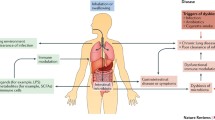
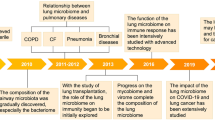
Abstract
Once thought to be a sterile environment, it is now established that lungs are populated by various microorganisms that participate in maintaining lung function and play an important role in shaping lung immune surveillance. Although our comprehension of the molecular and metabolic interactions between microbes and lung cells is still in its infancy, any event causing a persistent qualitative or quantitative variation in the composition of lung microbiome, termed “dysbiosis”, has been virtually associated with many respiratory diseases. A deep understanding of the composition and function of the “healthy” lung microbiota and how dysbiosis can cause or participate in disease progression will be pivotal in finding specific therapies aimed at preventing diseases and restoring lung function. Here, we review lung microbiome dysbiosis in different lung pathologies and the mechanisms by which these bacteria can cause or contribute to the severity of the disease. Furthermore, we describe how different respiratory disorders can be caused by the same pathogen, and that the real pathogenetic mechanism is not only dependent by the presence and amount of the main pathogen but can be shaped by the interaction it can build with other bacteria, fungi, and viruses present in the lung. Understanding the nature of this bacteria crosstalk could further our understanding of each respiratory disease leading to the development of new therapeutic strategies.


Similar content being viewed by others
Abbreviations
- URT:
-
Upper respiratory tract
- LRT:
-
Lower respiratory tract
- BE:
-
Bronchiectasis
- CF:
-
Cystic fibrosis
- COPD:
-
Chronic obstructive pulmonary disease
- PDE:
-
Phosphodiesterase
- CFTR:
-
Cystic fibrosis transmembrane regulator
- AMP:
-
Antimicrobial peptide
- BAL:
-
Bronchoalveolar lavage
- SCLC:
-
Small cell lung cancer
- NSCLC:
-
Non small cell lung cancer
- EBC:
-
Exhaled breath condensate
References
Zitvogel L, Daillère R, Roberti MP, Routy B, Kroemer G (2017) Anticancer effects of the microbiome and its products. Nat Rev Microbiol 15(8):465–478
Huffnagle GB, Dickson RP, Lukacs NW (2017) The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol 10(2):299–306
Yagi K, Huffnagle GB, Lukacs NW, Asai N (2021) The lung microbiome during health and disease. Int J Mol Sci 22(19):1–13
Chotirmall SH, Gellatly SL, Budden KF, Mac Aogain M, Shukla SD, Wood DLA et al (2017) Microbiomes in respiratory health and disease: an Asia-Pacific perspective. Respirology 22(2):240–250
Budden KF, Shukla SD, Rehman SF, Bowerman KL, Keely S, Hugenholtz P et al (2019) Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med 7(10):907–920
Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA et al (2011) Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE 6(2):e16384
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C et al (2010) Disordered microbial communities in asthmatic airways. PLoS ONE 5(1):e8578
Gomes S, Cavadas B, Ferreira JC, Marques PI, Monteiro C, Sucena M et al (2019) Profiling of lung microbiota discloses differences in adenocarcinoma and squamous cell carcinoma. Sci Rep 9(1):1–11
Valverde-Molina J, García-Marcos L (2023) Microbiome and asthma: microbial dysbiosis and the origins, phenotypes, persistence, and severity of asthma. Nutrients 15(3):486
Hu T, Dong Y, Yang C, Zhao M, He Q (2021) Pathogenesis of children’s allergic diseases: refocusing the role of the gut microbiota. Front Physiol 12:1–10
Natalini JG, Singh S, Segal LN (2023) The dynamic lung microbiome in health and disease. Nat Rev Microbiol 21:222–235
O’Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR et al (2019) Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med 199(9):1127–1138
Dickson RP, Erb-Downward JR, Huffnagle GB (2013) The role of the bacterial microbiome in lung disease. Expert Rev Respir Med 7(3):245–257
Esposito V, Lucariello A, Savarese L, Cinelli MP, Ferraraccio F, Bianco A et al (2012) Morphology changes in human lung epithelial cells after exposure to diesel exhaust micron sub particles (PM1.0) and pollen allergens. Environ Pollut 171:162–167
Brune K, Frank J, Schwingshackl A, Finigan J, Sidhaye VK (2015) Pulmonary epithelial barrier function: some new players and mechanisms. Am J Physiol-Lung Cell Mol Physiol 308(8):L731–L745
Boccia M, Aronne L, Celia B, Mazzeo G, Ceparano M, D’Agnano V et al (2020) COVID-19 and coagulative axis: review of emerging aspects in a novel disease. Monaldi Arch Chest Dis. https://doi.org/10.4081/monaldi.2020.1300
Roca J, Vargas C, Cano I, Selivanov V, Barreiro E, Maier D et al (2014) Chronic obstructive pulmonary disease heterogeneity: challenges for health risk assessment, stratification and management. J Transl Med 12(Suppl 2):S3
Prasetyo A, Sadhana U, Budiman J (2021) Nasal mucociliary clearance in smokers: A systematic review. Int Arch Otorhinolaryngol 25(1):160–169
Sethi S, Maloney J, Grove L, Wrona C, Berenson CS (2006) Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173(9):991–998
Hirschmann JV (2000) Do bacteria cause exacerbations of COPD? Chest 118(1):193–203
Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE (2012) The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS ONE 7(10):e47305
Lin L, Li J, Song Q, Cheng W, Chen P (2022) The role of HMGB1/RAGE/TLR4 signaling pathways in cigarette smoke-induced inflammation in chronic obstructive pulmonary disease. Immunity, Inflamm Dis 10(11):e711
Gangemi S, Casciaro M, Trapani G, Quartuccio S, Navarra M, Pioggia G, et al (2015) Association between HMGB1 and COPD: a systematic review. Mediat Inflamm
Alpkvist H, Athlin S, Mölling P, Norrby-Teglund A, Strålin K (2018) High HMGB1 levels in sputum are related to pneumococcal bacteraemia but not to disease severity in community-acquired pneumonia. Sci Rep 8(1):1–9
Polosukhin VV, Cates JM, Lawson WE, Zaynagetdinov R, Milstone AP, Massion PP et al (2011) Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184(3):317–327
Baraldo S, Turato G, Badin C, Bazzan E, Beghé B, Zuin R et al (2004) Neutrophilic infiltration within the airway smooth muscle in patients with COPD. Thorax 59(4):308–312
Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV et al (2012) The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 185(10):1073–1080
Dang AT, Marsland BJ (2019) Microbes, metabolites, and the gut-lung axis. Mucosal Immunol 12(4):843–850
Chen L-W, Chen P-H, Hsu C-M (2011) Commensal microflora contribute to host defense against Escherichia coli pneumonia through Toll-like receptors. Shock 36(1):67–75
Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJTH, de Boer JD et al (2016) The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65(4):575–583
Sprooten RTM, Lenaerts K, Braeken DCW, Grimbergen I, Rutten EP, Wouters EFM et al (2018) Increased small intestinal permeability during severe acute exacerbations of COPD. Respiration 95(5):334–342
Qu L, Cheng Q, Wang Y, Mu H, Zhang Y (2022) COPD and gut-lung axis: how microbiota and host inflammasome influence COPD and related therapeutics. Front Microbiol 13:868086
Zou M, Zhang W, Xu Y, Zhu Y (2022) Relationship between COPD and GERD: a bibliometrics analysis. Int J Chron Obstruct Pulmon Dis 17:3045–3059
Crowell MD, Zayat EN, Lacy BE, Schettler-Duncan A, Liu MC (2001) The effects of an inhaled beta(2)-adrenergic agonist on lower esophageal function: a dose-response study. Chest 120(4):1184–1189
Huang C, Liu Y, Shi G (2020) A systematic review with meta-analysis of gastroesophageal reflux disease and exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med 20(1):2
Yu F, Huang Q, Ye Y, Zhang L (2022) Effectiveness of proton-pump inhibitors in chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Front Med 9:841155
Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P et al (2023) Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J 61(4):2300239
Contoli M, Pauletti A, Rossi MR, Spanevello A, Casolari P, Marcellini A et al (2017) Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J 50(4):1700451
Carrera-Salinas A, González-Díaz A, Ehrlich RL, Berbel D, Tubau F, Pomares X et al (2023) Genetic adaptation and acquisition of macrolide resistance in Haemophilus spp. during persistent respiratory tract colonization in chronic obstructive pulmonary disease (COPD) Patients receiving long-term azithromycin treatment. Microbiol Spectr. 11(1):e0386022
Nigro E, Daniele A, Scudiero O, Monaco ML, Roviezzo F, D’Agostino B, Mazzarella G, Bianco A (2015) Adiponectin in asthma: implications for phenotyping. Curr Protein Pept Sci 16(3):182–187
Scherzer R, Grayson MH (2018) Heterogeneity and the origins of asthma. Ann Allergy Asthma Immunol 121(4):400–405
Martinez FD, Guerra S (2018) Early origins of asthma role of microbial dysbiosis and metabolic dysfunction. Am J Respir Crit Care Med 197(5):573–579
Durack J, Boushey HA, Lynch SV (2016) Airway microbiota and the implications of dysbiosis in asthma. Curr Allergy Asthma Rep 16(8)
Loss GJ, Depner M, Hose AJ, Genuneit J, Karvonen AM, Hyvärinen A et al (2016) The early development of wheeze environmental determinants and genetic susceptibility at 17q21. Am J Respir Crit Care Med 193(8):889–897
Martín R, Heilig GHJ, Zoetendal EG, Smidt H, Rodríguez JM (2007) Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J Appl Microbiol 103(6):2638–2644
Losol P, Park HS, Song WJ, Hwang YK, Kim SH, Holloway JW et al (2022) Association of upper airway bacterial microbiota and asthma: systematic review. Asia Pac Allergy 12(3):1–15
Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N et al (2015) The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17(5):704–715
Jacquet A (2011) Interactions of airway epithelium with protease allergens in the allergic response. Clin Exp Allergy 41(3):305–311
Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD (2015) Asthma. Nat Rev Dis Prim 1:1–22
Petersen C, Round JL (2014) Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 16(7):1024–1033
Denner DR, Sangwan N, Becker JB, Hogarth DK, Oldham J, Castillo J et al (2016) Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol 137(5):1398–1405
Richardson H, Dicker AJ, Barclay H, Chalmers JD (2019) The microbiome in bronchiectasis. Eur Respir Rev 28(153):190048
Purcell P, Jary H, Perry A, Perry JD, Stewart CJ, Nelson A et al (2014) Polymicrobial airway bacterial communities in adult bronchiectasis patients. BMC Microbiol 14(1):1–11
King P (2011) Pathogenesis of bronchiectasis. Paediatr Respir Rev 12(2):104–110
Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA et al (2000) An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 162(4I):1277–1284
Flume PA, Chalmers JD, Olivier KN (2018) Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 392(10150):880–890
Tung JP, Fraser JF, Wood P, Fung YL (2009) Respiratory burst function of ovine neutrophils. BMC Immunol 10:1–11
Whitters D, Stockley R (2012) Immunity and bacterial colonisation in bronchiectasis. Thorax 67(11):1006–1013
Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, Román-Sánchez P, Soriano J (2007) Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 132(5):1565–1572
Byun MK, Chang J, Kim HJ, Jeong SH (2017) Differences of lung microbiome in patients with clinically stable and exacerbated bronchiectasis. PLoS ONE 12(8):1–18
Rogers GB, Van Der Gast CJ, Cuthbertson L, Thomson SK, Bruce KD, Martin ML et al (2013) Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax 68(8):731–737
Tunney MM, Einarsson GG, Wei L, Drain M, Klem ER, Cardwell C et al (2013) Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med 187(10):1118–1126
Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD (2015) A comprehensive analysis of the impact of pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 12(11):1602–1611
Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W et al (2017) Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 49(6)
Cox MJ, Turek EM, Hennessy C, Mirza GK, James PL, Coleman M et al (2017) Longitudinal assessment of sputum microbiome by sequencing of the 16S rRNA gene in non-cystic fibrosis bronchiectasis patients. PLoS ONE 12(2):1–17
Mac Aogáin M, Narayana JK, Tiew PY, Ali NABM, Yong VFL, Jaggi TK et al (2021) Integrative microbiomics in bronchiectasis exacerbations. Nat Med 27(4):688–699
Vanfleteren LEGW, Spruit MA, Wouters EFM, Franssen FME (2016) Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med 4(11):911–924
Narayana JK, Aliberti S, Mac Aogáin M, Jaggi TK, Ali NABM, Ivan FX et al (2023) Microbial dysregulation of the gut-lung axis in bronchiectasis. Am J Respir Crit Care Med 207(7):908–920
Narayana JK, Aliberti S, Aogáin MM, Jaggi TK, Ali NABM, Xaverius IF et al (2022) Dysregulation of the microbial ‘gut-lung’ axis in bronchiectasis. Eur Respir J 60(Suppl 66):1646
Koh W-J, Lee JH, Kwon YS, Lee KS, Suh GY, Chung MP et al (2007) Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest 131(6):1825–1830
Ahn B, Lee DH, Lee CM, Hwang JJ, Yoon H, Shin CM et al (2016) Effect of proton pump inhibitors in bronchiectatic patients with gastroesophageal reflux disease. Korean J Gastroenterol 68(1):10–15
Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ et al (2013) Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 309(12):1251–1259
Malhotra S, Hayes D, Wozniak DJ (2019) Cystic fibrosis and pseudomonas aeruginosa: the host-microbe interface. Clin Microbiol Rev 32(3):1–46
Françoise A, Héry-Arnaud G (2020) The microbiome in cystic fibrosis pulmonary disease. Genes 11(5):536
Scialo F, Amato F, Cernera G, Gelzo M, Zarrilli F, Comegna M et al (2021) Lung microbiome in cystic fibrosis. Life 11(2):1–7
Shah VS, Meyerholz DK, Tang XX, Reznikov L, Alaiwa MA, Ernst SE et al (2016) Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351(6272):503–507
Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO et al (2012) Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487(7405):109–113
Scudiero O, Galdiero S, Cantisani M, Di Noto R, Vitiello M, Galdiero M et al (2010) Novel synthetic, salt-resistant analogs of human beta-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob Agents Chemother 54(6):2312–2322
Carnovale V, Castaldo A, Di Minno A, Gelzo M, Iacotucci P, Illiano A et al (2022) Oxylipin profile in saliva from patients with cystic fibrosis reveals a balance between pro-resolving and pro-inflammatory molecules. Sci Rep 12(1):1–10
Castaldo A, Iacotucci P, Carnovale V, Cimino R, Liguori R, Comegna M et al (2020) Salivary cytokines and airways disease severity in patients with cystic fibrosis. Diagnostics 10(4):222
Crovella S, Segat L, Amato A, Athanasakis E, Bezzerri V, Braggion C et al (2011) A polymorphism in the 5’ UTR of the DEFB1 gene is associated with the lung phenotype in F508del homozygous Italian cystic fibrosis patients. Clin Chem Lab Med 49(1):49–54
Tomaiuolo R, Ruocco A, Salapete C, Carru C, Baggio G, Franceschi C et al (2012) Activity of mannose-binding lectin in centenarians. Aging Cell 11(3):394–400
Castaldo A, Cernera G, Iacotucci P, Cimbalo C, Gelzo M, Comegna M et al (2020) TAS2R38 is a novel modifier gene in patients with cystic fibrosis. Sci Rep 10(1):5–10
Hogan DA, Willger SD, Dolben EL, Hampton TH, Stanton B, Morrison HG et al (2016) Analysis of lung microbiota in bronchoalveolar lavage, protected brush and sputum samples from subjects with Mild-To- Moderate cystic fibrosis lung disease. PLoS ONE 11(3):1–23
Rieber N, Hector A, Carevic M, Hartl D (2014) Current concepts of immune dysregulation in cystic fibrosis. Int J Biochem Cell Biol 52:108–112
Klepac-Ceraj V, Lemon KP, Martin TR, Allgaier M, Kembel SW, Knapp AA et al (2010) Relationship between cystic fibrosis respiratory tract bacterial communities and age, genotype, antibiotics and Pseudomonas aeruginosa. Environ Microbiol 12(5):1293–1303
Keravec M, Mounier J, Guilloux CA, Fangous MS, Mondot S, Vallet S et al (2019) Porphyromonas, a potential predictive biomarker of Pseudomonas aeruginosa pulmonary infection in cystic fibrosis. BMJ Open Respir Res 6(1):1–5
Van Der Gast CJ, Walker AW, Stressmann FA, Rogers GB, Scott P, Daniels TW et al (2011) Partitioning core and satellite taxa from within cystic fibrosis lung bacterial communities. ISME J 5(5):780–791
Keravec M, Mounier J, Prestat E, Vallet S, Jansson JK, Burgaud G et al (2015) Insights into the respiratory tract microbiota of patients with cystic fibrosis during early Pseudomonas aeruginosa colonization. Springerplus 4(1):1–8
Goss CH, Burns JL (2007) Exacerbations in cystic fibrosis·1: epidemiology and pathogenesis. Thorax 62(4):360–367
Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S et al (2015) Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 5:1–12
Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF et al (2012) Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA 109(15):5809–5814
Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML et al (2012) Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci USA 109(34):13769–13774
Whelan FJ, Surette MG (2015) Clinical insights into pulmonary exacerbations in cystic fibrosis from the microbiome what are we missing? Ann Am Thorac Soc 12(6):S207–S211
Fonseca C, Bicker J, Alves G, Falcão A, Fortuna A (2020) Cystic fibrosis: physiopathology and the latest pharmacological treatments. Pharmacol Res 162:105267
Heirali AA, Acosta N, Storey DG, Workentine ML, Somayaji R, Laforest-Lapointe I et al (2019) The effects of cycled inhaled aztreonam on the cystic fibrosis (CF) lung microbiome. J Cyst Fibros 18(6):829–837
Perrotta F, Rocco D, Vitiello F, De Palma R, Guerra G, De Luca A et al (2019) Immune checkpoint blockade for advanced NSCLC: a new landscape for elderly patients. Int J Mol Sci 20(9):2258
Nigro E, Perrotta F, Monaco ML, Polito R, Pafundi PC, Matera MG et al (2020) Implications of the adiponectin system in non-small cell lung cancer patients: a case-control study. Biomolecules 10(6):926
Stella GM, Scialò F, Bortolotto C, Agustoni F, Sanci V, Saddi J et al (2022) Pragmatic expectancy on microbiota and non-small cell lung cancer: a narrative review. Cancers 14(13):3131
Nigro E, Perrotta F, Scialò F, D’Agnano V, Mallardo M, Bianco A et al (2021) Food, nutrition, physical activity and microbiota: which impact on lung cancer? Int J Environ Res Public Health 18(5):2399
Dong Q, Chen ES, Zhao C, Jin C (2021) Host-microbiome interaction in lung cancer. Front Immunol 12:1–9
Mao Q, Jiang F, Yin R, Wang J, Xia W, Dong G et al (2018) Interplay between the lung microbiome and lung cancer. Cancer Lett 415:40–48
Bianco A, Malapelle U, Rocco D, Perrotta F, Mazzarella G (2018) Targeting immune checkpoints in non small cell lung cancer. Curr Opin Pharmacol 40:46–50
Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K et al (2019) Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51(2):285–329
Holmes E, Li JV, Marchesi JR, Nicholson JK (2012) Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab 16(5):559–564
McLean AEB, Kao SC, Barnes DJ, Wong KKH, Scolyer RA, Cooper WA et al (2022) The emerging role of the lung microbiome and its importance in non-small cell lung cancer diagnosis and treatment. Lung Cancer 165:124–132
Tsay JCJ, Wu BG, Badri MH, Clemente JC, Shen N, Meyn P et al (2018) Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am J Respir Crit Care Med 198(9):1188–1198
Yu G, Gail MH, Consonni D, Carugno M, Humphrys M, Pesatori AC et al (2016) Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol 17(1):1–12
Dickson RP, Martinez FJ, Huffnagle GB (2014) The role of the microbiome in exacerbations of chronic lung diseases. Lancet 384(9944):691–702
Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB et al (2015) Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc 12(6):821–830
Goto T (2020) Airway microbiota as a modulator of lung cancer. Int J Mol Sci 21(9):3044
Yan X, Yang M, Liu J, Gao R, Hu J, Li J et al (2015) Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res 5(10):3111–3122
Erb-Downward JR, Falkowski NR, D’souza JC, McCloskey LM, McDonald RA, Brown CA et al (2020) Critical relevance of stochastic effects on low-bacterialbiomass 16s rrna gene analysis. MBio 11(3):1–12
D’Agnano V, Scialò F, Perna F, Atripaldi L, Sanduzzi S, Allocca V et al (2022) Exploring the role of Krebs von den Lungen-6 in severe to critical COVID-19 patients. Life 12:1141
Pattaroni C, Watzenboeck ML, Schneidegger S, Kieser S, Wong NC, Bernasconi E et al (2018) Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe 24(6):857–865
Funding
There are no funding to associate to this work.
Author information
Authors and Affiliations
Contributions
AB: Conceptualization. VD, DM: Software. FS, MV, AC, SFMC, FP: Writing—original draft preparation, AB, MC, LP, SFMC, FS: Writing—review and editing; visualization, FS, VD, DM. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scialò, F., Vitale, M., D’Agnano, V. et al. Lung Microbiome as a Treatable Trait in Chronic Respiratory Disorders. Lung 201, 455–466 (2023). https://doi.org/10.1007/s00408-023-00645-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-023-00645-3