, massive sulfides, and late fluorite veins. We studied 4 fluid inclusions from the prograde stage (1 FIA), 39 from the ore stage (11 FIAs), and 38 from the late stage (10 FIAs) (Fig. 9b). Photomicrographs of the analyzed FIAs are provided in Fig. S5 in the Supplementary Material.
Prograde stage
FIAs hosted by garnet are confined to specific rims inside zoned crystals or occur as isolated fluid inclusions up to 20 μm in diameter, and display oval or polygonal shapes. Normally, they are L + V (F at 0.85–0.95), although some contain small translucent and opaque trapped crystals. Small translucent solids show a greenish tint and isotropic character. One Te at –40.0 °C indicates the occurrence of bivalent cation chlorides (e.g., FeCl2 or MgCl2; Steele-MacInnis et al. 2016). One FIA displays Tmi at –11.3 °C, equaling 15.0 wt% NaCl equiv., and Th at 597 °C. However, several fluid inclusions remained L + V at 600 °C.
Ore stage
Primary and pseudosecondary FIAs in fluorite and calcite from the massive ore occurs as trails along sealed fractures. These consist of L + V or L + V + daughter solids, attain up to 30 μm in diameter, exhibit rectangular or round shapes, and locally form negative crystals. Daughter and trapped crystals are frequent and include halite, sylvite, greenish irregular crystals, and colorless tabular crystals (both unknown minerals; Fig. S5f–h). F varies between 0.75 and 0.85 and necking is frequent. Te was only observed in two inclusions at about –38.4 °C and at –100.0 °C, which suggests the presence of MgCl2 or FeCl2 (Steele-MacInnis et al. 2016) in addition to NaCl, KCl, and, eventually, methane. In fluorite, Tmi and Th average –14.1 ± 2.2 °C and 404 ± 26 °C (n = 7), whereas in calcite Tmi and Th average –14.1 and 433.1 °C. In both minerals, halite dissolves at 363 ± 44 °C (44.6 ± 3.8 wt% NaCl equiv.), the unknown tabular mineral at ~ 235 °C, and sylvite between 195 and 299 °C (44.9–55.1 wt% KCl; Sterner et al. 1988).
A group of secondary FIAs enclosed in quartz phenocrysts from the rhyolite porphyry (endoskarn zone) were also analyzed. Fluid inclusions are rounded, consist of L + V + halite + sylvite (F ~ 0.85), and are aligned across continuous trails (Fig. S5i). One Te at –26.1 °C indicates that the main solutes are NaCl and KCl, probably with additional MgCl2 (Steele-MacInnis et al. 2016). Tmi averages –9.2 ± 2.9 °C and Th averages 495 ± 11 (n = 3). Temperatures of halite solubilization average 512 ± 39 °C, equivalent to salinities of 60.5 ± 3.4 wt% NaCl equiv. Based on similarities between these data and those of ore-stage fluid inclusions, it is likely that fluid inclusions in quartz phenocryst contain retrograde-stage fluids.
Late stage
Late FIAs encompass pseudosecondary fluid inclusions in fluorite veins and secondary fluid inclusions in ore-stage calcite. Those in fluorite are L + V (F at 0.90–0.95), average 20 µm in diameter, and display rectangular shapes. One Te occurs around –20.8 °C, which is similar to that of the NaCl-H2O system (–21.2 °C; Steele-MacInnis et al. 2016). Tmi averages –14.7 ± 1.6 °C (salinity of 18.3 ± 1.4 wt% NaCl equiv.) and Th averages 223 ± 30 °C (n = 9). Secondary fluid inclusions in calcite display rhombohedral shapes, high F (~ 0.95), and are distributed along intragranular trails. They seldom contain trapped opaque and translucent solids. Te occurs around –84.1 °C, and unidentified cryogenic solids melt at about –53.2 °C, thus indicating a chemical system that likely involves methane and bivalent cation chlorides (Steele-MacInnis et al. 2016). Tmi around –12.7 °C corresponds to 16.5 wt% NaCl equiv., and Th averages 143 °C.
ATR-FT-IR analyses
We analyzed one sample of ore-stage fluorite (SM-58–11), which contained abundant primary and pseudosecondary fluid inclusions. The spectrum displays bands at 2,960 and 2,870 cm−1 that are typical of the asymmetric and symmetric stretching in vibrations of the C-H bond (Fig. S6). Other bands at 1,460 and 1,380 cm–1 are related to the asymmetric and symmetric torsion of vibrations of the H–H bond. In addition, the absence of diagnostic bands of -CH2 bonds points toward methane as the main volatile, instead of a more complex hydrocarbon. The characteristic band of the stretching vibration of the C = O bond at 1,700 cm–1 is also visible, due to calcite in association with fluorite.
Stable isotopes
Carbon and oxygen isotopes
Carbon and oxygen stable isotopes were analyzed in calcite from limestones, marbles, exoskarns, endoskarns, altered aplites, massive sulfides, post-ore veins, and breccias (Table S4 in the Supplementary Material). The C-O isotopic composition of the fluid from which calcite crystallized was calculated following Bottinga (1968) and O’Neil et al. (1969). The results are shown in Fig. 10.
δ18O versus δ13C plot for fluids calculated in calcite samples from Santa María and Antares compared to various C and O sources (Eichman and Schindlowski 1975; Zheng and Hoefs 1993; Campbell and Larson 1998; González-Partida et al. 2019; Huston et al. 2023). δ18O and δ13C of calcite from limestone and marble are plotted as δ18OVSMOW and δ13CVPDB
Santa María: In unaltered limestones of the Cuesta del Cura Formation, δ18OVSMOW equals 19.46‰ and δ13CVPDB equals 0.65‰. In recrystallized limestones, δ18OVSMOW is between 18.47‰ and 18.92‰, and δ13CVPDB between –3.43‰ and –3.21‰. In fine-grained marble, δ18OVSMOW ranges between 14.56‰ and 21.26‰, and δ13CVPDB between –7.99‰ and –0.49‰. Retrograde calcite from the exoskarn shows the lowest δ values (δ18OVSMOW between 14.81‰ and 18.08‰, and δ13CVPDB between –6.87‰ and –3.25‰), equaling fluids with δ18Ofluid between 11.93‰ and 15.19‰ and δ13Cfluid between –4.59‰ and –0.98‰. Calcite from massive sulfide assemblages records δ18OVSMOW between 14.61‰ and 21.05‰ (δ18Ofluid between 13.85‰ and 15.46‰), and δ13CVPDB between –2.57‰ and –0.20‰ (δ13Cfluid between –3.86‰ and 2.07‰). Similarly, aplites with adularia + fluorite + carbonate + sericite alteration (ore stage) host calcite that displays δ18OVSMOW between 16.00‰ and 23.28‰ (δ18Ofluid between 13.12‰ and 20.40‰), and δ13CVPDB between –4.56‰ and –0.06‰ (δ13Cfluid between –2.29‰ and 2.21‰). Calcite in post-ore veins shows δ18OVSMOW between 14.61‰ and 21.05‰ (δ18Ofluid between 11.39‰ and 17.82‰), and δ13CVPDB between –4.21‰ and –0.61‰ (δ13Cfluid between –1.99‰ and 1.61‰). In breccias, calcite δ18OVSMOW ranges between 13.87‰ and 22.71‰ (δ18Ofluid between 10.65‰ and 19.49‰), and δ13CVPDB between –3.38‰ and –0.34‰ (δ13Cfluid between –1.17‰ and 1.88‰).
Antares
The least-altered limestones display δ13CVPDB at 14.05‰ and δ18OVSMOW at –0.30‰, whereas calcite from marbles yielded δ18OVSMOW at ~ 13.06‰ and δ13CVPDB between –5.64‰ and –0.05‰. Retrograde calcite from endoskarns yielded δ18OVSMOW between 10.67‰ and 21.37‰ (δ18Ofluid between 8.07‰ and 18.77‰), and δ13CVPDB between –8.29‰ and –5.13‰ (δ13Cfluid between –5.97‰ and –2.81‰), and from exoskarns yielded δ18OVSMOW between 9.35‰ and 15.38‰ (δ18Ofluid between 6.75‰ and 12.78‰), and δ13CVPDB between –6.95‰ and –0.59‰ (δ13Cfluid between –4.63‰ and 1.73‰). Ore-stage calcite from massive sulfides yielded δ18OVSMOW between 12.16‰ and 20.13‰ (δ18Ofluid between 9.56‰ and 17.53‰), and δ13CVPDB between –8.08‰ and 0.39‰ (δ13Cfluid between –5.76‰ and 1.93‰). Calcite from altered aplites (retrograde-ore stage) yielded δ18OVSMOW between 10.72‰ and 17.23‰ (δ18Ofluid between 8.12‰ and 14.64‰), and δ13CVPDB between –6.48‰ and –0.28‰ (δ13Cfluid between –4.17‰ and 2.04‰).
Sulfur isotopes
Santa María
δ34SVCDT was obtained in sulfides from ore (arsenopyrite, galena, pyrite, pyrrhotite, and sphalerite), post-ore (pyrite), and late stages (pyrite-marcasite; Table S4 in the Supplementary Material). δ34SVCDT in arsenopyrite ranges between –2.61‰ and –0.26‰, in pyrite between –2.77‰ and 0.49‰, in pyrrhotite between –0.67‰ and –0.22‰, in sphalerite between –3.20‰ and 1.21‰, in galena –1.38‰, in post-ore pyrite –0.33‰, and in late pyrite-marcasite –0.40‰ (Fig. 11).
Histograms for δ34SVCDT in sulfides from the ore stage at Santa María and Antares. Magmatic sulfur range after Chaussidon and Lorand (1990). Data from sedimentary wall rocks and skarn deposits at the Santa María and San Lorenzo ranges from Gilmer et al. (1988) and Jiménez-Franco et al. (2020). Key: Apy = arsenopyrite, Gn = galena, Mrc = marcasite, Mol = molybdenite, Po = pyrrhotite, Py = pyrite, Py* = pyrite from the post-ore stage at Santa María, Sp = sphalerite
Antares
δ34SVCDT was obtained in sulfides from the ore stage (Table S4). δ34SVCDT in arsenopyrite ranges between –1.54‰ and –0.60‰, in chalcopyrite between –1.35‰ and 1.20‰, in galena between –0.91‰ and 1.69‰, in pyrrhotite between –2.80 and 0.36‰, in pyrite between 0.04 and 0.41‰, in sphalerite between –1.87‰ and 1.54‰, in marcasite –0.43‰, and in molybdenite 0.8‰ (Fig. 11).
Dating
40Ar/39Ar dating was performed on two samples (SM-43–12 and SM-58–12) of pseudo-rhombohedral adularia from the ore stage at Santa María (Table S5 in the Supplementary Material). Adularia (size fraction 180–150 µm) from the sample SM-43–12 showed a flat degassing pattern with indication of some Ar loss in the early degassing steps (Fig. 12). The steps in the last part of the spectrum were fairly homogeneous and allow calculating a weighted mean age or “mini plateau” date of 37.48 ± 0.09 Ma (2σ; MSWD = 1.41; 40.1% of gas; 21 of 39 steps). Adularia from sample SM-58–12 (same mineral size fraction, 180–150 µm) presented an almost identical Ar degassing pattern (Fig. 12). With the last ~ 40% of the gas used to calculate a “mini plateau” date of 37.62 ± 0.09 Ma (2σ; MSWD = 4.18; 15 of 34 steps). We interpret these dates as indicating that the mineralization event occurred during the late Eocene.
Discussion
Typology and evolutionary process of the metallogenic system
The Santa María and Antares Zn-Pb(-Ag) skarn deposits developed during the Eocene–Oligocene with the intrusion of felsic subalkaline–alkaline magma into hypabyssal Cretaceous carbonates of the Sector Transversal de Parras (Fig. 1; Jiménez-Franco et al. 2020). Our 40Ar/39Ar dates on ore-stage adularia (37.5 Ma) are older than K/Ar cooling ages on magmatic biotite (33.1–33.4 Ma) of the Velardeña Intrusive Complex (Felder 1979). This implies that either the causative intrusion for these skarn deposits is linked to older magmatic events or K/Ar cooling ages were reset by younger magmatic or hydrothermal pulses at ~ 33 Ma. Rather, our adularia dates are similar to a 38 ± 1.2 Ma date in the Concepción del Oro skarn deposit (K/Ar in adularia; Buseck 1966), a date at 36.1 Ma (unknown method) for skarn deposits at Mapimí, and U–Pb zircon dates of 36.2–37.1 Ma for intrusions at Mazapil-Peñasquito (see references in Camprubí 2013). This aligns with other Eocene U–Pb zircon dates from intrusions along the Sector Transversal de Parras (Sosa-Valdés 2011), such as Mineral de Providencia (43.5 ± 5.6 Ma), Concepción del Oro (42.6 ± 0.5 Ma), Noche Buena (35.1 ± 0.6 Ma), and Santa Rosa (32.1 ± 0.6 Ma).
According to Industrias Peñoles (2010), the Santa María and Antares deposits are separated by a NW-striking sub-vertical normal fault/calcite vein that displaced Santa María downward with respect to Antares (Fig. 2). Pinet and Tremblay (2009) suggested that post-mineralization NW-striking faults in the Santa María and San Lorenzo ranges are associated with the Basin and Range Province and calculated apparent vertical displacements exceeding 300 m. Therefore, for the evolutionary model presented in Fig. 13, we restored Santa María to its likely original position based on the cross-section presented in Fig. 2 and considering an almost pure vertical displacement of ~ 300 m as in Pinet and Tremblay (2009).
Schematic genetic model for the Santa María and Antares skarn deposits at 37.5 Ma (late Eocene), with position of fault blocks restored to their position before the post-mineralization displacement of the fault/calcite vein. Quartz and calcite are ubiquitous in retrograde assemblages. Key: Act = actinolite, Adl = adularia, Apy = arsenopyrite, Bt = biotite, Cal = calcite, Ccp = chalcopyrite, Chl = chlorite, Di = diopside, Ep = epidote, Ttr = tetrahedrite-group minerals, Flr = fluorite, Gn = galena, Grt = garnet, Kfs = K-feldspar, Mrc = marcasite, Ms = muscovite, Po = pyrrhotite, Prh = prehnite, Py = pyrite, Ser = sericite, Sp = sphalerite, Tr = tremolite, Wol = wollastonite
Along lithological contacts, deeply-sourced magmatic hydrothermal fluids upwelled and reacted with limestones and felsic rocks (Fig. 13). According to primary fluid inclusions in garnet, metasomatism was produced by single-phase fluids with moderate salinities (~ 15 wt% NaCl equiv.; exoskarns and endoskarns in Fig. 9b), which generated high-temperature calc-silicate assemblages of garnet (grossular > andradite), wollastonite, and diopside in exoskarns and endoskarns (i.e., prograde skarn), and biotite ± K-feldspar alteration in igneous rocks (Figs. 5a and 6a–b, d). These mineral associations are diagnostic of temperatures > 500 °C (Sillitoe 2010; Bucher and Grapes 2011; Kouzmanov and Pokrovski 2012), which agree well with fluid inclusions in garnet > 600 °C. In addition, as garnet is more abundant than pyroxene (especially at Santa María), the prograde stage was likely produced by metasomatic fluids that exsolved from deep-seated oxidized igneous bodies (Meinert et al. 2005). The association of magmatism with both skarn systems is further underlined by O and C isotopic signatures, which are closer to the magmatic endmember in Antares than in Santa María (Fig. 10).
The retrograde stage at Santa María and Antares was produced by successive fluid pulses that crystallized tremolite, actinolite, epidote, chlorite, muscovite, scapolite, pseudo-rhombohedral adularia (restricted to Santa María), quartz, calcite, fluorite, and massive sulfide ore (Figs. 5 and 6). The abundance of tremolite-actinolite and crystalline epidote + muscovite suggests minimum temperatures of 300 °C for retrograde fluids (Reyes 1990; Corbett and Leach 1998), which are consistent with fluid inclusion data in fluorite (327–450 °C), sphalerite (349–435 °C), calcite (370–433 °C), and quartz (350–415 °C) (Fig. 9).
The ore stage at Santa María and Antares is coeval with the retrograde stage (Fig. 4), as is usually found in skarn deposits worldwide (Burt 1977; Meinert et al. 2005; Kouzmanov and Pokrovski 2012). At Santa María, the ore is dominated by pyrite and sphalerite (both Fe-rich and Fe-poor), whereas at Antares it is dominated by pyrrhotite and Fe-rich sphalerite (Figs. 5d–i and 6e, g–j). Ores in both deposits contain arsenopyrite, pyrrhotite, galena, chalcopyrite, molybdenite, Pb-Bi-Sb sulfosalts, and magnetite (Fig. 7b–c). The dominance of pyrrhotite + Fe-rich sphalerite + arsenopyrite assemblages at Antares suggests a low-sulfidation state, whereas pyrite + Fe-poor sphalerite + arsenopyrite assemblages at Santa María agree with an intermediate-sulfidation state (Einaudi et al. 2003). However, ore-stage fluids from both deposits likely cooled close to the limit between intermediate and low-sulfidation states—defined by the pyrite–pyrrhotite reaction (Einaudi et al. 2003)—as suggested by (1) pyrite and pyrrhotite invariably occurring in ore assemblages from both skarn deposits, and (2) similar sulfur fugacity and temperature estimations on arsenopyrite from Santa María (–11.0 to –4.5 log fS2, ~ 310–520 °C) and Antares (–12.4 to –4.4 log fS2, 300–500 °C; Table 1). The differences in the sulfidation state in both deposits were probably caused by variations in the S availability of ore-bearing fluids rather than in temperature, as microthermometry of ore-stage fluid inclusions (~ 350–450 °C) and arsenopyrite geothermometry data (~ 300–500 °C) from both deposits yielded similar results (Fig. 9; Table 1). Noteworthy, these temperatures for the ore stage are atypically high compared to what has been found in many Zn-Pb skarns worldwide (< 400 °C; e.g., Shu et al. 2017, 2021), although other relatively “high”-temperature deposits have been documented (e.g., El Mochito, Honduras; Samson et al. 2008).
The post-ore event at Santa María is recorded by calcite veins that crosscut earlier assemblages and locally carry arsenopyrite, pyrite, and late tetrahedrite-group minerals (tennantite Fe-tetrahedrite Zn; Figs. 3a and 5j). Arsenopyrite coprecipitation with pyrite at 345–415 °C and –8.7 to –6.4 log fS2 suggests an intermediate-sulfidation state, which was approximately constant as the fluids cooled as indicated by the occurrence of late tetrahedrite-group minerals that crystallized at < 170 °C (group 1 in Fig. 8; Einaudi et al. 2003; Camprubí and Albinson 2007). Fluid inclusion data from post-ore calcite at 375 ± 51 °C (1σ; n = 104; Fig. 9a) and fluid compositions in the system H2O-CaCl2-NaCl-CH4 are similar to those of the ore-stage fluid inclusions (350–450 °C). Therefore, post-ore calcite veins probably record a late hydrothermal pulse associated with the retrograde stage.
During the late stage at Santa María and Antares, bird’s-eye-textured pyrite + marcasite and tetrahedrite-group minerals (tetrahedrite Fe-tetrahedrite Zn-freibergite) replaced ore-stage sulfides, coeval with the formation of sericite + epidote + chlorite assemblages (Figs. 5g–h, 6j and 8). Pyrite + tetrahedrite-group minerals + marcasite assemblages, tetrahedrite geothermometry (< 300 °C for group 2; Fig. 8), and secondary FIAs in ore-stage fluorite and quartz (< 250 °C; Fig. 9) are altogether consistent with fluids at low temperature and an intermediate-sulfidation state (Einaudi et al. 2003). The existence of poorly crystalline epidote marks temperatures in the range of 200–240 °C (type 1, as of Reyes 1990), thus providing further evidence for a low-temperature environment as the system waned. Moreover, this stage may correspond to the epithermal event ~ 200 °C reported by Jiménez-Franco et al. (2020) in nearby ore bodies.
Nature of ore-stage fluids
The occurrence of Ca-silicates (tremolite-actinolite and epidote) and ubiquitous calcite in the ore stages at Santa María and Antares indicate dominant neutral-to-alkaline pH conditions for the fluids, which may have varied locally to slightly acidic given the occurrence of sericite-rich alterations (Henley and Ellis 1983; Corbett and Leach 1998). Moreover, the virtual absence of hypogene oxides, the presence of chlorite + pyrrhotite + pyrite associations, and the occurrence of methane in fluid inclusions, particularly at Santa María (Fig. S6), are suggestive of reduced hydrothermal fluids (Einaudi et al. 2003; Simmons et al. 2005; Zhang et al. 2019), which is in good agreement with the inferred low to intermediate-sulfidation states.
There are striking differences between the composition of ore-stage fluids at Antares and Santa María (Table 2). At Antares, ore-stage fluids consist of H2O, NaCl, KCl, and lesser bivalent cation chlorides (e.g., FeCl2 or MgCl2) or methane, and display salinities up to > 50 wt% NaCl equiv. (Fig. 9b). In addition, triphase (liquid + vapor + daughter crystal) and multi-solid fluid inclusions are common (Fig. S5f–i). These characteristics are similar to the “hypersaline liquid inclusions” interpreted by Kouzmanov and Pokrovski (2012) as frequently associated with metalliferous porphyries and proximal skarns. These fluids can thus represent aliquots of a boiled-off magmatic hypersaline fluid exsolved from a cooling body at depth. As a consequence, we infer that the input of magmatic fluids did not cease after the prograde stage and was prevalent during the ore stage, as indicated by δ18Ofluid of 10‰ to 15‰ in retrograde calcite from exoskarns, endoskarns, and massive sulfides (Fig. 10). The input of magmatic components is also evidenced by sulfides with δ34SVCDT from –2.8‰ to 1.7‰ (Fig. 11), which match the typical range of magmatic sulfur (–3‰ to 3‰; Ohmoto 1972; Shimazaki and Sakai 1984; Chaussidon and Lorand 1990). Further, the narrow variation of δ34SVCDT in sulfides indicates that the sulfur sources in both areas remained unchanged during the ore stage, whose physicochemical conditions were approximately constant, and the precipitation of the ore did not cause significant changes in the total isotope composition of the solution (Shimazaki and Yamamoto 1979).
At Santa María, ore-stage fluids recorded by ore and post-ore FIAs can be ascribed to the H2O-CaCl2-NaCl system and are dominantly calcic brines with salinities ranging between 17 and 30 wt% CaCl2 (Table 1; Fig. 9a). Fluid inclusions contain no daughter crystals, except for assemblages that were formed due to boiling (Fig. S3i). These features indicate a single-phase intermediate-salinity fluid, which is normally found in base-metal distal skarns (Kouzmanov and Pokrovski 2012). The magmatic affinity of ore-bearing fluids is supported by (a) the spatial link between skarn ore bodies at Santa María and igneous rocks (i.e., Santa María dike; Fig. 2), (b) the moderate-to-high salinities and temperatures of ore-stage inclusion fluids, and (c) the consistency between ages of ore-stage adularia and those reported in the adjoining magmatic suite. In this way, δ18Ofluid of 14‰ to 23‰ suggests that magmatic-derived fluids probably lost their original isotopic traits (e.g., δ18O between 6‰ and 10‰; Zheng and Hoefs 1993; Campbell and Larson 1998) by equilibrating with the carbonate sequence (Fig. 10). Moreover, δ13Cfluid between –4‰ and 2‰ indicate that C was mostly provided by marine carbonates and, to some extent, by interlayered pelitic rocks (Fig. 10; Eichman and Schindlowski 1975). The occurrence of thermogenic methane (see next section; Fig. S6) and the dominance of CaCl2 in ore-stage fluids support this interpretation, as these components are probably derived from sedimentary wall rocks. The conspicuous presence of calcic brines and thermogenic methane at Santa María, along with a stronger isotope affinity toward sedimentary sources than at Antares (Fig. 10), indicate that local carbonate rocks were sources for chemical species in the ore-bearing fluids. If the original magmatic fluids bore similar compositions to those at Antares, calcic waters were likely formed by interaction (i.e., cationic exchange reactions or dissolution) between the local limestones and NaCl ± KCl bearing fluids, as documented in skarn deposits elsewhere, such as El Mochito in Honduras (Samson et al. 2008), Baiyinnuo’er and Haobugao in China (Shu et al. 2017, 2021), and Cantung in Canada (Legros et al. 2020). This probably resulted from different water-to-rock ratios, which would be reasonably expected from ore fluids associated with the smallest intrusions (i.e., the one at Santa María) versus ore fluids associated with the largest hypabyssal bodies (i.e., Antares, La Industria, La Esperanza, San Lorenzo range; see Fig. 2 in Jiménez-Franco et al. 2020).
Origin of methane and incorporation into ore-stage fluids
Methane production and incorporation in hydrothermal fluids are related to abiotic and biotic mechanisms (Welhan 1988; Salvi and Williams-Jones 1997; Potter and Konnerup-Madsen 2003; Etiope and Sherwood-Lollar 2013). Abiotic mechanisms are (1) Fischer–Tropsch reactions, (2) inorganic synthesis of compounds with CO2 and H2 (or C-H species) derived from external sources (e.g., carbonate rocks), and (3) direct magmatic degassing. Fischer–Tropsch synthesis occurs very slowly unless catalyzed by group-VIII metals (e.g., Co, Ru, or Fe; Van Der Laan and Beenackers 1999) and requires higher temperatures than those found in the ore stage in order to take place efficiently (Ren et al. 2020). Also, both Fischer–Tropsch and inorganic syntheses generate complex hydrocarbons (Zhang et al. 2019). The absence of -CH2 features in infrared spectrograms (Fig. S6) denotes a lack of heavier hydrocarbons. Thus, Fischer–Tropsch and inorganic syntheses are unlikely mechanisms in this case. Direct exsolution of magmatic methane is also unlikely because temperatures of ~ 400 °C of ore-stage fluids are far too low to represent unmodified magmatic fluids. Also, methane is more common in reduced ilmenite-series igneous rocks (e.g., Zhang et al. 2019) rather than oxidized intrusions such as the Velardeña Intrusive Complex.
Biotic processes are (1) biological production by bacterial activity, and (2) thermogenesis of carbonaceous rocks and sediments. Bacterial production of methane occurs at low temperatures and hence can be discarded due to the temperatures of ore-stage fluids. Consequently, intermediate- to high-temperature thermogenesis of organic-matter-bearing horizons (e.g., carbonaceous mudstones) interlayered with carbonate rocks would be the most likely mechanism to produce methane. This process might have happened during (1) the broadening of marble fronts (i.e., contact metamorphism), and/or (2) the subsequent hydrothermal fluid circulation across the sedimentary sequence (Fig. 13). In addition, the regional Jurassic and Cretaceous basin sequences across northern and northeastern Mexico host numerous epigenetic mineral deposits (MVT-like and red-bed hosted). In those deposits, hydrocarbons are frequently found in fluid inclusions (González-Sánchez et al. 2007, 2009; Camprubí 2013, 2019), thus providing evidence for a feasible source for hydrocarbons, their circulation across the basins, and the chance that thermal events induced their breakdown.
Evolution of mineralizing fluids and mechanisms of ore precipitation
Our data from fluid inclusions at Antares suggest evolutionary trends similar to trends determined in the Santa María and Reyna de Cobre skarn-to-epithermal deposits by Gilmer et al. (1988) and Jiménez-Franco et al. (2020) (Fig. 9b), whereas our data from fluid inclusions at Santa María behave otherwise (Fig. 9a). The Antares-like fluid-inclusion data indicate the following:
-
(1)
Deep boiling below the brittle–ductile transition. Deep boiling trends are deduced between prograde and ore-stage FIAs, based on increments in salinity from 15 to ~ 40–60 wt% NaCl equiv. as temperature declined from > 600° to 350–500 °C (path 1 in Fig. 9b). This process occurred at > 600 °C, well below (deeper) the brittle–ductile transition, which is normally acknowledged at rock temperatures of ~ 400 °C (Fournier 1999). The boiling fluids must have been magmatic, since deeply circulated meteoric fluids could not cross the brittle–ductile transition; this is supported by the magmatic isotopic compositions of O in both prograde and retrograde associations at Antares (Fig. 10). Given that ore-stage fluorite and calcite—minerals hosting ore-stage FIAs—coprecipitated with the ore (Fig. 4), we conclude that boiled-off hypersaline brines found at Antares probably promoted the earliest stages of ore precipitation, in agreement with observations by Gilmer et al. (1988) in nearby skarn deposits. In retrograde/ore-stage calcite, δ18Ofluid between 13‰ and 17‰ (Fig. 10) suggests that these brines equilibrated and neutralized with the host carbonate rocks, and this process possibly enhanced metal deposition.
-
(2)
Dilution of boiled-off brines. Dilution occurred between the ore stage and late stage and is supported by declines in salinity and temperature between stages (path 2 in Fig. 9b). Salinity fell from ~ 40–50 wt% NaCl equiv. in the ore sage to ~ 20 wt% NaCl equiv. in the late stage. Temperature fell from 350–500 °C in the ore stage to < 250 °C in the late stage. This dilution would have generated < 20 wt% NaCl equiv. fluids after deep boiling irrespective of their starting salinity and would be the result of the interaction of deep upwelling brines with deeply circulated cooler fluids of meteoric origin before their thermal equilibration with country rocks.
-
(3)
Conductive cooling of pre-boiled fluids. Alternatively, conductive cooling of pre-boiled fluids was another plausible evolutionary trend, provided that inclusion fluids in garnet at ~ 15 wt. % NaCl equiv. and ~ 600 °C probably cooled down into late-stage fluids at 15–20 wt% NaCl equiv. and < 300 °C (path 3 in Fig. 9b). This conductive cooling pathway, which would not require boiling or dilution along the way, may also explain the collective distribution of data of prograde-stage fluid inclusions from previous studies in Fig. 9b.
-
(4)
Conductive cooling of late fluids. Finally, an isosalinity trend around 17 wt% NaCl equiv. in late-stage FIAs (path 3 in Fig. 9) indicates that conductive cooling of late-stage fluids marks the end of hydrothermal activity in this deposit.
Data from fluid inclusions at Santa María in this study suggest evolutionary trends different from Antares (Fig. 9). The Santa María-like fluid inclusion data is characterized as due to (1) conductive cooling and, probably, (2) isothermal mixing. Scarce data on prograde-stage FIAs from Santa María preclude putting forth deep boiling as an early process in fluid evolution. However, temperatures above 600 °C in primary fluid inclusions hosted by garnet at Santa María indicate a clear magmatic affinity for prograde fluids (pre-boiled fluids?).
-
(1)
Conductive cooling of ore- and late-stage fluids. Conductive cooling is indicated by fluid inclusions in ore-stage fluorite, calcite, quartz, and sphalerite, which roughly define a temperature decrease trend at 20–30 wt% CaCl2 from 450 to 320 °C (path 1 in Fig. 9a). In retrograde/ore-stage calcite, δ18O (Fig. 10) up to 20‰ suggests that ore-bearing fluids equilibrated and/or neutralized with the host carbonate rocks, and this process was more conspicuous than at Antares. Therefore, the paragenetic position of fluorite, calcite, quartz, and sphalerite with respect to ore minerals (Fig. 4) indicates that conductive cooling and neutralization were the likeliest mechanisms for ore deposition at Santa María. Further, late fluids with < 250 °C and 12–25 wt% CaCl2 were probably the result of uninterrupted conductive cooling of ore-stage fluids (Fig. 9a).
-
(2)
Isothermal mixing of ore-stage fluids. The focusing of ore-stage fluid inclusion data around 400 ± 50 °C is suggestive of isothermal mixing (path 2 in Fig. 9a) between upwelling deep magmatic brines and thermally equilibrated meteoric fluids that circulated near the brittle–ductile threshold (i.e., deeply circulated meteoric fluids; e.g., Fuentes-Guzmán et al. 2023). Despite being a reasonable possibility, the range of salinities between ~ 20 and 30 wt% CaCl2 is not broad enough to confidently put forth isothermal mixing as a plausible interaction between these differently sourced fluids, although some isothermal mixing cannot be ruled out.
At Santa María, boiling occurred near the brittle–ductile transition (~ 450 °C) in ore and post-ore stages, and can be described as relatively shallow boiling. In the ore stage, this process is characterized by the occurrence of pseudo-rhombohedral adularia and by the coexistence of liquid- and vapor-rich inclusions within the same FIAs (Figs. 5f and S3e; Steiner 1970; Simmons et al. 2005; Camprubí and Albinson 2006, 2007; Libbey and Williams-Jones 2016). However, adularia and boiling-suggestive FIAs rarely coexist in the same sample, thus suggesting that adularia crystallization probably resulted from boiling and other processes. According to Hofstra and Cline (2000, and references therein), K-metasomatism by fluids at near neutral pH and high aK+/aH+ can account for adularia crystallization in the absence of boiling. If this were the case at Santa María, K-metasomatism would have been favored by near-neutral pH conditions of ore-stage fluids and the high availability of K in the aplite enhanced by sericite- and biotite-rich alterations. Nonetheless, with the available evidence, we cannot assert to what degree each process contributed to adularia crystallization. In any case, since pseudo-rhombohedral adularia and sulfides are intergrown (Fig. 5f), it is likely that boiling produced ore precipitation. Moreover, in the post-ore stage, evidence of boiling is provided by fluid inclusions with contrasting liquid/vapor ratios in the same FIA (Fig. S3i) and by the occurrence of breccias with angular clasts and moderate cement abundance, which probably resulted from hydraulic brecciation and hence are indicative of sudden pressure release (Hedenquist et al. 2000).
Conclusions
-
The Santa María and Antares ore deposits in the Velardeña Mining District display most of the diagnostic features of Zn-Pb(-Ag) skarn deposits worldwide and are spatially linked to aplite dikes and rhyolitic stocks located 470 m apart. These intrusions and concomitant skarn mineralization (adularia 40Ar/39Ar dates at ca. 37.5 Ma) are coeval with the initial silicic magmatism of the Sierra Madre Occidental that took place in the Eocene–early Oligocene, during which other polymetallic skarn deposits occurred along the Sector Transversal de Parras in the northern Mexican Orogen.
-
The Santa María and Antares deposits were formed in three major stages: prograde, retrograde/ore, and late. In addition, Santa María registers a post-ore stage. The prograde stage records the passage of dominantly magmatic fluids that generated high-temperature (> 600 °C) Ca-silicates, such as garnet (grossular-andradite), wollastonite, and diopside, as well as biotite ± K-feldspar alterations. Later, cooler ore-rich fluids (~ 300–500 °C) produced the retrograde/ore stage, in which tremolite-actinolite, chlorite, epidote, muscovite, scapolite, fluorite, pseudo-rhombohedral adularia, quartz, and calcite crystallized in association with the massive ore consisting of sphalerite (Fe-rich and Fe-poor), pyrrhotite, pyrite, arsenopyrite, chalcopyrite, molybdenite, tetrahedrite-group minerals, and Pb-Bi-Sb sulfosalts. The post-ore stage is represented by poorly mineralized calcite veins that are probably linked to the retrograde stage. Finally, late assemblages of pyrite + marcasite + tetrahedrite-group minerals + epidote + chlorite were produced by low-temperature (< 250 °C) fluids.
-
The ore assemblages, fluid inclusion data, and arsenopyrite composition reveal that fluids had an intermediate-sulfidation state during the ore-stage at Santa María and a low-sulfidation state at Antares.
-
Carbon and oxygen isotope data indicate a modified magmatic signature of fluids from Antares, contrasting with data from Santa María that are greatly influenced by the host limestones. These data may have resulted from higher water-to-rock ratios at Antares compared to Santa María, as expected from association with the main intrusion (Antares) versus a satellite dike (Santa María). At Santa María, lower water-to-rock ratios allowed efficient interaction with the limestones, ultimately increasing the proportion of CaCl2 and thermogenic methane in the fluids. In both deposits, sulfur isotope data highlight the input of magmatic S.
-
The evolution of the fluids in each skarn deposit followed different paths. At Santa María, ore-bearing fluids cooled and neutralized due to interactions with wall rocks and probably underwent isothermal mixing with deeply circulated meteoric fluids at ~ 400 °C. These processes triggered massive ore precipitation. At Antares, magmatic-derived fluids underwent deep boiling that generated hypersaline boiled-off brines from which metals precipitated due to neutralization with carbonate rocks. Later, boiled-off fluids experienced dilution with meteoric waters, until further cooling generated the late-stage assemblages.
References
Ángeles-Villeda ME, Hinojosa-Espinosa JJ, López-Oliva JG, Valdés-González A, Livas-Vera M (2005) Estratigrafía y microfacies de la parte sur del Cañón La Boca, Santiago, Nuevo León, México. Rev Mex Cienc Geol 22:272–281
Baker T, Van Achterberg E, Ryan CG, Lang JR (2004) Composition and evolution of ore fluids in a magmatic-hydrothermal skarn deposit. Geology 32:117–120. https://doi.org/10.1130/G19950.1
Bakker RJ (1999) Optimal interpretation of microthermometrical data from fluid inclusions: Thermodynamic modelling and computer programming. Habilitation Thesis. Ruprecht-Karls-Universität Heidelberg
Bakker RJ (2003) Package FLUIDS 1. Computer programs for analysis of fluid inclusion data and for modelling bulk fluid properties. Chem Geol 194:3–23. https://doi.org/10.1016/S0009-2541(02)00268-1
Bottinga Y (1968) Calculation of fractionation factors for carbon and oxygen isotopic exchange in the system calcite-carbon dioxide-water. J Phys Chem 72:800–808
Bryan S (2007) Silicic large igneous provinces. Episodes 30:20–31. https://doi.org/10.18814/epiiugs/2007/v30i1/004
Bucher K, Grapes R (2011) Petrogenesis of metamorphic rocks. Springer, Berlin Heidelberg
Burt DM (1977) Mineralogy and petrology of skarn deposits. Società Italiana Di Mineralogia e Petrologia 33:859–873
Buseck PR (1966) Contact metasomatism and ore deposition: Concepcion del Oro, Mexico. Econ Geol 61:97–137. https://doi.org/10.2113/gsecongeo.61.1.97
Buzatu A, Damian G, Dill HG, Buzgar N, Apopei AI (2015) Mineralogy and geochemistry of sulfosalts from Baia Sprie ore deposit (Romania) - New bismuth minerals occurrence. Ore Geol Rev 65:132–147. https://doi.org/10.1016/j.oregeorev.2014.09.016
Campbell A, Larson P (1998) Introduction to stable isotope applications in hydrothermal systems. Reviews in Econ Geol 10:173–193. https://doi.org/10.5382/Rev.10.08
Camprubí A (2009) Major metallogenic provinces and epochs of Mexico. SGA News 25:6–50
Camprubí A (2013) Tectonic and metallogenic history of Mexico. Soc Eco Geo Spc Pub 17:201–243. https://doi.org/10.5382/SP.17.06
Camprubí A, Albinson T (2006) Depósitos epitermales en México: Actualización de su conocimiento y reclasificación empírica. B Soc Geol Mex 58:27–81. https://doi.org/10.18268/bsgm2006v58n1a2
Camprubí A, Albinson T (2007) Epithermal deposits in México - Update of current knowledge and an empirical reclassification. Geol S Am S 422:377–415. https://doi.org/10.1130/2007.2422(14)
Camprubí A, González-Partida E, Richard A, Boiron MC, González-Ruiz LE, Aguilar-Ramírez CF, Fuentes-Guzmán E, González-Ruiz D, Legouix C (2019) MVT-like fluorite deposits and Oligocene magmatic-hydrothermal fluorite–Be–Mo–P–V–U overprints in northern Coahuila, Mexico. Minerals 9:1–27. https://doi.org/10.3390/min9010058
Chang Z, Shu Q, Meinert LD (2019) Skarn Deposits of China. Soc Eco Geo Spc Pub 22:189–234
Chaussidon M, Lorand JP (1990) Sulphur isotope composition of orogenic spinel lherzolite massifs from Ariege (North-Eastern Pyrenees, France): An ion microprobe study. Geochim Cosmochim Ac 54:2835–2846. https://doi.org/10.1016/0016-7037(90)90018-G
Chi GX, Ni P (2007) Equations for calculation of NaCl/(NaCl + CaCl2) ratios and salinities from hydrohalite-melting and ice-melting temperatures in the H2O-NaCl-CaCl2 system. Acta Petrol Sin 23:33–37
Chu H, Chi G, Chou IM (2016) Freezing and melting behaviors of H2O-NaCl-CaCl2 solutions in fused silica capillaries and glass-sandwiched films: implications for fluid inclusion studies. Geofluids 16:518–532. https://doi.org/10.1111/gfl.12173
Coplen TB (1988) Normalization of oxygen and hydrogen isotope data. Chem Geol 72:293–297. https://doi.org/10.1016/0168-9622(88)90042-5
Coplen TB, Brand WA, Gehre M, Gröning M, Meijer HAJ, Toman B, Verkouteren RM (2006) New guidelines for δ13C measurements. Anal Chem 78:2439–2441. https://doi.org/10.1021/ac052027c
Corbett GJ, Leach TM (1998) Southwest Pacific rim gold–copper systems: structure, alteration and mineralization. Soc Eco Geo Spc Pub 6:1–214. https://doi.org/10.5382/SP.06
Cruz-Pérez R, López-Escalona J (1981) Estudio minero de las sierras de Santa María y San Lorenzo, municipio de Cuencamé, estado de Durango. Servicio Geológico Mexicano historical archive
Dunham RJ (1962) Classification of carbonate rocks according to depositional texture. In: Ham WE (ed) Classification of carbonate rocks. AAPG, Tulsa, pp 108–121
Eguiluz y de Antuñano S, Aranda-García M, Marrett R (2000) Tectónica de la Sierra Madre Oriental, México. B Soc Geol Mex 53:1–26. https://doi.org/10.18268/BSGM2000v53n1a1
Eguiluz y de Antuñano S, Aranda-Gómez JJ, Juárez-Arriaga E (2022) Estratigrafía y ambientes de depósito de la Formación Ahuichila en el Sector Transversal de Parras, Sierra Madre Oriental. B Soc Geol Mex 74:A111121. https://doi.org/10.18268/BSGM2022v74n1a111121
Eichman R, Schindlowski M (1975) Isotopic fractionation between coexisting organic carbon-carbonate pairs in Precambrian sediments. Geochim Cosmochim Ac 39:585–595. https://doi.org/10.1016/0016-7037(75)90004-6
Einaudi MT, Burt DM (1982) Introduction-terminology, classification and composition of skarn deposits. Econ Geol 77:745–754. https://doi.org/10.2113/gsecongeo.77.4.745
Einaudi MT, Hedenquist JW, Inan EE (2003) Sulfidation state of fluids in active and extinct hydrothermal systems: transitions from porphyry to epithermal environments. Soc Eco Geo Spc Pub 10:1–51. https://doi.org/10.5382/SP.10.15
Etiope G, Sherwood-Lollar B (2013) Abiotic methane on earth. Rev Geophys 51:276–299. https://doi.org/10.1002/rog.20011
Felder, F., 1979, Estudio de algunas estructuras mineralizadas en el domo de Santa María, Velardeña, Durango. Convención Nacional de la Asociación de Ingenieros de Minas, Metalurgistas y Geólogos de México, XIII, Acapulco, Mexico, 1979, Extended Abstracts, 192–224.
Ferrari L, Valencia-Moreno M, Bryan S (2005) Magmatismo y tectónica en la Sierra Madre Occidental y su relación con la evolución de la margen occidental de Norteamérica. B Soc Geol Mex 57:343–378. https://doi.org/10.18268/bsgm2005v57n3a5
Ferrari L, Valencia-Moreno M, Bryan S (2007) Magmatism and tectonics of the Sierra Madre Occidental and relation with the evolution of the western margin of North America. Geol S Am S 422:1–39. https://doi.org/10.1130/2007.2422(01)
Ferrari L, Orozco-Esquivel T, Bryan SE, López-Martínez M, Silva-Fragoso A (2018) Cenozoic magmatism and extension in western Mexico: Linking the Sierra Madre Occidental silicic large igneous province and the Comondú Group with the Gulf of California rift. Earth-Sci Rev 183:115–152. https://doi.org/10.1016/j.earscirev.2017.04.006
Fitz-Díaz E, Lawton TF, Juárez-Arriaga E, Chávez-Cabello G (2018) The Cretaceous-Paleogene Mexican orogen: Structure, basin development, magmatism and tectonics. Earth-Sci Rev 183:56–84. https://doi.org/10.1016/j.earscirev.2017.03.002
Fournier RO (1999) Hydrothermal processes associated to movement of fluid from plastic into brittle rock in the magmatic-epithermal environment. Econ Geol 94:1193–1211. https://doi.org/10.2113/gsecongeo.94.8.1193
Fuentes-Guzmán E, Camprubí A, González-Partida E, Hernández-Avilés G, Alfonso P, Cienfuegos-Alvarado E, Mesino-Hernández JC, Ortega-Obregón C, Otero-Trujano FJ, Vázquez Ramírez JT (2023) The Tatatila-Las Minas IOCG skarn (Veracruz, Mexico): Mineralogical, fluid inclusion and stable isotope constraints. J S Am Earth Sci 122:104112. https://doi.org/10.1016/j.jsames.2022.104112
Gilmer AL, Clark KF, Conde CJ, Hernández CI, Figueroa SJI, Porter EW (1988) Sierra de Santa María, Velardeña Mining District, Durango, Mexico. Econ Geol 83:1802–1829. https://doi.org/10.2113/gsecongeo.83.8.1802
Goldstein RH, Reynolds TJ (1994) Systematics of fluid inclusions in diagenetic mineral. Soc Sediment Geol Short Course Series 31:1–199. https://doi.org/10.2110/scn.94.31
González-Partida E, Camprubí A, Carrillo-Chávez A, Díaz-Carreño EH, González-Ruiz LE, Farfán-Panamá JL, Cienfuegos-Alvarado E, Morales-Puente P, Vázquez-Ramírez JT (2019) Giant fluorite mineralization in central Mexico by means of exceptionally low salinity fluids: An unusual style among MVT deposits. Minerals 9:1–23. https://doi.org/10.3390/min9010035
González-Sánchez F, Puente-Solís R, González-Partida E, Camprubí A (2007) Estratigrafía del Noreste de México y su relación con los yacimientos estratoligados de fluorita, barita, celestina y Zn-Pb. B Soc Geol Mex 59:43–62. https://doi.org/10.18268/bsgm2007v59n1a4
González-Sánchez F, Camprubí A, González-Partida E, Puente-Solís R, Canet C, Centeno-García E, Atudorei V (2009) Regional stratigraphy and distribution of epigenetic stratabound celestine, fluorite, barite, and Zn-Pb deposits in the MVT province of Northeastern Mexico. Miner Deposita 44:343–361. https://doi.org/10.1007/s00126-008-0212-4
Gvozdev VI, Grebennikova AA, Vakh AS, Goryachev NA, Fedoseev DG (2020) Mineral evolution during formation of gold–rare-metal ores in the Sredne-Golgotay Deposit (Eastern Transbaikalia). Russ J Pac Geol 14:66–86. https://doi.org/10.1134/S1819714020010054
Hawthorne FC (2021) On the calculation of the relative amounts of endmember constituents for garnet. Can Mineral 59:169–176. https://doi.org/10.3749/canmin.2000074
Hedenquist JW, Arribas A, González-Urien E (2000) Exploration for epithermal gold deposits. Soc Econ Geol Rev 13:245–277. https://doi.org/10.5382/Rev.13.07
Henley RW, Ellis AJ (1983) Geothermal systems ancient and modern: a geochemical review. Earth-Sci Rev 19:1–50. https://doi.org/10.1016/0012-8252(83)90075-2
Henry CD, Aranda-Gomez JJ (2000) Plate interactions control middle-late Miocene, proto-Gulf and Basin and Range extension in the southern Basin and Range. Tectonophysics 318:1–26. https://doi.org/10.1016/S0040-1951(99)00304-2
Hernández I (1991) Economic geology of the Velardeña Mining District, Durango. In Salas GP (ed) The Geology of North America. Geological Society of America, Boulder, 269–278. https://doi.org/10.1130/DNAG-GNA-P3.269
Hoffmann E (2003) Le système de veines épithermales et de failles normales au sein de la propriété Velardeña, Mexique: analyse structurale et métallogénique. M.Sc. thesis, Canada, Université du Québec
Hofstra AH, Cline JS (2000) Characteristics and models for Carlin-type gold deposits. Soc Econ Geol Rev 13:163–220. https://doi.org/10.5382/Rev.13.05
Huston D, Trumbull B, Beaudoin G, Ireland T (2023) Light stable isotopes (H, B, C, O and S) in ore studies—methods, theory, applications and uncertainties. In: Huston D, Gutzmer J (eds) Isotopes in economic geology metallogenesis and exploration. Springer, Reston, pp 209–238
Imlay RW (1937) Geology of the middle part of the Sierra de Parras, Coahuila, Mexico. Geol Soc Am Bull 48:588–630. https://doi.org/10.1130/GSAB-48-587
Industrias Peñoles (2010) Proyecto Velardeña, estudio de prefactibilidad: Industrias Peñoles, internal report, 145
Ishihara S (1977) The magnetite-series and ilmenite-series granitic rocks. Mining Geol 27:293–305. https://doi.org/10.11456/shigenchishitsu1951.27.293
Jiménez-Franco A (2012) Estudio de la paragénesis, inclusiones fluidas e isotopía del skarn de Velardeña, Durango. M.Sc. thesis, Univerisdad Nacional Autónoma de México
Jiménez-Franco A, Canet C, Alfonso P, González-Partida E, Rajabi A, Escalante E (2020) The Velardeña Zn-(Pb-Cu) skarn-epithermal deposits, central-northern Mexico: New physical-chemical constraints on ore-forming processes. B Soc Geol Mex 72:1–26. https://doi.org/10.18268/bsgm2020v72n3a270719
Koppers AAP (2002) ArArCALC-software for 40Ar/39Ar age calculations. Comput Geosci 28:605–619. https://doi.org/10.1016/S0098-3004(01)00095-4
Koppers AAP, Staudigel H, Pringle MS, Wijbrans JR (2003) Short-lived and discontinuous intraplate volcanism in the South Pacific: Hot spots or extensional volcanism? Geochem, Geophy, Geosy 4(10):1087. https://doi.org/10.1029/2003GC000533
Kouzmanov K, Pokrovski GS (2012) Hydrothermal controls on metal distribution in porphyry Cu (-Mo-Au) systems. Soc Eco Geo Spc Pub 16:573–618. https://doi.org/10.5382/SP.16.22
Kretschmar U, Scott SD (1976) Phase relations involving arsenopyrite in the system Fe-As-S and their application. Can Mineral 14:364–386
Kuiper KF, Deino A, Hilgen FJ, Krijgsman W, Renne PR, Wijbrans JR (2008) Synchronizing rock clocks of earth history. Science 320:500–504. https://doi.org/10.1126/science.1154339
Legros H, Lecumberri-Sanchez P, Elongo V, Laurent O, Falck H, Adlakha E, Chelle-Michou C (2020) Fluid evolution of the Cantung tungsten skarn, Northwest Territories, Canada: Differentiation and fluid-rock interaction. Ore Geol Rev 127:1–11. https://doi.org/10.1016/j.oregeorev.2020.103866
Libbey RB, Williams-Jones AE (2016) Compositions of hydrothermal silicates and carbonates as indicators of physicochemical conditions in the Reykjanes geothermal system, Iceland. Geothermics 64:15–27. https://doi.org/10.1016/j.geothermics.2016.04.007
McCrea JM (1950) On the isotopic chemistry of carbonates and a paleotemperature scale. J Chem Phys 18:849–857. https://doi.org/10.1063/1.1747785
Megaw PKM, Ruiz J, Titley SR (1988) High-temperature, carbonate-hosted Ag-Pb-Zn (Cu) deposits of northern Mexico. Econ Geol 83:1856–1885. https://doi.org/10.2113/gsecongeo.83.8.1856
Meinert LD, Dipple GM, Nicolescu S (2005) World skarn deposits. Econ Geol 100th Anniversary volume:299–336. https://doi.org/10.5382/AV100.11
Min K, Mundil R, Renne PR, Ludwig KR (2000) A test for systematic errors in 40Ar/39Ar geochronology through comparison with U/Pb analysis of a 1.1-Ga rhyolite. Geochim Cosmochim Ac 64:73–98. https://doi.org/10.1016/S0016-7037(99)00204-5
National Institute of Standards and Technology (NIST), 2021, NIST Chemistry WebBook, SRD 69, Methane https://www.nist.gov/. Accessed 14 February 2023
Nieto-Samaniego ÁF, Alaniz-Álvarez SA, Camprubí A (2005) La Mesa Central de México: estratigrafía, estructura y evolución tectónica cenozoica. B Soc Geol Mex 57:285–318. https://doi.org/10.18268/bsgm2005v57n3a3
Nieto-Samaniego ÁF, Alaniz-Álvarez SA, Camprubí A (2007) Mesa Central of México: Stratigraphy, structure, and Cenozoic tectonic evolution. Geol S Am S 422:41–70. https://doi.org/10.1130/2007.2422(02)
O’Neil JR, Clayton RN, Mayeda TK (1969) Oxygen isotope fractionation in divalent metal carbonates. J Chem Phys 51:5547–5558. https://doi.org/10.1063/1.1671982
Ohmoto H (1972) Systematics of sulfur and carbon isotopes in hydrothermal ore deposits. Econ Geol 67:551–578. https://doi.org/10.2113/gsecongeo.67.5.551
Ortega-Gutiérrez F, Elías-Herrera M, Morán-Zenteno DJ, Solari L, Weber B, Luna-González L (2018) The pre-Mesozoic metamorphic basement of Mexico, 1.5 billion years of crustal evolution. Earth-Sci Rev 183:1–37. https://doi.org/10.1016/j.earscirev.2018.03.006
Ortega-Gutiérrez F, Mitre-Salazar LM, Roldán-Quintana J, Aranda-Gómez JJ, Morán-Zenteno D, Alaniz-Álvarez SA, Nieto-Samaniego AF (1992) Carta geológica de la República Mexicana, quinta edición, escala 1:2,000,000: Consejo de Recursos Minerales and Instituto de Geología
Pinet N, Tremblay A (2009) Structural analysis of the Velardeña Mining District, Mexico: A faulted Au-Ag-rich hydrothermal system. Can J Earth Sci 46:123–138. https://doi.org/10.1139/E09-007
Potter J, Konnerup-Madsen J (2003) A review of the occurrence and origin of abiogenic hydrocarbons in igneous rocks. Geol Soc Am Spc Pub 214:151–173. https://doi.org/10.1144/GSL.SP.2003.214.01.10
Ramírez-Peña CF (2014) Análisis del contexto tectónico del emplazamiento de intrusivos en el transpaís de la Sierra Madre Oriental. M.Sc. thesis, Universidad Autónoma de Nuevo León
Ren T, Zhong H, Xing-Chun Z (2020) Fluid inclusion and stable isotope (C, O and S) constraints on the genesis of the high-grade Langdu Cu skarn deposit in Yunnan. SW China Ore Geol Rev 118:103354. https://doi.org/10.1016/j.oregeorev.2020.103354
Révész KM, Keybl J, Landwehr JM (2001) Measurement of 13C and 18O isotopic ratios of CaCO3 using a Thermoquest Finnigan GasBench II Delta Plus XL continuous flow isotope ratio mass spectrometer with application to Devils Hole core DH-11 calcite. U.S. Geological Survey open-file report 01–257. https://doi.org/10.3133/ofr01257
Révész KM, Landwehr JM (2002) δ13C and δ18O isotopic composition of CaCO3 measured by continuous flow isotope ratio mass spectrometry: statistical evaluation and verification by application to Devils Hole core DH-11 calcite. Rapid Commun Mass Spectrom 16:2102–2114. https://doi.org/10.1002/rcm.833
Reyes AG (1990) Petrology of Philippine geothermal systems and the application of alteration mineralogy to their assessment. J Volcanol Geoth Res 43:279–309. https://doi.org/10.1016/0377-0273(90)90057-M
Roedder E (1984) Fluid inclusions. Mineralogical Society of America, Reviews in Mineralogy 12
Sack RO (2005) Internally consistent database for sulfides and sulfosalts in the system Ag2S-Cu2S-ZnS-FeS-Sb2S3-As2S3: Update. Geochim Cosmochim Ac 69:1157–1164. https://doi.org/10.1016/j.gca.2004.08.017
Sack RO, Fredericks R, Hardy LS, Ebel DS (2005) Origin of high-Ag fahlores from the Galena Mine, Wallace, Idaho, U.S.A. Am Mineral 90:1000–1007. https://doi.org/10.2138/am.2005.1651
Salvi S, Williams-Jones AE (1997) Fischer-Tropsch synthesis of hydrocarbons during sub-solidus alteration of the strange lake peralkaline granite, Quebec/Labrador, Canada. Geochim Cosmochim Ac 61:83–99. https://doi.org/10.1016/S0016-7037(96)00313-4
Samson IM, Williams-Jones AE, Ault KM, Gagnon JE, Fryer BJ (2008) Source of fluids forming distal Zn-Pb-Ag skarns: Evidence from laser ablation-inductively coupled plasma-mass spectrometry analysis of fluid inclusions from El Mochito, Honduras. Geology 36:947–950. https://doi.org/10.1130/G25214A.1
SGM, Servicio Geológico Mexicano (1997) Carta geológica-minera Velardeña G13-D44, scale 1:50,000
SGM, Servicio Geológico Mexicano (2013) Geological mining monograph of the state of Durango
Sharp ZD, Essene EJ, Kelly WC (1985) A re-examination of the arsenopyrite geothermometer: pressure considerations and applications to natural assemblages. Can Mineral 23:517–534
Shimazaki H, Sakai H (1984) Regional variation of sulfur isotopic composition of skarn deposits in the westernmost part of the Inner Zone of southwest Japan. Mining Geology 34:419–424. https://doi.org/10.11456/shigenchishitsu1951.34.419
Shimazaki H, Yamamoto M (1979) Sulfur isotope ratios of some Japanese skarn deposits. Geochem J 13:261–268. https://doi.org/10.2343/geochemj.13.261
Shu Q, Chang Z, Hammerli J, Lai Y, Huizenga JM (2017) Composition and evolution of fluids forming the Baiyinnuo’er Zn-Pb Skarn deposit, northeastern China: Insights from laser ablation ICP-MS study of fluid inclusions. Econ Geol 112:1441–1460. https://doi.org/10.5382/econgeo.2017.4516
Shu Q, Chang Z, Mavrogenes J (2021) Fluid compositions reveal fluid nature, metal deposition mechanisms, and mineralization potential: An example at the Haobugao Zn-Pb skarn, China. Geology 49:473–477. https://doi.org/10.1130/G48348.1
Sillitoe RH (2010) Porphyry Copper Systems. Econ Geol 105:3–41. https://doi.org/10.2113/gsecongeo.105.1.3
Simmons SF, White NC, John DA (2005) Geological characteristics of epithermal precious and base metal deposits. Econ Geol 100th Anniversary volume:485–522. https://doi.org/10.5382/AV100.16
Sosa-Valdés R (2011) Geocronología U-Pb del Cinturón de Intrusivos de Concepción del Oro. B.Sc. thesis, Universidad Autónoma de Nuevo León
Spurr JE, Garrey GH (1908) Ore deposits of the Velardeña District, Mexico. Econ Geol 3:688–725. https://doi.org/10.2113/gsecongeo.3.8.688
Staude JMG, Barton MD (2001) Jurassic to Holocene tectonics, magmatism, and metallogeny of northwestern Mexico. Geol Soc Am Bull 113:1357–1374. https://doi.org/10.1130/0016-7606(2001)113%3c1357:JTHTMA%3e2.0.CO;2
Steele-MacInnis M, Lecumberri-Sanchez P, Bodnar RJ (2012) HokieFlincs_H2O-NaCl: A Microsoft Excel spreadsheet for interpreting microthermometric data from fluid inclusions based on the PVTX properties of H2O-NaCl. Comput Geosci 49:334–337. https://doi.org/10.1016/j.cageo.2012.01.022
Steele-MacInnis M, Ridley J, Lecumberri-Sanchez P, Schlegel TU, Heinrich CA (2016) Application of low-temperature microthermometric data for interpreting multicomponent fluid inclusion compositions. Earth-Sci Rev 159:14–35. https://doi.org/10.1016/j.earscirev.2016.04.011
Steiger RH, Jäger E (1977) Subcommission on geochronology: Convention on the use of decay constants in geo- and cosmochronology. Earth Planet Sci Lett 36(3):359–362. https://doi.org/10.1016/0012-821X(77)90060-7
Steiner A (1970) Genesis of hydrothermal K-feldspar (adularia) in an active geothermal environment at Wairakei, New Zealand. Mineral Mag 37:916–922. https://doi.org/10.1180/minmag.1970.037.292.07
Sterner SM, Hall DL, Bodnar RJ (1988) Synthetic fluid inclusions. V. Solubility relations in the system NaCl-KCl-H2O under vapor-saturated conditions. Geochim Cosmochim Ac 52:989–1005. https://doi.org/10.1016/0016-7037(88)90254-2
Taylor JR (1997) An introduction to error analysis: The study of uncertainties in physical measurements. California, University Science Books, Mill Valley
Van den Kerkhof AM, Hein UF (2001) Fluid inclusion petrography. Lithos 55:27–47. https://doi.org/10.1016/S0024-4937(00)00037-2
Van der Laan GP, Beenackers AACM (1999) Kinetics and selectivity of the Fischer-Tropsch synthesis: A literature review. Catal Rev Sci E 41:255–318. https://doi.org/10.1081/CR-100101170
Voudouris PC, Spry PG, Mavrogonatos C, Sakellaris GA, Bristol SK, Melfos V, Fornadel AP (2013) Bismuthinite derivatives, lillianite homologues, and bismuth sulfotellurides as indicators of gold mineralization in the Stanos shear-zone related deposit, Chalkidiki, Northern Greece. Can Mineral 51:119–142. https://doi.org/10.3749/canmin.51.1.119
Warr LN (2021) IMA–CNMNC approved mineral symbols. Mineral Mag 85:291–320. https://doi.org/10.1180/mgm.2021.43
Welhan JA (1988) Origins of methane in hydrothermal systems. Chem Geol 71:183–198. https://doi.org/10.1016/0009-2541(88)90114-3
Xu G (2000) Fluid inclusions with NaCl-CaCl2-H2O composition from the Cloncurry hydrothermal system, NW Queensland, Australia. Lithos 53:21–35. https://doi.org/10.1016/S0024-4937(00)00008-6
York D (1968) Least squares fitting of a straight line with correlated errors. Earth Planet Sci Lett 5:320–324. https://doi.org/10.1016/S0012-821X(68)80059-7
Zhang W, Williams-Jones AE, Leng CB, Zhang XC, Chen WT, Qin CJ, Su WC, Yan JH (2019) The origin of CH4-rich fluids in reduced porphyry–skarn Cu–Mo–Au systems. Ore Geol Rev 114:103135. https://doi.org/10.1016/j.oregeorev.2019.103135
Zheng YF, Hoefs J (1993) Carbon and oxygen isotopic covariations in hydrothermal calcites - Theoretical modeling on mixing processes and application to Pb-Zn deposits in the Harz Mountains, Germany. Miner Deposita 28:79–89. https://doi.org/10.1007/BF00196332
Acknowledgements
The Consejo Nacional de Humanidades, Ciencia y Teconología (CONAHCyT) supported N.C. with a grant during his M.Sc. studies, and this paper constitutes a substantial part of his associated research. This study was financially supported by means of personal allocations to UNAM academicians. Additional funding was provided by the CONACyT-SENER PT. 4.1 Gemex-EU research grant to E.G.P., and CONACyT-Ciencia Básica A1-S-14574 and PAPIIT IA-101419 research grants to Vanessa Colás Ginés (Departamento de Ciencias de la Tierra, University of Zaragoza). We thank UNAM academicians from the Instituto de Geología, Centro de Geociencias, and Instituto de Geofísica: María Colín, Carlos Linares, Adriana Meléndez, Francisco Otero, Augusto Rodríguez, and Juan Tomás Vázquez for their kind assistance in sample preparation and laboratory analyses. We also thank Industrias Peñoles and, in particular, Eng. Efrén Sánchez for the help during the stay at the mine facilities and for allowing us to access the studied drill holes. Rodrigo Delgado, José Juan Guarneros, Maricarmen Morales, and Scarlett Montoya are acknowledged for their help during sample preparation. We also thank the Associate Editor, Nils F. Jansson, and reviewers Steven Bussey, Sibila Borojević Šoštarić, and Lisard Torró, for their insightful comments and suggestions, which have helped to significantly improve our manuscript. Aaron Hantsche provided an insightful review on an earlier version of this manuscript.
Author information
Authors and Affiliations
Contributions
Antoni Camprubí, Ana K. González-Ambrocio, Néstor Cano, and Eduardo González-Partida conceived the study. Ana K. González-Ambrocio collected the samples at the mine. Néstor Cano and Ana K. González-Ambrocio selected and prepared the samples. Néstor Cano, Antoni Camprubí, Eduardo González-Partida, Ana K. González-Ambrocio, Pura Alfonso, Daniel P. Miggins, Edith Fuentes-Guzmán, Edith Cienfuegos-Alvarado, and Alexander Iriondo conducted laboratory analyses and organized data. Néstor Cano wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editorial handling: N. F. Jansson
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cano, N., Camprubí, A., González-Partida, E. et al. Metallogenic model of the Eocene Santa María and Antares Zn-Pb(-Ag) skarn deposits, Velardeña Mining District, Durango, Mexico. Miner Deposita 59, 671–698 (2024). https://doi.org/10.1007/s00126-023-01225-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-023-01225-4