Abstract
Magnetite-(apatite) ore deposits are interpreted as being formed by the crystallization of iron-rich ultrabasic melts, dominantly generated by the interaction of silicate melts with oxidized P-F-SO4-bearing sedimentary rocks. This hypothesis is supported by geologic evidence, experimental studies, numerical modeling, stable and radiogenic isotope geochemistry, mineralogy, and melt- and mineral-inclusion data. Assimilation of crustal rocks during ascent promotes separation from a silicate magma of Fe-rich, Si-Al-poor melts with low solidus temperatures and viscosities, allowing coalescence, migration, and emplacement at deep to subaerial crustal environments. When the iron-rich melt attains neutral buoyancy, fractional crystallization leads to melt immiscibility similar to that observed in industrial blast furnaces, which promotes separation of massive magnetite ore overlain by different types of “slag” containing actinolite or diopside ± phosphates ± magnetite ± feldspar ± anhydrite ± scapolite, commonly enriched in high field strength elements. The mineralogy and morphology of this iron-depleted cap strongly depend on the depth of emplacement and composition of the iron-rich magma. Most of these systems exhibit high oxygen fugacity, which inhibits the precipitation of significant sulfide mineralization. The initially high fO2 of these systems also promotes the formation of low-Ti (< 1 wt%) magnetite: Ti acts as an incompatible component and is enriched in the iron-poor caps and in the hydrothermal aureole. High fluid-phase pressures produced during massive crystallization of magnetite from the melt further facilitate the exsolution of magmatic-hydrothermal fluids responsible for the formation of aureoles of alkali-calcic-iron alteration with hydrothermal replacement-style iron mineralization. On the whole, these systems are dramatically different from the magmatic-hydrothermal systems related to intermediate to felsic igneous rocks; they are more akin to carbonatite and other ultramafic rocks.
Similar content being viewed by others
Introduction
Magnetite-(apatite) deposits are the second largest resource of iron globally, after the deposits related to banded iron formations, and can also host significant resources of rare-earth elements (REE), uranium, cobalt, and phosphorus. Magnetite-(apatite) rocks are found in discrete districts worldwide, span geologic time, and are among the most enigmatic of ore types. At present, there is not even a basic consensus on their ore genesis. These systems infill tensional structures or are deposited subaerially. They share an uncommon but diagnostic mineral assemblage dominated by low-Ti magnetite (< 1 wt% TiO2), variable proportions of fluorapatite, and Ca-Mg-(Fe) silicates (dominantly diopside or actinolite), accompanied by lesser amounts of scapolite, anhydrite, fluorite, and locally, feldspar, phlogopite, andradite, and olivine. The mineral assemblage is similar, except for the low Ti contents in magnetite, to that found in nelsonite (Kolker 1982; Dymek and Owens 2001; Charlier et al. 2015; He et al. 2016) or magnetite-(apatite) rocks in carbonatites and alkaline complexes (Mikhailova et al. 2016; Hurai et al. 2017; Chen et al. 2019; Lan et al. 2022). These latter deposits are widely interpreted as the product of the separation of iron-rich melts from alkaline or tholeiitic liquids.
Magnetite-(apatite) rocks are formed at variable crustal depths from mesozonal environments in high-temperature-low-pressure metamorphic belts (e.g., Adirondacks; Lupulescu et al. 2017) to the more common epizonal and subaerial settings. At the district scale, individual deposits can form over a depth interval of more than 10 km (Tornos et al. 2021).
Here, we present new mineralogical and chemical data and a genetic model that reconciles current geological and geochemical knowledge and explains many of the features observed in magnetite-(apatite) systems. We compare various magnetite-(apatite) systems worldwide, with emphasis on the subvolcanic rocks of El Laco, Chile. We contend that the formation of magnetite-(apatite) systems can only be explained by the crystallization of highly evolved, Si-Al-poor (ultrabasic) iron-rich melts. As they cool, these systems behave as natural analogs for industrial blast furnaces, in which a low-viscosity, high-density iron-rich melt separates and accumulates below an immiscible and less dense Ca-Mg-Si-P-(Fe) slag enriched in elements other than iron.
The magnetite-(apatite) mineral system
Magnetite-(apatite) rocks are grouped in well-defined clusters that are mostly related to continental magmatic arcs and extensional environments (Table 1). The host rocks are dominantly igneous, calc-alkaline to alkaline, and ranging in composition from felsic to mafic, with a predominance of the former (Table 1). Early studies (Meyer 1988; Hitzman et al. 1992) included magnetite-(apatite) systems as a part of the IOCG group; only later were they recognized as an end member of the IOCG clan or an independent style of mineralization with debatable mutual links (Williams et al. 2005; Groves et al. 2010; Skirrow 2022). At the global scale, most magnetite-(apatite) districts lack of significant Cu-Au (IOCG) mineralization (Table 1). The mineralization interpreted as of the IOCG type in some localities is considered to be a late replacement of earlier magnetite-(apatite) rocks, such as at Kiruna and Malmberget in the Nörrbotten region of northern Sweden (Bauer et al. 2018). In fact, significant IOCG mineralization coexists with magnetite-(apatite) systems in a few places, like the Coastal Cordillera of the Andes (Espinoza 1990; Sillitoe 2003; Mateo et al. 2023) and the Carajás district of Brazil (Monteiro et al. 2008).
Basically, magnetite-(apatite) systems form in three different settings: (1) deep intrusive systems that are isolated from the surface and permit slow cooling; (2) subaerial or extrusive systems with widespread degassing and quenching; and (3) subvolcanic systems with transitional features (Table 1). Intrusive and extrusive systems are well described in many places, but the scarcer shallow subvolcanic geologic setting has some key features critical for a full understanding of these systems.
Intrusive deposits
Uneroded and well-preserved intrusive deposits commonly show a striking vertical zonation from a lower, dominant zone of massive magnetite with low P (< 1 wt% P2O5) that constitutes the bulk of the mined iron ore (Fig. 1) through an intermediate zone of magnetite-bearing ore with a pegmatitic texture, into an upper zone rich in Ca-Mg silicates (diopside or actinolite), and apatite.
Iron mineralization occurs as bodies of massive magnetite infilling open spaces, such as discordant dikes to sill-like mineralization, breccia pipes, or irregular to dome-shaped bodies (Fig. 2a,b). Massive magnetite is perhaps the least diagnostic and monotonous part of these systems.
Main features of magnetite-(apatite) systems. (a) Discordant vein of magnetite-(apatite) crosscutting andesite with pervasive K-Ca-Fe alteration and magnetite-rich stockwork. The contact is intruded by a diorite porphyry. West Vein, Los Colorados open pit (Chile). (b) Massive magnetite veins infilling tension structures with local presence of mingling textures with coeval dacite. Marcona Mina 16 (Peru). (c) Andesite replaced by K-feldspar, actinolite, magnetite, and epidote with stockwork-like mineralization. Los Colorados open pit (Chile). (d) Esfordi apatite mine overlying massive magnetite (Iran). (e) Steam-heated alteration dominated by alunite; note the significant iron depletion. El Laco (Chile). (f) Magnetite and apatite infilling open spaces in dacite with argillic alteration. Cerro Huanaquino (Bolivia)
The pegmatite zone is composed of coarse-grained apatite, diopside/actinolite, anhydrite, and, more rarely, andradite, feldspar, and scapolite intergrown with magnetite. It includes large euhedral crystals up to 50-cm long of apatite and diopside or actinolite, commonly with unidirectional growth or comb textures, in some cases including vertical mineralogical banding (see Fig. 4h of Tornos et al. 2021) similar to that in Sn-W deposits (Charoy and Noronha 1996). Textural evidence and the coexistence of inclusions of melt and aqueous fluid (see below) suggest that they are analogous to pegmatite in felsic magmatic systems. As such, they suggest formation by late crystallization of flux-rich melts under fluctuating fluid pressures and high concentrations of volatiles (Dingwell 1985; Nabelek et al. 2010).
Pegmatite is probably the most diagnostic rock in magnetite-(apatite) systems. It is found in almost every district (Table 1; Fig. 3a–l). To our knowledge, this distinctive combination of mineralogical and textural features has not been described in other styles of mineralization, such as sulfide-poor but magnetite-rich facies of IOCG systems or other types of mineralization with massive magnetites, such as skarns or ironstones.
Textures and assemblages of pegmatite. (a) Magnetite-apatite, Acropolis prospect, Olympic Dam (Australia). (b) Vein of diopside-apatite; near Mina Justa (Peru). (c) Magnetite-andradite, Iron Mountain (USA). (d) Actinolite-apatite-magnetite, Los Colorados (Chile). (e) Magnetite-apatite, Chogart (Iran). (f) Actinolite-apatite-ilmenite, Maria Ignacia (Chile). (g) Diopside-magnetite-apatite, Gaucun (China). (h) Diopside-apatite, Cerro del Mercado (Mexico). Note that apatite is concentrated in the lower part. S: soluble salts, dominantly anhydrite; V: vapor. (i) Magnetite-apatite, Abovian (Armenia). (j) Apatite-actinolite vein, Morro de Sossego, Carajás (Brazil). (k) Massive fluorapatite supporting fragments of magnetite-apatite pegmatite, Esfordi (Iran). (l) Magnetite-actinolite-scapolite, Cerro Negro Norte (Chile). See also Fig. 6
In many deposits, the pegmatite grades upward into a zone rich in apatite with variable proportions of diopside/actinolite and anhydrite, with traces of ilmenite, rutile, and titanite. In some cases, this pegmatite directly caps the iron-rich ore (Fig. 2d; Esfordi, Iran; Daliran 2002; Jami 2005) or occurs as abundant subvertical veins and breccia pipes, as in the Coastal Cordillera of the Andes (Tornos et al. 2021). This upper zone may contain up to 30–40% P2O5 and thus constitutes mineable phosphate bodies.
The mineralization is commonly surrounded by an aureole of alkali-calcic and iron-rich alteration dominated by alkali feldspar, actinolite/diopside, magnetite, scapolite, and significant amounts of Ti-bearing minerals including titanite and rutile (Fig. 2c); quartz, where present, is found only in the most external parts of the aureole. The alteration is centered on, and zoned around, the bodies of massive magnetite; the altered host rock can host large bodies of disseminated, stockwork-like, or massive magnetite.
Extrusive deposits
The less common subaerial magnetite mineralization includes large stratabound bodies with volcanic structures similar to those of low-viscosity mid-ocean ridge basalt (MORB; Henriquez and Martin 1978; Lyons 1988; Naslund et al. 2002).
Extrusive magnetite-(apatite) deposits commonly have structures typical of explosive volcanism with pyroclastic eruptions and diatreme and maar complexes of variable size (Table 1). Along with the maar-diatreme at El Laco (Tornos et al. 2017a, b), we have observed analogous systems worldwide, including Abovian (Armenia), Vergenoeg (South Africa) (Fig. 4f; Goff et al. 2004), Gushan (China; Fig. 4c), Per Geijer (Sweden), Cerro del Mercado, Artillero (Levresse et al. 2020), Peña Colorada (Tritlla et al. 2003), La Perla (Mexico), several deposits in the Bafq district (Iran; Förster and Jafarzadeh 1994; Heidarian et al. 2017), and Pilot Knob (Missouri, USA; Fig. 4e, Panno and Hood 1983). Giant breccia pipes have also been described in the Tunguska Basin (Neumann et al. 2017), Kostega (Berdnikov et al. 2021), and Angara-Ilim (Von der Flaass and Naumov 1995; Soloviev 2010) districts (Russia). Other enigmatic deposits that plausibly have similar origins include the giant Wernecke Breccia, Canada (Thorkelson et al. 2001; Hunt et al. 2010), and even the Olympic Dam Breccia Complex, Australia (Reeve 1990).
Explosive vent facies in magnetite-(apatite) systems. (a) Vent facies including highly altered welded rhyolite(?) supported by fragmental magnetite deposited in a subaqueous environment, likely in a maar structure, Artillero (Mexico). (b) Subaqueous deposit of hematite and diadochite, Laco Sur (Chile). (c) Magnetite-rich explosion breccia, Gushan (China). (f) Banded hematite-apatite rocks, Per Geiger (Sweden). (e) Polymictic breccia, including some welded fragments, supported by hematite, Pilot Knob (USA). (f) Stratabound fluorite-magnetite breccia supported by fine-grained magnetite. Spill Zone, Vergenoeg (South Africa)
In many cases, vent facies retain their original airfall textures (Panno and Hood 1983; Nyström et al. 2016) including welded fragments at Artillero, Mexico (Fig. 4a; Levresse et al. 2020), Pilot Knob, USA, and Vergenoeg, South Africa (Fig. 4f), ballistic fragments at Hormuz Island, Iran (Faramarzi et al. 2019), well-preserved spindle bombs at El Laco (Henríquez and Nyström 1998), remnants of pollen indicative of deposition in an environment lacking liquid water (Corona-Esquivel et al. 2010), or complex P-Fe or Fe-S phosphates (e.g., lipscombite-destinezite; Fig. 5f) that have been interpreted by Mungall et al. (2018), de Fourestier (2019), and Velasco et al. (2020) as ejected immiscible melts. These geologic lines of evidence leave little doubt that these deposits are primary tephra and not the product of hydrothermal replacement of a putative host rock.
Evidence of melt immiscibility in magnetite-(apatite) systems. (a) Melt inclusion in phenocryst of andesite. Immiscible droplets now formed by magnetite, enstatite, and fluorapatite (ap) and sometimes with a droplet of an immiscible copper sulfide are supported by a groundmass of glassy rhyolite with phenocrysts of clinopyroxene; the melt inclusion shows a subtle aureole of albitization of the host phenocryst of plagioclase. El Laco (Chile); sample LACO-AND. (b) Blebs of magnetite hosted by microdiorite and enclosed in an aureole of albitization. Low-Ti magnetite (0.01–0.94 wt%Ti), including exsolution of titanomagnetite (Supplementary Table 3), coexists with small amounts of fluorapatite, albite, diopside (Fe/Fe + Mg, 0.04–028), and titanite. Los Colorados (Chile) sample COL-4. (c) Large droplets of diopside (Di32-40) + low-Ti magnetite in a groundmass of dacitic composition with pervasive alteration to K-feldspar. Note the large cavities in the mafic phase, likely evidence of a gas-rich phase and now partially infilled with chalcedony and platy calcite. (d) Dacite with abundant droplets of diopside + magnetite. Kiruna (Sweden) sample DDH 6876. (e) Apatite + actinolite assemblage (“blebs”) inside the massive magnetite zone. Carmen de Fierro Mine (Chile), underground level 2. (f) Fragment of diadochite interpreted as the product of a pyroclastic ejection of an immiscible P-Fe-S melt (Mungall et al. 2018) and interbedded with fragmental magnetite; note the load cast structures in the base, suggesting that it was deposited under a non-fully consolidated state. Laco Sur (Chile). (g) Apatite including small amounts of a rhyolitic melt showing devitrification spherulites now replaced by chalcedony and ankerite; Los Colorados (Chile). (h) Magnetite and partially devitrified rhyolite showing mingling structures. Spill zone, Vergenoeg (South Africa)
We interpret these explosive structures as due to the degassing of a low-density vapor emitted from the ascending iron-rich melt (see also Keller et al. 2022). Incidentally, the interaction of molten iron with even small amounts of water is one of the causes of large explosions in blast furnaces, owing to the sudden vaporization of water (Babaitsev and Kuznetsov 2001).
Magnetite is commonly replaced by maghemite, which can be the predominant phase in many deposits. Hematite makes up only a small part of the mineralization and almost invariably is in zones rich in subaerial fragmental ore, probably pyroclastic in origin like at Per Geijer (Fig. 4d; Martinsson 2015), Cerro del Mercado and La Perla (Lyons 1988; Corona-Esquivel et al. 2010), Pilot Knob (Panno and Hood 1983), or El Laco (Fig. 4b; Henríquez and Nyström 1998). The maar structures, where preserved, may be infilled by hematite-rich surge fall-out deposits, in some cases with subaqueous traction structures indicative of deposition below the water level. Otherwise, hematite in magnetite-(apatite) systems is late and formed by hydrothermal replacement or supergene alteration.
The El Laco system
The El Laco deposit
The well-known El Laco iron deposit, the most recent and best-preserved magnetite-(apatite) system, has attracted much attention over the past 60 years (Park 1961; Frutos and Oyarzun 1975; Henriquez and Martin 1978; Broman et al. 1999; Rhodes and Oreskes 1999; Rhodes et al. 1999; Naslund et al. 2002; Sillitoe and Burrows 2002; Naranjo et al. 2010; Nyström et al. 2016; Tornos et al. 2016, 2017b; Velasco et al. 2016; Mungall et al. 2018; Ovalle et al. 2018; Childress et al. 2020; Bain et al. 2021; Xie et al. 2021, among others).
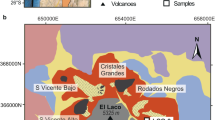
The El Laco volcanic system includes a 2.18 ± 0.03 to 1.83 ± 0.09 My old (Brown et al. 2021) andesitic stratovolcano with coeval iron-oxide bodies arranged along an annular structure that is probably related to a caldera-collapse structure (Frutos and Oyarzun 1975; Keller et al. 2022). The dominantly stratiform mineralization hosts various types of volcanic facies such as spatter cones (San Vicente Alto), lava lakes (Laco Norte), and volcanic edifices, including alternating lava and pyroclastic and ash flows crosscut by diatreme-maar structures (Laco Sur) (Nyström et al. 2016; Tornos et al. 2017b). These bodies are rooted in subvertical dikes of massive magnetite and in breccia pipes; irregularly eroded subvolcanic roots are found at San Vicente Bajo, Rodados Negros, Cristales Grandes, and Pasos Blancos areas (Henriquez and Martin 1978; Naslund et al. 2002; Naranjo et al. 2010; Nyström et al. 2016; Tornos et al. 2017b). The magnetometric data of Alva-Valdivia et al. (2003) show that there is a large, deep zone of mineralization; it is likely that the current El Laco orebody is the uppermost part of a large subvolcanic magnetite-(apatite) system.
Near the surface at El Laco, metasomatic alteration is dominated by extensive steam-heated alteration replacing andesite (Fig. 2e). The most advanced alteration-induced mineral assemblage is dominated by illite–smectite, tridymite, and sulfates (natro-alunite, gypsum), with lesser amounts of fluorite, P-rich minerals (variscite, florencite, phosphosiderite, woodhouseite) and high field strength element (HFSE) bearing phases such as monazite, anatase, and thorite. The 87Sr/86Sr values of the steam-heated alteration zone are between those of the unaltered andesite and the ore assemblages (Tornos et al. 2017b), suggesting that the SO2, F, and P-rich fluid that formed this alteration was directly related to the ore-forming event.
It is worthwhile to note that the andesite is bleached and depleted in iron as a result of the steam-heated alteration, which implies that the acidic vapor was not the main agent of iron transport at El Laco as has been proposed by Rhodes and Oreskes (1994) and Ovalle et al. (2022). Iron solubility in low-density magmatic vapor equilibrated with dacite and andesite is low, even at extremely high temperatures (< 10−6 XFe, gas over the interval > 800–500 °C; Symonds et al. 1987; Africano et al. 2002). Furthermore, iron minerals have seldom been reported near fumaroles (Kodosky and Keith 1995). In fact, elements like iron, Cu, K, Na, Sb, Ni, Ga, V, Mn, and Li have low distribution coefficients between magma and magmatic gas (Symonds et al. 1987) and would strongly fractionate in a coexisting chloride-rich brine (if present). Furthermore, the transport of Fe by gas should decouple it from elements like Na, K, Ca, and Mg, which are an integral part of the ore-related alteration. Minor magnetite deposited by rapid cooling of a magmatic gas is probably what is observed in the gas-escape tubes of Laco Sur along with fluorite, fluorapatite, and a range of sulfates and other minerals including REE and Nb-bearing phases (Henriquez and Martin 1978; Tornos et al. 2017b). Such assemblages have also been observed in open cracks in volcanic rocks affected by steam-heated alteration; their walls become coated by small amounts of euhedral magnetite and apatite (e.g., Huañaquino, Bolivia; Payún Matrú, Argentina; Fig. 2f).
The subvolcanic system at El Laco
The uneroded Pasos Blancos subvolcanic system includes voluminous and well-preserved coarse-grained rocks that we have grouped into the generic name of pegmatite based on mineral textures, paragenesis, and zonation (see also Naranjo et al. 2010; Tornos et al. 2017b) (Fig. 6). Pasos Blancos has only been sparsely drilled, but one drillhole crosscuts a subvertical body of massive magnetite at 324-m depth (Fig. 7a). This and other nearby drillholes intersected an igneous complex including diopside-magnetite, plagioclase-diopside-magnetite, magnetite-apatite, scapolite-magnetite-diopside, and diopside-anhydrite-ilmenite rocks (Fig. 7b–h). Similar rocks are found beneath Laco Sur down to depths of 300 m. All these rocks and the host andesite are crosscut by massive anhydrite forming large vein-like bodies up to tens of meters thick. At Pasos Blancos, we have not yet observed the actinolite/diopside-apatite-magnetite facies that is typical elsewhere, but large bodies of this rock can be observed in other areas of the El Laco complex (Fig. 7g). Below the massive magnetite, and at depths below 370 m, the drillholes intersected plagioclase-phyric andesite with abundant blebs of low-Ti magnetite and cm-sized drop-like inclusions of anhydrite and quartz (Tornos et al. 2017b).
Massive mineralization and pegmatite at El Laco (Chile). (a) Massive magnetite mineralization in sharp contact with andesite with diopside-magnetite alteration. 343–345-m depth (Pasos Blancos). (b) Magnetite-diopside pegmatite, 345-m depth (Pasos Blancos). (c) Diopside-anhydrite pegmatite, 274-m depth (Pasos Blancos). (d) Diorite, 306-m depth (Pasos Blancos). (e) Veins of magnetite-scapolite (marialite) pegmatite supporting fragments andesite replaced by albite and minor diopside, 47-m depth (Pasos Blancos). (f) Vein of massive hedenbergite (pyroxenite) crosscutting massive, fine-grained andesine-oligoclase, 153-m depth. (g) Diopside-apatite pegmatite (southern sector). (h) Vein of magnetite-(apatite) (Cristales Grandes). Drillcore has an HQ diameter (63.5 mm)
The pegmatite typically exhibits gradational, but in some locations, sharp, contacts with the massive magnetite and, in most cases, intrusive contacts with andesite. Pasos Blancos also includes large bodies of breccia with fragments of pegmatite and variably altered andesite supported by an assemblage of diopside, magnetite, scapolite, anhydrite, and apatite that may correspond to the deep roots of the diatreme pipes.
The massive magnetite
The large subvolcanic body, likely a vertical vein some 30–35 m in true thickness, is formed by poorly vesicular fine-grained magnetite showing locally unidirectional cooling structures similar to those in the Rodados Negros area. Crystals of fluorapatite up to 1-cm long and irregularly replaced by chlorapatite occur among the large crystals of magnetite. The magnetite analyzed so far is Ti-poor with contents of 0.0 to 0.71 wt% TiO2 (Supplementary Table 3; Fig. 8). Such low contents of Ti in magnetite are consistent even of the deepest samples, collected between 349 and 369 m depth (Supplementary Table 2).
Composition of magnetite from El Laco (Chile). Dashed fields correspond to the surface samples of stratabound mineralization (Laco Sur, Laco Norte, and San Vicente Alto), discordant dikes (Rodados Negros and Pasos Blancos) (Ovalle et al. 2018), and andesite (Velasco et al. 2016; Ovalle et al. 2022). The position of magnetite and hematite are at the lowest TiO2 plotted. Samples from the bibliography from unknown depths or lithologies are not included
Our systematic petrographic, SEM, EPMA, and confocal Raman spectroscopy work shows that although magnetite is free of exsolved phases, it hosts abundant 20–200 µm inclusions of subcalcic pyroxene (En21-77 Fs13-65 Wo0-14) (Figs. 9 and 10a; Supplementary Table 3), diopside, euxenite(?), titanite, monazite, and carbonates with an intermediate composition between ankerite and dolomite. The largest grains of enstatite coexist with µm-sized roundish glassy inclusions partially altered to chlorite and intergrown with quartz and magnetite.
Composition of pyroxene from the Pasos Blancos orebody compared with that of the phenocrysts in the andesite (Velasco et al. 2016). Note that while the large crystals in the pegmatite are of diopside to high-Ca augite composition, the inclusions in coexisting magnetite are subcalcic and in the massive magnetite are enstatite. For comparison, the diagram includes the composition of pyroxene in melt inclusions in the andesite from Pietruszka et al. (2023a)
Inclusions in magnetite-(apatite) mineralization at El Laco (Chile). (a) Zoned magnetite including cores of Ti-poor magnetite with abundant micro-inclusions, dominantly of enstatite to diopside, and a second generation of Ti-poor magnetite devoid of inclusions. Diopside-magnetite pegmatite. LCO-222–5, 368-m depth (Pasos Blancos). (b) Multi-phase silicate-rich melt inclusion in massive magnetite. LCO-222, 368-m depth (Pasos Blancos). (c) Massive, low-Ti magnetite hosting abundant melt inclusions with compositions close to diopside (Si-Ca-Mg > Fe) and albite (Si-Al-Na > K). Some of them include two phases separated by a meniscus (arrow). Deep magnetite orebody, Pasos Blancos. Sample LCO-222–1, 358 m depth (Pasos Blancos). (d) Melt inclusions in apatite including likely Ca-Mg sulfates, magnetite, and a large bubble of gas-rich phase. Diorite. Sample LCO-312, 227.5-m depth (Pasos Blancos). (e) Inclusions in the fluorapatite of the diorite, including mono- to polyphase inclusions of vapor, salts, diopside, anhydrite, and magnetite. Sample LCO-312, 227.5-m depth (Pasos Blancos). (f) Inclusions of (Fe-)columbite (cb) and an unknown Nb-Th-(Fe)-rich phase, possibly aspedamite or ekerbergite (?) in massive stratabound magnetite. LCO-213 (Laco Sur). (g) Zoned crystal including a core of ilmenite and a rim of hematite; m; the edge of the ilmenite crystal is partially altered to maghemite. Diopside-anhydrite pegmatite. LCO-204, 207-m depth (Laco Sur). (h) Inclusion in diopside including halides and anhydrite. Massive magnetite with inclusions of diopside. LCO-222–3, 366-m depth (Pasos Blancos)
The diopside-magnetite pegmatite
The diopside-magnetite pegmatite forms the most immediate host rock to the dikes of massive magnetite (Fig. 7b) but also occurs as veins of up to meters thick within the massive magnetite. It is one of the most common rock types at Pasos Blancos, but it is also especially abundant in the footwall of the Laco Sur and Laco Norte stratabound bodies. The rock is composed of euhedral crystals of diopside up to 1-cm long and interstitial magnetite, minor fluorapatite, and large euhedral crystals (> 2 mm) of titanite. This pegmatite sharply grades into massive magnetite, pyroxenite, and jigsaw-like and chaotic breccias with cm- to m-sized fragments of andesite and supported by the same, but fine-grained, mineral assemblage (Fig. 7f). Equivalent rocks have been observed elsewhere in magnetite-(apatite) systems such as in eastern China, the Carajás district in Brazil, and the Esfordi mine in the Bafq district of Iran.
The composition of the large crystals of clinopyroxene is rather constant, En38-45 Fs8-16 Wo43-49 (Fig. 9). Petrographically, early iron-rich augite is replaced by later diopside. The magnetite has Ti contents on average ten times higher than the massive magnetite (3.0 ± 8.8 wt% TiO2; Fig. 8). The magnetite also contains exsolution lamellae of Mg-rich ilmenite, ulvöspinel, and rutile along the {111} planes. Magnetite crystals show a relatively simple internal zonation with an mm-sized irregular core defined by the presence and local alignment along zone edges of myriad 1–10 µm elongate drop-like inclusions of clinopyroxene of variable composition ranging from enstatite to diopside (En16-76 Fs10-68 Wo0-48; Figs. 9 and 10a,b). In general, the inclusions of diopside are subhedral to euhedral and larger than those of subcalcic pyroxene. Other mineral inclusions include zircon and plates of Ti–rich phlogopite. Outer zones of the magnetite grains are devoid of inclusions.
Chemical analyses (SEM–EDS) and Raman spectra reveal that the magnetite and diopside grains host abundant 1–50 µm inclusions of anhydrite, Nb-rich (ca. 4 wt% Nb2O5) ilmenite, phlogopite, thorite, zircon, rutile, apatite, ankerite-dolomite, Th-La-Ce silicates, niobian titanite, and small drop-like inclusions of quartz. In the diopside and magnetite (dominantly in the former), there are 50–300 µm inclusions of plagioclase (Ab64-92An1-27Or4-26) showing roundish to elongated shape (Fig. 11 and Supplementary Table 3). Plagioclase is commonly replaced by later K-feldspar along edges and fissures. Very locally, both are intergrown with quartz. The plagioclase has Raman spectra typical of high triclinic albite, likely formed above 500 °C and typical of quenched volcanic rocks (Tuttle and Bowen 1950).
Another group of small (3–10 µm) roundish to elongate inclusions contain two or even three phases (Fig. 10b). The two-phase inclusions contain diopside-enstatite, diopside-ilmenite, enstatite-ilmenite, albite-enstatite, albite-diopside, and albite-ilmenite. The three-phase inclusions comprise diopside, enstatite, and ilmenite. Figure 10c shows a complex inclusion with enstatite, albite, K-feldspar, and minor amounts of quartz, Na–K chlorides, and titanite with a small bubble. The abundance of silicates, some meniscus-like contacts (Fig. 10b), and the presence of pyroxene with enstatite to pigeonite composition confirm that these are melt inclusions.
Inclusions in the large diopside crystals are different than those in the magnetite and have been studied in detail by Broman et al. (1999) and Bain et al. (2021). Diopside does not host enstatite inclusions, but hosts abundant polycrystalline inclusions interpreted to have crystallized from a trapped melt (Broman et al. 1999; Bain et al. 2021). Crystal cores host up to 50-µm inclusions with anhydrite, hematite, and apatite constituting half of each inclusion, and albite, K-feldspar, and quartz making up the remaining ∼50 volume %. The inclusions at the edge of the crystals are dominated by anhydrite with lesser amounts of hematite, glauberite, baryte, natrite, halite, allanite, ilmenite, and pyrite. The low-density vapor phase is dominated by H2O (Broman et al. 1999). Inclusions in the diopside suggest that it crystallized from a melt having ∼6–17 wt% Fe, ∼7–14 wt% Si, and ∼9–11 wt% oxidized S (Bain et al. 2021). Some diopside grains include roundish inclusions with variable proportions of magnetite ± Ca-Mg sulfate ± vapor.
The diorite
Two drillholes at Pasos Blancos have intersected a highly heterogeneous igneous unit dominated by cm-scale crystals of euhedral plagioclase with intergranular diopside, magnetite, and fluorapatite with a gabbro-like texture (Fig. 7d). In detail, the intercrystal spaces between a network of euhedral andesine to oligoclase (Ab35-85An9-65Or0-6; Supplementary Table 3 and Fig. 11) are infilled by a later assemblage of diopside (En25-42Fs9-26Wo44-50) plus minor amounts of fluorapatite, Ti-bearing magnetite (0.50–3.82 wt% TiO2), titanite, and dolomite. This coarse-grained facies dominates the core of the unit, but toward the margin, the plagioclase is more fine-grained and the plagioclase-rich rock is brecciated and supported by massive pyroxenite (Fig. 7f), texturally similar to that adjacent to the diopside-magnetite pegmatite. However, it is made up of medium-grained hedenbergite (En4-10Fs42-50Wo46-48) and more accessory andradite (Adr84-97Slo0-9; calculated following Locock 2008; Supplementary Table 3), low-Ti magnetite, fluorapatite, dolomite, and titanite.
Oligoclase hosts abundant droplets of magnetite ± Ca-Mg sulfate, whereas late albite is inclusion-free. In the clinopyroxene and garnet, abundant inclusions are equivalent to the salt-rich inclusions described above and contain Ca-Mg sulfate, magnetite, and a vapor bubble. In the fluorapatite, there are abundant inclusions (Fig. 10d,e). Some are ellipsoidal and show consistent volumetric proportions of minerals, thus can be interpreted as melt inclusions. They include up to five phases, including variable proportions of low-Ti (< 1% wt%) magnetite, diopside, zircon, anhydrite, monazite, titanite, and undetermined iron phosphates with a transparent groundmass, likely a Ca-Mg sulfate or chloride. The volume proportions of the bubbles in the inclusions are highly variable, in some cases occupying up to 95% of the inclusion volume.
The texture of the diorite with the ferromagnesian mineral assemblage infilling intercrystal spaces and corroding plagioclase is similar to that described by Hayes et al. (2017) and Yao and Mungall (2022) as taking place in magma chambers hosting two or more immiscible melts. This hybrid rock was probably formed by the infiltration of a dense and low-viscosity iron-rich melt into a previous mush zone of oligoclase crystals with interstices originally occupied by residual (sulfate-carbonate?) melt. Such infiltration of the iron-rich melt would dissolve and displace the original melt and corrode the earlier crystals.
The Sr–Nd radiogenic isotopes of two whole-rock samples of the diorite (Supplementary Table 4) plot within the field of the ores at El Laco; they have similar 87Sr/86Sr values (0.7080–0.7081 vs. 0.7080–0.7083) and εNd values (− 5.0 and − 5.1 vs. − 5.4 to − 4.6) but distinctly higher 87Sr/86Sr signatures than the host volcanic suite (0.7066–0.7076) confirming that these rocks acquired radiogenic Sr that is not present in the host andesite (Tornos et al. 2017b).
We interpret that plagioclase in the diopside-magnetite pegmatite and in the diorite is equivalent to the feldspar-quartz fraction of melt inclusions described by Bain et al. (2021) in the core of the diopside crystals. This plagioclase-rich fraction is similar to the albitite described in some magnetite-(apatite) and IOCG districts such as in the Bafq district (Jami 2005), the Adirondacks (Buchanan 2015), the Coastal Cordillera of the Andes (Tornos et al. 2021), or in the Lower Yangtze district (Zeng et al. 2022). The origin of the albitite is controversial and, in many instances, interpreted as being hydrothermal, but the presence of melt inclusions (Carriedo and Tornos 2010) strongly suggests that at least some of these rocks crystallized from a Na-rich silicate melt.
The diopside-anhydrite pegmatite
The pyroxene-anhydrite pegmatite forms curvilinear veins 10- to 50-cm thick hosted by altered andesite. These veins show large (up to 5-cm long) euhedral crystals of diopside or augite with signs of unidirectional growth (Fig. 7c) and interstitial mm-scale grains of ilmenite replaced by maghemite with hematite-rich rims (Fig. 10g). The pyroxene has a monotonous composition, En42-44Fs10-13Wo45-46, equivalent to that of the most evolved Mg-rich member of the diopside-magnetite pegmatite (Fig. 9). The pyroxene includes some large (> 100 µm) inclusions of fluorapatite and grains of rutile up to 30 µm in length, plus thorite, calcite, Ti-poor magnetite, and wollastonite. Non-silicate polycrystalline inclusions contain anhydrite, carbonate, quartz, and ilmenite similar to those in the rim of the diopside in the diopside-magnetite rock. The diopside also hosts subrounded inclusions of albite (Ab90-93An4-6Or3-5). As a whole, there is an evolution from andesine-oligoclase in the diorite toward oligoclase-albite in the diopside-magnetite pegmatite and albite in the diopside-anhydrite pegmatite.
Occupying the core of the veins and located interstitially between the diopside crystals is coarse-grained, massive anhydrite. The anhydrite is rich in minute (< 100 µm) roundish inclusions of quartz and is widely replaced by supergene bassanite and gypsum.
Scapolite-rich rock
This rock is similar to the diorite and shows mutual complex intergrowths (Fig. 7e). It consists of a coarse-grained, equigranular assemblage dominated by unoriented, euhedral, mm- to cm-sized crystals of marialite with intergranular inclusions of diopside and magnetite and minor fluorapatite and anhydrite. This rock is further characterized by abundant scapolite-rich veins. It has been extensively described by Naranjo et al. (2010).
The magnetite-apatite rocks
Unlike magnetite-(apatite) systems elsewhere, El Laco lacks an apatite-rich cap and has a paucity of apatite-rich pegmatite. Large crystals of apatite intergrown with magnetite are, however, found in the magnetite dikes of Rodados Negros, Cristales Grandes, and Pasos Blancos or in a diopside-magnetite pegmatite in Cristales Grandes (Fig. 7h) and other nearby areas (Fig. 7g).
All of these rocks are crosscut by trails of secondary fluid inclusions that, when opened, reveal a cavity with crystals lining the walls (Fig. 10h). SEM–EDS analysis shows that they are dominated by chlorides, halite with lesser proportions of sylvite and FeCl2, more random MgCl2 and CaCl2, and smaller proportions of anhydrite. The inclusions are sporadically enriched in Zn and Sn. The strikingly variable proportions of Cl/(SO4 + CO2) in the polycrystalline and fluid inclusions suggest that Cl was preferentially incorporated into the aqueous phase. As a whole, the data suggest that there is a continuum in the El Laco system between silicate and salt melts and aqueous fluids (Veksler and Lentz 2006; Audetat and Lowenstern 2014).
Mineralogy of the subvolcanic system
Pyroxene as a tracer of the evolution of the system
The composition of pyroxene at El Laco provides a connection between the host andesite, the melt inclusions, the massive magnetite, and the pegmatite (Fig. 9). To some extent, our results match the previous data of Velasco et al. (2016) and Ovalle et al. (2022), but the melt inclusions in the massive magnetite significantly expand composition toward the subcalcic field and reveal compositions that are otherwise exclusively found in mafic–ultramafic rocks. Velasco et al. (2016) described both ortho- and clinopyroxene in andesite that presumably crystallized as two phases along the pyroxene immiscibility surface; this solvus pair is also found in the deep massive-magnetite ore and the diopside-magnetite pegmatite, where both diopside or augite and enstatite are abundant as micro-inclusions (Fig. 9). However, the presence of pigeonite in the diopside-magnetite pegmatite shows that some of these rocks were trapped above the pyroxene solvus at temperatures above 1200 °C (see Wells 1977 and Lindsley 1983).
The large crystals in most samples of pegmatite have compositions close to diopside. Comparison of the composition of the pyroxene in the pegmatite with that in the immiscible iron-rich phase in the melt inclusions of the andesite (Fe# = 0.39–0.56; Pietruszka et al. 2023a) suggests a gradual evolution, first by Fe–Mg depletion at a rather constant ratio above solvus surface from ca. 1100° to ca. 900 °C during cooling, and later toward more Ca-Mg-rich compositions. We interpret this pattern of evolution as related to Fe sequestration during magnetite crystallization along the pyroxene solvus. The only exception is the massive pyroxenite, dominated by hedenbergite; the high iron content is probably related to pyroxene crystallization at magnetite-undersaturated conditions.
The Ti-in-magnetite problem
The extensive dataset on the composition of iron oxides at El Laco (Fig. 8) shows that they can vary significantly in Ti content (see Supplementary Table 3; Dare et al. 2015, Velasco et al. 2016, Ovalle et al. 20182022 and La Cruz et al. 2020). In brief, the vast majority of the massive magnetite ore has a TiO2 content below 1 wt% TiO2, including all the stratabound ores and their deep roots. Only 4% of the analyzed magnetite of the massive ore, regardless of the depth of emplacement, shows TiO2 values exceeding 1 wt% TiO2. Remarkably, the dike of massive magnetite intersected at depth has the same low Ti contents (bdl–0.87 wt% TiO2) as the magnetite that crops out at the surface. Similar low TiO2 contents are also found in the magnetite grains in the andesite porphyry beneath El Laco (e.g., 0.25–0.87 wt% TiO2). These low values are typical of magnetite-(apatite) systems elsewhere (Heidarian et al. 2016; Broughm et al. 2017; Nie et al. 2017; Palma et al. 2020).
In contrast, iron oxides in the pegmatite have higher TiO2 contents, also regardless of the depth. The contents range from the limit of detection to compositions close to that of ulvöspinel and ilmenite (see also Bain et al. 2021). However, some of these late values should be normalized to higher contents to determine the original bulk composition since these crystals contain abundant exsolution structures involving Ti-bearing minerals. The diopside-anhydrite pegmatite hosts ilmenite as the dominant Fe-Ti oxide (Fig. 10g). Ilmenite and hematite, but no magnetite, are also present in the apatite-actinolite caps at the Maria Ignacia and California apatite mines in the Coastal Cordillera of the Andes in Chile (Tornos et al. 2021). The magnetite in the alkali-calcic-iron alteration also has relatively high TiO2 contents (see Velasco et al. 2016).
Bulk analyses of rocks at El Laco (Supplementary Table 1 and Supplementary Fig. 1) corroborate these relationships between mineral assemblages and the Ti content of magnetite and show that the feeder structures and the stratiform ore invariably have low Ti contents. The stratabound ore at Laco Sur has Ti contents of less than 800 µg/g, which are similar to those of the subvolcanic bodies at Pasos Blancos (bdl–770 µg/g) and the feeder dikes at Rodados Negros (bdl–0.7 wt% Ti). The diopside-magnetite pegmatite has significantly higher Ti contents (360 µg/g to 2.4 wt% Ti), as do the rocks affected by alkali-calcic-iron alteration (0.15–2 wt% Ti). In fact, whole rock normalized ratios of total iron (Supplementary Fig. 1) show that in this system, Ti is not accommodated solely by magnetite. The widespread presence of inclusions of titanite, rutile, and ilmenite in the massive magnetite ore, the pegmatite, and the altered andesite suggests that the El Laco system was close to saturation or even saturated with one or more Ti–rich mineral phases.
These results are also consistent with the database of Compañia Minera del Pacífico (unpublished, 2017), which shows that all the surficial and deep samples of massive magnetite at Laco Norte and Laco Sur have 0.0–0.14 wt% Ti (n = 1343) and 0.0–0.21 wt% Ti (n = 1175), respectively, and the number of Ti–rich samples is negligible. The massive ore in the deep orebodies at Pasos Blancos has equivalent values of 0.02 ± 0.01 wt% Ti (n = 24).
Most recent studies dealing with magnetite geochemistry relate the trace element contents of magnetite to the temperature of crystallization, a magmatic versus hydrothermal origin, or the type of mineralization (Dupuis and Beaudoin 2011; Nadoll et al. 2014; Huang and Beaudoin 2019); a major variable in the putative “discrimination diagrams” of these latter studies is the Ti content. Several authors have offered such interpretations of El Laco magnetite (Dare et al. 2015; Ovalle et al. 2018, 2022; La Cruz et al. 2020; Palma et al. 2020). However, such interpretations are largely speculative because they largely rest on empirical comparisons with compilations of magnetite compositions from other geologic environments rather than on the thermodynamic controls of Ti partitioning between fluids and magnetite. For example, the Ti-in-magnetite concentration can be strongly influenced by the redox state of the system, as has been demonstrated experimentally (Spencer and Lindsley 1981; Lindsley 1991) and theoretically (Ghiorso and Evans 2008). When in the presence of Fe-Ti rhombohedral oxides and at high fO2 (> NNO + 3), Ti behaves as an incompatible element in magnetite. The isocompositional curves are almost parallel to the fO2 axis, indicating that the effect of temperature is negligible. At such oxidizing conditions, the Ti-in-magnetite is below ca. 0.4 wt% and is predicted to be negligible at higher fO2 near the hematite-magnetite (HM) buffer. Small fluctuations in the redox state, therefore, can explain the variability observed in the Ti contents. At lower redox conditions, Ti incorporation into magnetite depends on both the temperature and the fO2 (Ghiorso and Evans 2008), as well as, of course, the Ti activity of the magnetite-forming fluid. Despite the lack of experimental work, thermodynamic principles demand that this is also the situation in the presence of other Ti-buffering phases such as rutile or titanite when the fO2 can be constrained externally. In the absence of a Ti-saturating phase, the degrees of freedom of the system do not allow one to predict temperature or redox state because the amount of Ti in magnetite also depends on the composition of the system and the KD mineral-fluid (see Tornos et al. 2023).
The presence at El Laco of high-temperature hematite replaced by “mushketovite” (magnetite pseudomorphic after hematite) or as inclusions in magnetite, and of the anhydrite + low Ti-magnetite assemblage in the melt inclusions and the massive ore, indicates that magnetite-(apatite) rocks started to crystallize at fO2 close to the HM buffer (Tornos et al. 2017b), and well above the redox state of typical calc-alkaline silicate melts in magmatic arcs (≈QFM + 1; Stagno and Fei 2020). The lack of pyrrhotite indicates that the system never crossed the pyrrhotite-pyrite-magnetite buffer, even at high degrees of crystallization. Thus, at the conditions predicted for the precipitation of massive magnetite at El Laco, early magnetite can only host small amounts of Ti. In such an iron-rich system, redox conditions are buffered by the Fe3+/Fe2+ ratio of 66.7 Fe3+/33.3% Fe2+ in magnetite. The crystallization of large amounts of magnetite draws down the fO2 by the general reaction
Progressive crystallization of magnetite thus depletes the iron-rich melt in Fe3+ relative to Fe2+ and allows for the subsequent formation of Ti–rich magnetite and ilmenite in the later pegmatites. Potential degassing of SO2,g from the melt would also cause a decrease in fO2 (Giggenbach 1987; Burgisser and Scaillet 2007).
Our results also show that none of the compositions of massive magnetite ore at El Laco have high contents of Ti, whereas the igneous magnetite in the adjacent host andesite shows high Ti (8–13 wt% TiO2 (Velasco et al. 2016; Broughm et al. 2017). Hence, these data strongly indicate that there is no inheritance of magmatic phenocrysts from the hosting andesite in the El Laco ore.
HFSE enrichment
The whole-rock pXRF survey on 98 samples of iron-rich ore from El Laco (Supplementary Table 1) shows that the content of base metals that are typically enriched in magmatic-hydrothermal systems related to intermediate to felsic igneous rocks in active subduction zones is low (Cu, avg. 1000 µg/g [bdl, 50 µg/g, to 20,000 µg/g]; Mo [bdl, 15 µg/g]; Zn, avg. 70 µg/g [bdl, 40 µg/g, to 160 µg/g]) (see Cooke et al. 2014). However, these ore samples have variable but locally high values of HFSE such as Sn, Nb, Th, and LREE. Niobium shows a variable but significant anomaly (15–800 µg/g; avg. 210 µg/g), as does Sn (50–2100 µg/g; avg. 200 µg/g). These latter values are well above those of average Cenozoic Andean andesite, which are 11 µg/g Nb and 2 µg/g Sn, respectively (data from GEOROC database, https://georoc.eu/, downloaded on 1 March 2022).
Enrichment in high field strength elements is consistent with the mineralogy of the El Laco system. The pegmatite and the subvolcanic massive ore contain several minerals rich in Nb, REE, Ti, Sn, Th, and Zr. At Laco Sur, we have found abundant < 100 µm inclusions of thorite, columbite, euxenite, Nb-Sn oxides (including foordite?), and various complex and as yet undetermined Nb-(Sn-Th-Ce-(U-Nd-Y)) oxides, phosphates, and carbonates ranging in size from a few µm to 190 µm. The degassing tubes in the stratabound ores of Laco Sur (Henriquez and Martin 1978) host abundant rutile, monazite, davidite, thorite, Fe-phosphate, and other REE-bearing minerals accompanying fluorite (Tornos et al. 2017b). The pegmatite has Nb-rich titanite and Nb-bearing ilmenite.
Not surprisingly, enrichment in HFSE is a characteristic of magnetite-(apatite) deposits worldwide, in some cases with a significant enhancement in U, such as at Kiruna, SE Missouri, Olympic Dam, the Adirondacks, and the Bafq district (Daliran 2002; Harlov et al. 2002, 2016; Moore and Modabberi 2003; Torab and Lehmann 2007; Valley et al. 2011; Ehrig et al. 2012; Taghipour et al. 2015a; Apukhtina et al. 2017; Mercer et al. 2020; Yan and Liu 2022). This enrichment in HFSE is also consistent with the late immiscibility of Nb-REE-rich carbonate melts in the melt inclusions of Pietruszka et al. (2023a) at El Laco. However, El Laco and probably most of the Andean magnetite-(apatite) deposits seem to be relatively less enriched in REE than other deposits worldwide.
Discussion: the origin and evolution of magnetite-(apatite) systems
Crystallization of iron-rich melts or hydrothermal origin?
The ultimate origin of magnetite-(apatite) systems is an ongoing topic of major debate. Proposed hypotheses include a hydrothermal origin, with replacement of intermediate-mafic volcanic rocks by sedimentary brines (e.g., Barton and Johnson 1996; Rhodes et al. 1999), or magmatic-hydrothermal fluids exsolved during the crystallization of underlying intermediate to basic silicate melts (e.g., Hildebrand 1986; Sillitoe and Burrows 2002; Corriveau et al. 2016). In a recent variant of this model, the massive magnetite is envisioned to have formed by the separation of magmatic phenocrysts of magnetite from an underlying, crystallizing, intermediate-basic composition silicate melt when the magnetite grains became attached to ascending bubbles of magmatic-hydrothermal fluid and were later cemented by hydrothermal magnetite precipitating from the same fluid (Knipping et al. 2015).
Other alternative models propose that the ore is the product of the crystallization of iron-rich melts immiscible from a parental silicate magma (Badham and Morton 1976; Förster and Jafarzadeh 1994; Naslund et al. 2002; Chen et al. 2010b; Mungall et al. 2011; Nold et al. 2013; Tornos et al. 2016; Xie et al. 2021 and references therein) or directly from the anatexis of sedimentary ironstones interbedded with phosphorus-rich carbonatic sediments (Frutos and Oyarzun 1975; Mungall et al. 2018) or evaporite-rich sequences (Bain et al. 2020) where crosscut by intruding andesitic dikes. Parak (1991) suggests that these deposits are of volcano-sedimentary origin.
The solvus surface in the melt system SiO2-TiO2-Al2O3-FeOt-CaO-MgO-Na2O-K2O-P2O5-H2O (Fig. 12) is well defined on the basis of extensive work on melt inclusions and experiments (Kamenetsky et al. 2013; Lester et al. 2013a; Hou et al. 2018; Honour et al. 2019; Lledo et al. 2020 and references therein). It shows that in primitive, H2O-poor systems (DSiO2LFe/LSi > 0.4), a melt of dacitic composition (LSi), is immiscible with an iron-rich melt (LFe) that contains significant amounts of silica (ca. 14–46 wt% SiO2) and a composition close to a ferrogabbro or ferrodiorite. This basic iron-rich melt has been observed in layered igneous complexes (McBirney and Nakamura 1974; Jakobsen et al. 2005) and in melt inclusions in andesitic-basaltic rocks (Hurai et al. 1998; Clark and Kontak 2004). These latter authors record the presence of droplets of magnetite ± pyroxene in a rhyolitic glass showing unambiguous immiscibility textures (see Roedder and Weiblen 1971; Philpotts 1978).
Composition-distribution coefficient of silica between iron-rich and silica-rich melts (DSiO2LFe/LSi) diagram showing the miscibility gap in the SiO2-TiO2-Al2O3-FeOt-CaO-MgO-Na2O-K2O-P2O5-H2O system, modified from Kamenetsky et al. (2013). The column on the right includes the approximate composition of the iron-rich melt from poorly evolved (A) to highly evolved (C) systems. Probably, only these late melts separate from the parental melt and form magnetite-(apatite) deposits
Similar melt inclusions are widespread in the host andesite related to the El Laco mineralization (Naslund et al. 2009; Velasco et al. 2016; Pietruszka et al. 2023a). They occur as sieve-like structures in the plagioclase phenocrysts or as roundish inclusions in pigeonite or augite. Their compositions are consistent with rather low DSiO2LFe/LSi values (≈ < 0.6; Fig. 5a). The recent study of Pietruszka et al. (2023a) shows that inclusions in plagioclase include globules that have a phase assemblage identical to that of the magnetite-(apatite) systems, with pigeonite or augite, magnetite, crystals of apatite 0.5–1 µm in size, and some anhydrite. The apatite includes < 200 nm immiscible roundish inclusions of REE-Nb carbonates, quartz, and a low-density gas phase. Macroscopic equivalents of these melt inclusions are found at Kiruna (Fig. 5d), where blebs of pyroxene are hosted by dacite-trachyte (Andersson 2013), and at Cerro del Mercado (Fig. 5c), where cm-sized spheroidal blebs of diopside with minor magnetite, fluorapatite, and anhydrite occur in large cavities, presumably originally infilled by gas, in hosting rhyolite. Recently, Berdnikov et al. (2021) described coexisting microspherules of silicate and magnetite in iron-rich pyroclastic rocks in the Kostenga deposit, Russia.
However, these melt inclusions and rocks are unlikely representatives of the iron-rich melt from which the massive magnetite-(apatite) ores formed; it must have lower DSiO2LFe/LSi values in order to increase the magnetite/pyroxene ratio. Experiments by Lester et al. (2013a), Hou et al. (2018), and Lledo et al. (2020) have shown that under highly oxidizing conditions, an increase in the activity of H2O and high P-F contents can drive DSiO2LFe/LSi to values well below 0.2, dramatically expanding the miscibility gap. The most evolved melts in the experiments of Hou et al. (2018) are iron-rich (up to 40 wt% FeO) and have silica and alumina contents as low as 3 wt% SiO2 and 2 wt% Al2O3; Lledo et al. (2020) have even generated P-Fe-Ca melts of even lower silica contents. The recent theoretical modeling by Keller et al. (2022) using ALPHAMELTS at 100 MPa and 900–1100 °C and various fO2 values reproduced the formation of these immiscible melts, closely matching the results of Hou et al. (2018) and adding support to the hypothesis that iron-rich melts can exist and can produce magnetite-(apatite) mineralization.
Homogenization of the melt inclusions in the host andesite takes place between 900 and 1145 °C (Pietruszka et al. 2023a), marking a minimum temperature of separation of the iron-rich melts. Stable isotope geothermometry at El Laco indicates that the pegmatite described above crystallized at ca. 900–1300 (± 50 °C) (Tornos et al. 2016), although the uppermost temperatures of that range are probably overestimated owing to the steep slopes of ∆18Omineral-fluid at these temperatures. Melt inclusions in the diopside homogenize to a non-aqueous liquid at temperatures as high as 800–950 °C (Bain et al. 2021), consistent with the lack of homogenization at 800 °C (Broman et al. 1999) and the stability of disordered albite (> ≈500 °C). Somewhat lower maximum temperatures of 700–800 °C are recorded at Marcona by Chen et al. (2010a), the Meishan magnetite-(apatite) deposit in China (ca. 780 °C; Li et al. 2015), and other deposits of the Lower Yangtze region, with subsolidus re-equilibration temperatures of 512–602 °C (Zeng et al. 2022).
It should be emphasized that attempts at geothermometry based on the Fe content of actinolite as suggested by Del Real et al. (2021), or the Mg content of magnetite following the methodology of Canil and Lacourse (2020) as has been used, for example, by Ovalle et al. (2022) at El Laco, are erroneous and should be discarded. According to basic thermodynamic definitions, a single element in one mineral alone cannot be used for thermometry. Rather, such calculations violate the fundamental requirements for thermobarometry based on the Gibbs Phase Rule.
The occurrence of magnetite-(apatite) systems at a wide range of crustal depths suggests that the density of the aqueous fluids varies greatly depending on P–T-X conditions (Driesner and Heinrich 2007). By analogy with other magmatic-hydrothermal systems, in the deepest portions (≈ > 200 MPa fluid pressure), the hydrothermal fluid would be dominated by a low- to intermediate-salinity fluid (Redmond et al. 2004). When this fluid intersects the two-phase surface, it would unmix two fluids of contrasting densities and, with further decompression, would intersect the liquid–vapor-halite curve. The liquid phase would be consumed, leaving only low-salinity, low-density vapor. The location of magnetite-(apatite) deposits at highly variable crustal depths suggests that their formation is independent of the phase state of the saline-aqueous hydrothermal fluid. This is also implied by the results of Bain et al. (2020 and 2021), who showed that polycrystalline melt inclusions coexist in some systems with only aqueous brine inclusions; in others with brine inclusions and vapor inclusions; and in yet others with only vapor inclusions and without coexisting brines. This wide variability in the phase state of the hydrothermal fluid has profound implications for the genetic models mentioned above. In deep systems such as the Adirondacks (Valley et al. 2011), magnetite cannot coexist with aqueous vapor, raising serious issues with any genetic model wherein magnetite could ascend attached to vapor-rich bubbles as proposed by Knipping et al. (2015). Indeed, recent studies have largely concluded that buoyant ascent of bubbles through relatively viscous silicate melts is inefficient and that the bulk of aqueous fluid release is only accomplished at high degrees of crystallinity where grain-supported channels can develop (Parmigiani et al. 2016; Lamy-Chappuis et al. 2020), again raising serious doubts that magnetite attached to bubbles could ascend through a magma chamber at all. Furthermore, experiments of Pleše et al. (2019) show that the long-distance transport of magmatic phenocrysts in a turbulent aqueous fluid is unlikely because of the rapid separation of gas and crystals. For this mechanism to work, several silicate phases with similar wetting angles to magnetite should be common in the magnetite-(apatite) systems (Pleše et al. 2018) and not just a special case for El Laco. Toward the surface, the relative proportion of the low-density fluid increases dramatically at the same time as its salinity decreases, leading at the surface to a fluid with > 99% gas and a (hydro)-saline melt. Models predict that at El Laco, as in any volcano, the existence of liquid water is thermodynamically unlikely (Tornos et al. 2016), inhibiting the precipitation of magnetite by the metasomatic replacement of andesite by hydrothermal fluids. A hydrothermal origin for the massive magnetite-(apatite) and pegmatite ores is also not supported by the abundance of melt inclusions in magnetite, diopside, and apatite, the melt inclusions in the andesite, the high temperature of formation, the lack of liquid-rich fluid inclusions (Bain et al. 2021), and the presence in the massive ore and pegmatite of unequivocal magmatic phases such as pigeonite and enstatite.
Crustal contamination is the key control for magnetite-(apatite) systems
At El Laco, magnetite-(apatite) rocks are in isotopic disequilibrium with the host andesite; Sr–Nd-Pb isotopes reveal a significant upper crustal component not found in the adjacent andesite. The Sr isotopic ratios of the massive ore (0.7080–0.7085) are indicative of mixing of andesite melt with upper crustal continental rocks that could well be the shallow marine to terrestrial sedimentary rocks of the Salta Group (Hauterivian-Oligocene) with 87Sr/86Sr values of 0.714–0.7156 (Sial et al. 2001) and located ca. 1.5–6 km beneath the volcanic arc (Scheuber et al. 2006). Alternatively, crustal contamination could also involve deeper Silurian-Devonian basement rocks including ironstones, carbonates, and phosphatic levels (Frutos and Oyarzun 1975; Tornos et al. 2017b; Pietruszka et al. 2023b). In fact, the andesite at El Laco and the nearby Lascar volcano host enclaves of former carbonate rocks (Matthews et al. 1996; Naranjo et al. 2010). Hou et al. (2012) have also reported radiogenic Sr values in magnetite-(apatite) deposits in the Lower-Middle Yangtze district.
The magnetite ore at El Laco shows significantly lower 206Pb/204Pb values than the host andesite, and the Late Paleozoic-Early Mesozoic model ages (Pietruszka et al. 2023b) suggest that at least some of the Fe is inherited from a U-Th-poor reservoir formed at ca. 370–170 Ma, thus significantly pre-dating the host andesite. Deposits in the American Cordillera also show a systematic shift in 87Sr in comparison to their associated volcanic rocks (Tornos et al. 2017b, 2021; Levresse et al. 2020). The δ18Omagnetite values from El Laco (Rhodes and Oreskes 1999; Nyström et al. 2008; Tornos et al. 2017b; Childress et al. 2020), as well as in many other magnetite-(apatite) systems (see Jonsson et al. 2013; Tornos et al. 2017b; Troll et al. 2019; Childress et al. 2020; Weis et al. 2021), are consistent with precipitation from melts or high-temperature magmatically derived hydrothermal aqueous fluids. They are non-diagnostic, however, as they only reflect equilibration at high temperatures with isotopically heavy reservoirs. However, the recalculated triple oxygen isotope data of Childress et al. (2020) at El Laco plot in a MIF-O (Mass Independent Fractionation-Oxygen) trend of ∆,17O0.528 (ppm) values between − 58 and − 189 for a rather constant δ18Omagnetite values of 3.0 to 3.7 per mil. The ∆,17O0.528 values are in the range of other magnetite-(apatite) deposits elsewhere (Peters et al. 2019, 2021), including ∆,17O0.528 signatures as low as − 357 at Kiruna. These values reflect oxygen inheritance from a MIF source like evaporites, but plot away from orthomagmatic and magmatic-hydrothermal magnetite in equilibrium with typical silicate melts. The δ56Fe values of the ores are within and slightly above values for basalt-andesite (Troll et al. 2019; Childress et al. 2020; Xie et al. 2021), but are inconclusive about the origin of these rocks as they are close to average crustal reservoir compositions including banded iron formations (Beard et al. 2003). Also, the variable values of the Fe3+/Fe2+ ratio predicted at El Laco would be expected to strongly affect the δ56Fe values (Dauphas et al. 2017).
Bain et al. (2020, 2021) and Steele-MacInnis et al. (2021) have shown that in many magnetite-(apatite) systems, including El Laco, the pegmatite and iron-poor caps host abundant inclusions enriched in sulfates, carbonates, and chlorides, with non-silicates making up a significant proportion of the inclusions. In shallow systems such as El Laco, they could theoretically represent hot (Th > 780 °C) hydrosaline melts separating from a dominant low-density gas phase during shallow boiling (Tornos et al. 2016). However, the existence of significant amounts of silicates that are only sparingly soluble in water, the high temperature of homogenization (> 780 °C) to a non-aqueous melt, and the variable depths of formation (in some cases above and in other cases below the two-phase brine-vapor immiscibility surface) exclude the possibility that they represent heterogeneously trapped aqueous fluids.
The mesoscopic equivalents of these salt-rich inclusions are perhaps the diopside-anhydrite pegmatite and anhydrite masses at El Laco (Fig. 7c) or the abundant anhydrite veins elsewhere such as Hemushan and Meishan in the Lower-Middle Yangtze district (Zhou et al. 2013; Li et al. 2015; Luo et al. 2015), Kiruna (Nordstrand and Andersson 2013), the Coastal Cordillera of the Andes (Velasco and Tornos 2009; Tornos et al. 2021), and the Bafq (Förster and Jafarzadeh 1994) and SE Missouri (Nold et al. 2014) districts, as well as the carbonate-apatite-titanite dikes at Buena Vista mine, Nevada (Bain et al. 2020).
Regional geology supports these relationships, as magnetite-(apatite) deposits worldwide are systematically located above evaporite-bearing sequences or their metamorphic equivalents (Table 1). The only exception is the Coastal Cordillera of the Andes, where the mineralization crosscuts various, mainly juvenile Paleozoic terranes. Here, the most likely source of intermediate melts is the lower crust in the upper plate, where it has been percolated by fluids derived from the dehydration of crustal rocks in the subducting slab (Tornos et al. 2021).
The HFSE enrichment of magnetite-(apatite) systems is also likely related to the interaction of the juvenile melts with continental crust. Lester et al. (2013b) have experimentally obtained high KD values of HFSE (Nb, Zr, Th) between iron-rich melts and felsic melts where the system is oxidized (fO2 ≥ NNO), phosphorus-fluorine-rich and H2O-saturated, and, thus, less strongly polymerized (Ryerson and Hess 1978).
An alternative model to crustal contamination is that proposed by Matthews et al. (1995), who suggested that the formation of immiscible droplets of magnetite at the Lascar Volcano is due to mixing between basaltic andesite and strongly oxidized, anhydrite-bearing dacitic melts.
If the crustal contamination hypothesis is correct, then magnetite-(apatite) rocks form by a process similar to that yielding magmatic sulfides by separation of metal-rich silica-poor melts due to contamination of juvenile melts by pyrite-rich shale (Naldrett 1989). The oxidized vs. reduced nature of the contaminant is what probably marks the difference between sulfide- and iron oxide-rich melts.
Magnetite-(apatite) deposits are volatile-rich but low-silica mineral systems
The abundance of anhydrite and scapolite in magnetite-(apatite) systems is indicative of major enrichment in SO4 and Cl. Fluorapatite is the key phase in most magnetite-(apatite) systems, whereas fluorite has been described at El Laco (Tornos et al. 2017b) and several mines in the Adirondacks (Valley et al. 2011). In several deposits, the presence of F-rich actinolite and titanite also suggests high F activities; extreme F enrichment is exemplified by Vergenoeg (South Africa), the largest fluorite resource on Earth; it probably represents a reduced, phosphate-poor, fluorite-rich end member of these systems but having many geological features akin to El Laco (see Fourie 2000; Goff et al. 2004).
The systematic association of magnetite with P-F-SO4-Cl-bearing minerals is interpreted here as a critical constraint for the formation of iron-rich melts. The presence of even small amounts of phosphorus as a key fluxing agent favors low DSiO2LFe/LSi values, depressing melting temperatures and viscosities and hence enabling efficient separation of low Si-Al iron-rich melts (Philpotts 1967; Visser and Koster Van Groos 1979; Philpotts and Doyle 1983). Fluorine and even sulfate produce equivalent effects (Lester et al. 2013a; Hou et al. 2018), whereas sulfate also imposes elevated redox conditions on the system. Mungall et al. (2018) have also proposed that contamination by carbon-rich rocks is key in promoting the formation of iron-rich melts, which is consistent with the presence of carbonates in the assemblage and melt inclusions (Bain et al. 2020, 2021).
Monomineralic magnetite melts at temperatures exceeding 1590 °C (Blaney 2007), well above the highest recorded temperatures of the upper crust of ~ 1345 °C at 0.2 GPa (Falloon et al. 2007) and making theoretically unlikely that an iron oxide-rich melt can form in the Earth’s crust. There are no data available for the solidus in the Fe3O4-P2O5 system, but that of the Fe–P system is close to 1040 °C (Okamoto 1990) and that of the Fe2O3-P2O5 system is 1070 °C (Zhang et al. 2011). The addition of fluorine on top of phosphorus drops the solidus of the system even more, to temperatures recorded in the magnetite-(apatite) systems, as low as 650 °C (Lledo et al. 2020). In addition, iron-rich melts can contain 2–4 wt% H2O (Hou et al. 2018), which can also further depress the solidus. These temperatures are more consistent with those observed in magmatic arcs and suggest that only the addition of fluxing elements can allow the formation, coalescence, and separation of the iron-rich melts from a parental contaminated andesitic melt.
In the iron-rich melts, the low alumina and silica contents inhibit the crystallization of quartz, feldspars, micas, and Al-bearing amphibole and pyroxene and promote the partitioning of incompatible Na and K into the residual (aqueous) phase. To our knowledge, feldspars are exclusively described in the pegmatite at El Laco, and quartz is rarely found elsewhere. Furthermore, the oxidized nature of the system inhibits the formation of the Fe-rich endmembers of hedenbergite, ferro-actinolite, and fayalite (Fig. 13). The low silica contents only allow the crystallization of small amounts of actinolite and diopside and limit the formation of the andradite-grossular solid solution, which are the stable calcic-iron silicates at high fO2 (Burton et al. 1982).
Estimation of silica content in an aqueous fluid in equilibrium with an evolved iron-rich melt. At high fO2, the presence of actinolite or diopside + magnetite without quartz or feldspars indicates that prior to water/rock interaction, the system was silica-alumina deficient. At a fixed fO2 (≈HM-1), calcic garnet + actinolite + magnetite are stable at higher silica contents than actinolite or diopside + magnetite, explaining why calcic garnet is found where magnetite-(apatite) systems are dominantly hosted by felsic rocks. The curve enstatite-forsterite marks the approximate limit between mafic and ultramafic rocks. Curve (1) represents silica content buffered by quartz saturation (Brooks and Steele-MacInnis 2019), whereas curves (2) and (3) represent silica contents buffered by the (quartz-absent) assemblages enstatite-forsterite and hedenbergite-andradite-magnetite, respectively. Equilibria of the latter two buffer assemblages were calculated using SUPCRTBL (Zimmer et al. 2016). Due to the lack of reliable thermodynamic data, ferro-actinolite is assumed to be similar to hedenbergite. The immiscibility surface of aqueous fluid from Driesner and Heinrich (2007)
The presence of “grandite” in the Iron Mountain deposit in Missouri (Fig. 3c; Nold et al. 2014), the Bafq district in Iran (Taghipour et al. 2015b), the Middle-Lower Yangtze area in China (Li et al. 2015), or at El Laco (Naranjo et al. 2010) could be interpreted as somewhat higher activity of SiO2, but even here the aSiO2 is insufficient to stabilize more siliceous minerals.
This outstanding Si-Al depletion of magnetite-(apatite) rocks stands in stark contrast to the Si-Al-rich mineralogy of magmatic-hydrothermal systems in porphyry settings, even in the more mafic-associated ones. Porphyry systems associated with diorite have an early assemblage including quartz, biotite, adularia, and magnetite (Sillitoe 2010), indicating that quartz saturation was quickly attained at hydrothermal conditions. The porphyry-skarn Grasberg-Ersberg deposits in Indonesia are related to diorite-monzodiorite at 55–56 wt% SiO2 (Loucks 2014), similar to the andesite at El Laco (52–63 wt% SiO2; Tornos et al. 2017b) or in the Coastal Ranges of Chile (50–62 wt% SiO2; Parada et al. 2007), which suggests that an aqueous fluid separating from an andesitic melt should precipitate an assemblage with significant amounts of quartz and Si-Al-bearing minerals. In fact, even hydrothermal systems associated with basaltic magmatism are generally associated with the formation of extensive quartz veins, for example, those associated with submarine black smokers (Schwarzenbach and Steele-MacInnis 2020). Hence, the general paucity and even complete absence of quartz from magnetite-(apatite) ores represents a serious objection to any genetic model that invokes a central role of aqueous hydrothermal fluids.
On the basis of these arguments, we infer that hydrothermal fluids in equilibrium with intermediate igneous rocks did not play a substantial role in the formation of these mineral systems. Simply put, authors that propose a key connection between magnetite-(apatite) systems with magmatic-hydrothermal fluids derived from degassing of silicate-rich melts are not able to explain why a fluid in chemical equilibrium with andesite would precipitate magnetite, actinolite or diopside, apatite, and scapolite only during simple cooling in an open space, rather than large amounts of quartz, feldspars, and biotite, nor can they explain why these systems are enriched in HFSE and not in Cu, Mo, Au, or Zn.
Magnetite-(apatite) ore systems as natural analogs of industrial smelting
Along with the presence of large bodies of massive magnetite, the characteristic feature of magnetite-(apatite) systems is the existence of an upper iron-depleted zone rich in Ca-Mg silicates and apatite (Fig. 1). Both features are unique in these systems and are not described in other mineral systems such as porphyries, skarns, and other magmatic-hydrothermal systems.
We interpret the distinct vertical zonation in many magnetite-(apatite) systems, especially at El Laco, as reflecting an evolution similar to that of the industrial process of iron smelting. This process involves the initial separation of two immiscible melts: a dense iron-rich one that forms a “matte” of pig iron and a less dense buoyant slag that is removed from the smelting system (e.g., Stoughton 2018; Cameron et al. 2019). In smelters, the formation of slag removes impurities and is promoted by the addition of carbonates and other fluxing agents and enhanced significantly by a high flux of air, run at a constant high temperature, and a desirable low silica content of the iron ore. The effectiveness of the smelting process is based on the quick and efficient separation of immiscible layers and the separation of the matte from a slag containing as little iron as possible. After it solidifies, the industrial slag is dominated by Ca-Mg pyroxene and calcium phosphates.
Upon the emplacement of the iron-rich magma in natural magnetite-(apatite) systems, geologic observations suggest that the dominant, less viscous, and denser (e.g., ≈5.0–5.2 g/cm3) liquid, with a composition close to that of iron oxide, separates from a subordinate, more viscous, and less dense (e.g., ≈2.9–3.2 g/cm3) phase that incorporates the available silica, calcium, magnesium, phosphorus, titanium, sulfate, and volatiles. When this latter liquid crystallizes, it forms the iron-depleted cap in the upper part of the system. We contend that the formation of this iron-poor cap is critical for the formation of iron-rich ores, as this process facilitates the separation of most of the impurities and increases the grade of the iron ore. Probably, optimal conditions are attained in highly evolved systems with low Si-Al contents (see below) that have the lowest viscosities, which facilitates efficient separation between layers. The magnetite-bearing pegmatite located between the “slag zone” and the massive magnetite is interpreted here as a transition zone caused by inefficient separation, which is not expected in highly productive engineered industrial blast furnaces, where the temperature and thermal gradient can be more carefully controlled. Natural systems are less thermally and chemically efficient than smelters, and the scale is vastly different; whereas smelters can accommodate on the order of 103 m3, individual natural systems such as Kiruna in Sweden can be up to 109 m3 in volume.
The zonation predicted in magnetite-(apatite) systems changes when the iron-rich melts arrive at the surface, quench, and degas with widespread partitioning of H2O, S, F, and P and S into the vapor phase (Gilbert and Williams-Jones 2008; Wallace et al. 2015). Quenching and degassing inhibit the formation of a slag zone and precipitation of apatite, which explains why shallow systems like El Laco are relatively apatite-poor.
The role of H 2 O in ore formation
Raman spectroscopic analyses of the experimental products of Hou et al. (2018) suggest that H2O partitions into the silica-rich melt rather than the iron-rich one, with an H2O concentration ratio between the two melts of approximately 2:1. To propose an anhydrous iron-rich melt would contradict several lines of evidence: (1) magnetite-(apatite) deposits formed under high H2O pressures, leading to the presence and abundance of hydrous minerals such actinolite and scapolite; (2) these deposits host abundant diatreme-like structures; and (3) aureoles of alteration are common and widespread around the melt inclusions (Fig. 5a), the immiscible droplets of melt quenched to magnetite (Fig. 5b) and even the orebodies themselves. Furthermore, the crystallization of magnetite plays a major role in the evolution of these systems. Mass balance estimates (Supplementary Fig. 2) suggest that even at low initial contents of H2O, the crystallization of magnetite dramatically enriches the residual melt in H2O. Modeling shows that the crystallization of 80% (by mass) of the melt as magnetite, which would be consistent with the high magnetite:cap ratio, could lead to residual melts with H2O contents above 20 wt%. These high values suggest that the iron-rich melt would generally exsolve large amounts of water from the early stages of crystallization onward.
The evolution of magnetite-(apatite) systems
There are few descriptions of high DSiO2LFe/LSi iron-rich melts before coalescing to form significant orebodies. The best examples so far are the melt inclusions described by Hurai et al. (1998), Clark and Kontak (2004), Velasco et al. (2016), and Pietruszka et al. (2023a). However, these melt inclusions are not strictly the parental melts to the ore but are possible “snapshots” of the system trapped on the solvus at high DSiO2LFe/LSi values; further cooling should drive compositions of the immiscible melts to the more extreme compositions where magnetite-(apatite) systems form.
More proximal to the massive ores are the droplet-shaped grains of magnetite at Kiruna (Andersson 2013), the Lascar volcano (Matthews et al. 1995), or in the Coastal Cordillera of the Andes, where droplets of an iron-rich melt are preserved in microgabbro (Fig. 5b; see also Aguirre 2001). The contrasting viscosities and densities between the iron-rich melt and the felsic end member promote their efficient physical separation and explain why felsic rocks are usually left behind. Zhang et al. (2020) have developed a model showing that the segregation, coalescence, and migration of low-viscosity, high-density iron-rich melts such as sulfide and carbonatitic melts from silicate melts is physically viable.
Emplacement of low-viscosity, high-density mature melts with low DSiO2LFe/LSi values at various crustal depths takes place after ascent along conduits in a tectonic regime dominated by horizontal stresses such as tensional fractures and dilatational jogs in intracontinental strike slip-structures (Coastal Cordillera Andes, Cembrano et al. 2005, Tornos et al. 2021; Bafq, Daliran 2002; Kiruna, Bauer et al. 2018) or by caldera collapse (Bafq, Förster and Jafarzadeh 1994; Missouri district, Day et al. 2016; Frutos and Oyarzun 1975, Keller et al. 2022; Lower to Middle Yangtze, Yu et al. 2011 and Cerro del Mercado Lyons 1988) (Table 1).
Steady-state cooling and crystallization should lead to the formation of large bodies of massive magnetite as discordant or sill-like bodies (Table 1); sometimes, evidence of comingling between dacitic and iron-rich melts has been reported (Chen et al. 2010b). In rather deep systems (> ca. 0.5 km), the cooling iron-rich melt should progress into a refining process with the separation of a dense iron-rich melt and the flotation of a slag. During cooling, the slag enters a complex and poorly understood regime in which several immiscible melts form in a similar fashion as in industrial smelters (Gaskell 2007; Zhang et al. 2011). They include, at least, the formation of Fe-(Ca)-P-SO4 (Mungall et al. 2018; de Fourestier 2019), P-Ca–Si-Mg-(Fe) (Hou et al. 2018; Lledo et al. 2020), REE-CO3-Cl (Pietruszka et al. 2023a), and sulfate-carbonate-rich melts (Bain et al. 2020, 2021) accompanied by variable amounts of felsic melts.
In our current interpretation, the melt inclusions described by Broman et al. (1999), Li et al. (2015), and Bain et al. (2020, 2021) do not record the original iron-rich melts from which the massive magnetite crystallized, but later melts formed after the crystallization of large amounts of magnetite. This interpretation is consistent with the findings of Pietruszka et al. (2023a), showing that carbonates and sulfates form only a small part of the bulk melt and are trapped as immiscible melts inside apatite. Local impoverishment of the elements on the x-axis of Fig. 12 due to magnetite-slag crystallization should eventually drive the residual melt again into the immiscibility field, leading to the separation of Si-Al-bearing liquids to form rocks such as the plagioclase-rich facies of the diorite, quartz, and feldspars in melt inclusions, or the late silica-rich rocks (de Fourestier 2019; Fig. 11f of Tornos et al. 2021).
In subaerial environments, quick ascent and explosion were promoted by high contents of volatile species undergoing low-pressure exsolution to form a frothy gas–melt slurry (Keller et al. 2022). This is a well-studied mechanism of emplacement of gas-saturated basalt into volcanoes, in which case the low viscosity of the melt is a more important factor than its high density (Wilson and Head III 1981; Woods and Cardoso 1997; Polozov et al. 2016).
Exsolution of large amounts of hot, alkaline, hypersaline, and iron-saturated fluids enriched in Na, K, Ca, Mg, and other elements transported as chloride complexes would produce a large alteration zone enriched in alkali feldspars, actinolite, and magnetite but depleted in quartz, which is characteristic of magnetite-(apatite) systems.
Facies of the El Laco system resemble the pyroclastic hematite described by Hynek et al. (2002) in Terra Meridiani, Mars, and the ferrovolcanism described by Soldati et al. (2021) on the 16 Psyche asteroid. Magnetite-(apatite) systems are also similar to magmatic magnetite ± apatite rocks found in carbonatites (Groves and Vielreicher 2001; Veksler and Lentz 2006; Chen et al. 2013; Mikhailova et al. 2016; Kamenetsky et al. 2021; Palmieri et al. 2022) and that host inclusions rich in carbonates, sulfates, halides, phosphates, minor oxides, and sulfides, reflecting complex processes of immiscibility (Kjarsgaard and Hamilton 1988) and the transition from magmatic to hydrothermal conditions (Vartiainen and Paarma 1979; Krasnova et al. 2004; Veksler and Lentz 2006; Guzmics et al. 2012; Chen et al. 2013; Nikolenko et al. 2018; Bodnar and Frezzotti 2020; Feng et al. 2020; Walter et al. 2021). The P-Ca–Si-Mg-(Fe) slag in magnetite-(apatite) systems has a composition similar to that of phoscorite, also interpreted as an immiscible melt in carbonatitic systems (Kjarsgaard and Hamilton 1988; Lee et al. 2004; Giebel et al. 2019), although their ultimate origin is different.
The compositions and amounts of crustal rocks assimilated by the silicate melt control the composition of the iron-rich melts and probably are the decisive factors determining the differences between districts in the size of the orebodies, depth of emplacement, amount of slag, and types and abundance of immiscible melts. As a result, the melt inclusions have various compositions, with sulfates more than carbonates at El Laco, predominantly carbonates at Buena Vista in Nevada, and carbonate plus sulfates in more or less equal amounts at Iron Springs in Utah (Bain et al. 2020, 2021).
The most important factor in the formation of high-grade orebodies is the efficiency of separation between the iron-rich melt and the slag, with only the most evolved systems leading to a well-developed vertical zonation (Fig. 1). At Kiruna, zones of almost pure magnetite with P values less than 0.1% P (the LKAB B ore) irregularly alternate with others enriched in phosphorus (> 0.1 to > 2.2 wt% P: the LKAB D ore; Niiranen 2006). In some other deposits, the massive magnetite ore also hosts large irregular to spheroidal masses of apatite ± actinolite ± diopside ± magnetite (Fig. 5e), interpreted here as “slag” trapped within the crystallizing magnetite-(apatite)-forming melt.
Conclusions
Geological observations, melt inclusion investigations, stable and radiogenic isotope geochemistry, experimental data, thermodynamic constraints, and numerical modeling are consistent with magnetite-(apatite) systems as the product of crystallization of Fe-rich but Si-Al-poor, dominantly oxidized, ultrabasic melts. These lines of evidence are broadly in agreement with the conclusions of early authors since Geijer (1910) and Park (1961), who suggested the existence of magmatic magnetite mineralization. These ultrabasic melts are more akin to carbonatites than to magmatic-hydrothermal systems related to intermediate to basic silicate magmatism.
In well-developed evolved systems, the vertical zonation includes a large magnetite-only zone capped by Ca-Mg-(Fe) silicates and apatite via an intermediate pegmatitic magnetite ± pyroxene ± actinolite ± apatite zone, identical to that observed in industrial iron smelters. This zonation is formed by the evolution and separation of several immiscible melts with contrasting compositions.
Although these rocks are saturated with Ti–rich phases such as titanite and ilmenite, the Ti concentration of the massive magnetite ore is invariably low (< 1 wt% Ti) and well below the Ti content of magnetite in the associated host igneous rocks. Gradual reduction of the highly oxidizing system leads to the formation of pegmatites with Ti–rich magnetite and, finally, ilmenite or rutile in the most evolved rocks, showing that Ti-in-magnetite is not solely dependent on temperature, magmatic vs. hydrothermal origin, or the depth of formation, but is dominantly controlled by the fO2 of the system.
Many of the features of magnetite-(apatite) systems cannot be explained by alternative models advocating buoyancy of magnetite phenocrysts within a melt, nearly complete replacement of the host andesite by magmatic-hydrothermal brines exsolving from intermediate silicate magmas, and convection of basinal fluids. The dearth of hydrothermal quartz is a critical oversight in any such hydrothermal model.
Iron-rich magnetite-(apatite) melts are dominantly formed by the interaction of silicate magma with oxidized sediments. The ultimate factor governing their formation is the existence of a miscibility gap between iron-rich melts enriched in fluxes like P, F, and SO4 and having a high density and low viscosity, coexisting with a felsic silicate melt of relatively low density and high viscosity. The enrichment in REE, Sn, Nb, and other HFSE is inherited from the contamination by upper crustal rocks and the preferential partitioning of HFSE in the iron-rich melt.
This genetic interpretation emphasizes the important role of melt as an ore-forming mechanism, a process that may be more common than previously recognized.
References
Africano F, Van Rompaey G, Bernard A, Le Guern F (2002) Deposition of trace elements from high temperature gases of Satsuma-Iwojima volcano. Earth Planet Space 54:275–286
Aguirre R (2001) Magmatic iron ore breccias in the Chilean Coastal Cordillera. La Falda and Bronce Sur Mines, Pleito Melón district, III Region Chile. Master Thesis, Binghampton University
Alva-Valdivia LM, Rivas ML, Goguitchaichvili A, Urrutia-Fucugauchi J, Gonzalez JA, Morales J, Gómez S, Henríquez F, Nyström JO, Naslund RH (2003) Rock-magnetic and oxide microscopic studies of the El Laco iron ore deposits, Chilean Andes, and implications for magnetic anomaly modeling. Int Geol Rev 45:533–547. https://doi.org/10.2747/0020-6814.45.6.533
Andersson UB (2013) Coeval iron oxide and silicate magmas; structural evidence for immiscibility and mingling at Kirunavaara and Luossavaara, Sweden. Proceedings 12th SGA Biennial Meeting 2013, Uppsala, pp 1635–1637
Apukhtina OB, Kamenetsky VS, Ehrig K, Kamenetsky MB, Maas R, Thompson J, McPhie J, Ciobanu CL, Cook NJ (2017) Early, deep magnetite-fluorapatite mineralization at the Olympic Dam Cu-U-Au-Ag deposit, South Australia. Econ Geol 112:1531–1542. https://doi.org/10.5382/econgeo.2017.4520
Atapour H, Aftabi A (2017) The possible synglaciogenic Ediacaran hematitic banded iron salt formation (BISF) at Hormuz Island, southern Iran: implications for a new style of exhalative hydrothermal iron-salt system. Ore Geol Rev 89:70–95. https://doi.org/10.1016/j.oregeorev.2017.05.033
Audetat A, Lowenstern JB (2014) Melt inclusions. In: Holland HD, Turekian KK (eds) Treatise on geochemistry. Elsevier Science, v. 13, pp 143–171
Babaitsev I, Kuznetsov O (2001) Energy of explosions occurring when water falls onto a layer of molten metal. Metallurgist 45:185–188
Badham JPN, Morton RD (1976) Magnetite–apatite intrusions and calc-alkaline magmatism, Camsell River, N.W.T. Canad J Earth Sci 13:348–354. https://doi.org/10.1139/e76-037
Bain WM, Steele-MacInnis M, Li K, Li L, Mazdab FK, Marsh EE (2020) Carbonate-sulfate melts in iron oxide-apatite deposits. Nat Geosci 13:751–757. https://doi.org/10.1038/s41561-020-0635-9
Bain WM, Steele-MacInnis M, Tornos F, Hanchar JM, Creaser EC, Pietruszka DK (2021) Evidence for iron-rich sulfate melt during magnetite(-apatite) mineralization at El Laco, Chile. Geology 49:1044–1048. https://doi.org/10.1130/G48861.1
Barton MD, Johnson DA (1996) Evaporitic source model for igneous-related Fe oxide-(REE-Cu-Au-U) mineralization. Geology 24:259–262. https://doi.org/10.1130/0091-7613
Barton MD, Girardi JD, Kreiner DC, Seedorff E, Zurcher L, Dilles JH, Haxel GB, Johnson DA, Steininger R, Pennell W (2011) Jurassic igneous-related metallogeny of southwestern North America. Geological Society of Nevada, Great Basin Evolution and Metallogeny, pp 373–396
Bastrakov EN, Skirrow RG, Didson GJ (2007) Fluid evolution and origins of iron oxide Cu-Au prospects in the Olympic Dam district, Gawler craton, South Australia. Econ Geol 102:1415–1440
Bauer TE, Andersson JBH, Sarlus Z, Lund C, Kearney T (2018) Structural controls on the setting, shape, and hydrothermal alteration of the Malmberget iron oxide-apatite Deposit, Northern Sweden. Econ Geol 113:377–395. https://doi.org/10.5382/econgeo.2018.4554
Beard BL, Johnson CM, Von Damm KL, Poulson RL (2003) Iron isotope constraints on Fe cycling and mass balance in oxygenated Earth oceans. Geology 31:629–632. https://doi.org/10.1130/0091-7613(2003)031
Berdnikov N, Nevstruev V, Kepezhinskas P, Krutikova V, Konovalova N, Astapov I (2021) Silicate, Fe-oxide, and Au–Cu–Ag microspherules in ores and pyroclastic rocks of the Kostenga iron deposit, in the Far East of Russia. Russ J Pac Geol 15:236–251
Blaney L (2007) Magnetite (Fe3O4): Properties, synthesis, and applications. Lehigh Rev 15:1–50
Bodnar RJ, Frezzotti ML (2020) Microscale chemistry: Raman analysis of fluid and melt inclusions. Elements 16:93–98
Bookstrom AA (1977) The magnetite deposits of El Romeral, Chile. Econ Geol 72:1101–1130. https://doi.org/10.2113/gsecongeo.72.6.1101
Broman C, Nyström JO, Henriquez F, Elfman M (1999) Fluid inclusions in magnetite-apatite ore from a cooling magmatic system at El Laco, Chile. GFF 121:253–267. https://doi.org/10.1080/11035899901213253
Brooks HL, Steele-MacInnis M (2019) A model for the solubility of minerals in saline aqueous fluids in the crust and upper mantle. Am J Sci 319:754–787. https://doi.org/10.2475/09.2019.02
Broughm SG, Hanchar JM, Tornos F, Westhues A, Attersley S (2017) Mineral chemistry of magnetite from magnetite-apatite mineralization and their host rocks: examples from Kiruna, Sweden, and El Laco, Chile. Miner Dep 52:1223–1244. https://doi.org/10.1007/s00126-017-0718-8
Brown L, Singer BS, Barquero-Molina M (2021) Paleomagnetism and 40Ar/39Ar chronology of ignimbrites and lava flows, Central Volcanic Zone, Northern Chile. J S Am Earth Sci 106:103037. https://doi.org/10.1016/j.jsames.2020.103037
Buchanan AL (2015) Timing and evolution of metasomatic alteration and mineralization in the Lyon Mountain Granite and related iron oxide apatite (IOA) ores; constraints from apatite and titanite U-Pb geochronology, Sm-Nd isotopes and trace-elements. Master Thesis, Memorial University of Newfoundland
Burgisser A, Scaillet B (2007) Redox evolution of a degassing magma rising to the surface. Nature 445:194–197
Burton JC, Taylor LA, Chou I (1982) The fO2-T and fS2-T stability relations of hedenbergite and of hedenbergite - johannsenite solid solutions. Econ Geol 77:764–783
Calvo G, Tornos F, Velasco F (2013) The geology of the giant Pampa del Pongo magnetite skarn (S Peru) and its relationship with IOCG systems. In: Proceedings 12th SGA Biennial Meeting, Uppsala, pp 1355–1358
Cameron I, Sukhram M, Lefebvre K, Davenport W (2019) Blast furnace ironmaking. Analysis, control, and optimization. Springer
Canil D, Lacourse T (2020) Geothermometry using minor and trace elements in igneous and hydrothermal magnetite. Chem Geol 541:119576
Carriedo J, Tornos F (2010) The iron oxide copper gold belt of the Ossa Morena Zone, SW Iberia: implications for IOCG genetic models. In: Porter TM (ed) Hydrothermal Iron Oxide Copper-Gold and Related Deposits: A Global Perspective. PGC Publishing, Adelaide, pp 441–460
Cembrano J, Gonzalez G, Arancibia G, Ahumada I, Olivares V, Herrera V (2005) Fault zone development and strain partitioning in an extensional strike-slip duplex: a case study from the Mesozoic Atacama fault system, Northern Chile. Tectonophysics 400:105–125
Charlier B, Namur O, Bolle O, Latypov R, Duchesne J-C (2015) Fe–Ti–V–P ore deposits associated with Proterozoic massif-type anorthosites and related rocks. Earth-Sci Rev 141:56–81. https://doi.org/10.1016/j.earscirev.2014.11.005
Charoy B, Noronha F (1996) Multistage growth of a rare element volatile-rich microgranite at Argemela (Portugal). J Petrol 37:73–94
Chen H, Clark AH, Kyser TK (2010a) The marcona magnetite deposit, ica, South-Central Peru: a product of hydrous, iron oxide-rich melts? Econ Geol 105:1441–1456. https://doi.org/10.2113/econgeo.105.8.1441
Chen HY, Clark AH, Kyser TK, Ullrich TD, Baxter R, Chen YM, Moody TC (2010b) Evolution of the giant Marcona-Mina Justa iron oxide-copper-gold district, South-Central Peru. Econ Geol 105:155–185
Chen W, Kamenetsky VS, Simonetti A (2013) Evidence for the alkaline nature of parental carbonatite melts at Oka complex in Canada. Nat Com 4:2687
Chen W, Ying Y-C, Bai T, Zhang J-J, Jiang S-Y, Zhao K-D, Shin D, Kynicky J (2019) In situ major and trace element analysis of magnetite from carbonatite-related complexes: implications for petrogenesis and ore genesis. Ore Geol Rev 107:30–40. https://doi.org/10.1016/j.oregeorev.2019.01.029
Childress T, Simon AC, Reich M, Barra F, Bilenker LD, La Cruz NL, Bindeman IN, Ovalle JT (2020) Triple oxygen (δ18O, Δ17O), hydrogen (δ2H), and iron (δ56Fe) stable isotope signatures indicate a silicate magma source and magmatic-hydrothermal genesis for magnetite orebodies at El Laco, Chile. Econ Geol 115:1519–1536. https://doi.org/10.5382/econgeo.4760
Clark AH, Kontak DJ (2004) Fe-Ti-P oxide melts generated through magma mixing in the Antauta Subvolcanic Center, Peru: implications for the origin of nelsonite and iron oxide-dominated hydrothermal deposits. Econ Geol 99:377–395. https://doi.org/10.2113/gsecongeo.99.2.377
Cooke D, Hollings P, Wilkinson J, Tosdal R (2014) Geochemistry of porphyry deposits In: Holland HD, Turekian KK (eds) Treatise on geochemistry. Elsevier Science, v. 13, pp 357–381
Corona Esquivel R, Tritlla J, Camprubi A, Centeno E, Reche J, Morales P, Cienfuegos E (2001) New data on the origin of the Peña Colorada iron deposit (Colima, Mexico): orthomagmatic vs skarn. Proceedings 6th SGA Biennial Meeting, Krakow, pp 889–892
Corona-Esquivel R, Martínez-Hernández E, Henríquez F, Nyström JO, Tritlla J (2010) Palynologic evidence for iron-oxide ash fall at La Perla, an Oligocene Kiruna-type iron ore deposit in northern Mexico. GFF 132:173–181. https://doi.org/10.1080/11035897.2010.519048
Corriveau L, Montreuil J-F, Potter EG (2016) Alteration facies linkages among iron oxide copper-gold, iron oxide-apatite, and affiliated deposits in the Great Bear Magmatic Zone, Northwest Territories, Canada. Econ Geol 111:2045–2072. https://doi.org/10.2113/econgeo.111.8.2045
Daliran F (2002) Kiruna-type iron oxide-apatite ores and "apatitites" of the Bafq District, Iran, with an emphasis on the REE geochemistry of their apatites. In: Porter TM (ed) Hydrothermal iron oxide copper-gold and related deposits: A global perspective. PGC Publishing, Adelaide, v 2, pp.303–320
Dare SAS, Barnes S-J, Beaudoin G (2015) Did the massive magnetite “lava flows” of El Laco (Chile) form by magmatic or hydrothermal processes? New constraints from magnetite composition by LA-ICP-MS. Min Dep 50:607–617. https://doi.org/10.1007/s00126-014-0560-1
Dauphas N, John SG, Rouxel O (2017) Iron isotope systematics. Rev Miner Geochem 82:415–510. https://doi.org/10.2138/rmg.2017.82.11
Day WC, Slack JF, Ayuso RA, Seeger CM (2016) Regional geologic and petrologic framework for Iron oxide ± apatite ± rare earth element and iron oxide copper-gold deposits of the mesoproterozoic St. Francois Mountains Terrane, Southeast Missouri, USA. Econ Geol 111:1825–1858. https://doi.org/10.2113/econgeo.111.8.1825
de Fourestier J (2019) Application of ATLAS 5 large-area and nano-scale imaging to purported volcanic products of the el Laco volcano, Chile. Bachelor Thesis, Carleton University
Del Real I, Reich M, Simon AC, Deditius A, Barra F, Rodríguez-Mustafa MA, Thompson JF, Roberts MP (2021) Formation of giant iron oxide-copper-gold deposits by superimposed, episodic hydrothermal pulses. Comm Earth Environ 2:192
Dingwell D (1985) The structures and properties of fluorine rich silicate melts: Implications for granite petrogenesis. In: Taylor RP, Strong DF (eds) Granite-Related Mineral Deposits: Geology, paragenesis and tectonic setting. Canadian Institute of Mining and Metallurgy, pp 72–81
Dmitrijeva M, Ciobanu CL, Ehrig KJ, Cook NJ, Metcalfe AV, Verdugo-Ihl MR, McPhie J (2019) Mineralization-alteration footprints in the Olympic Dam IOCG district, South Australia: the Acropolis prospect. J Geochem Explor 205:106333. https://doi.org/10.1016/j.gexplo.2019.106333
Driesner T, Heinrich CA (2007) The system H2O-NaCl. Part I. Correlation formulae for phase relations in temperature-pressure-composition space from 0 to 1000ºC, 0 to 5000 bar and 0 to 1 XNaCl. Geochim Cosmochim Acta 71:4880–4901. https://doi.org/10.1016/j.gca.2006.01.033
Dupuis C, Beaudoin G (2011) Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner Dep 46:319–335
Dymek RF, Owens BE (2001) Petrogenesis of apatite-rich rocks (nelsonites and oxide-apatite gabbronorites) associated with mafic anorthosites. Econ Geol 96:797–815
Ehrig K, McPhie J, Kamenetsky V (2012) Geology and mineralogical zonation of the olympic dam iron oxide Cu-U-Au-Ag deposit, South Australia. In: Hedenquist JW, Harris M, Camus F (eds) Geology and Genesis of Major Copper Deposits and Districts of the World: A Tribute to Richard H. Sillitoe. Society of Economic Geologists, pp 236–267
Espinoza S (1990) The Atacama-Coquimbo ferriferous belt, northern Chile. In: Fontboté L, Amstutz GC, Cardozo M, Cedillo E, Frutos J (eds) Special Publication SGA. Springer Verlag, pp 353–364
Falloon TJ, Danyushevsky LV, Ariskin A, Green DH, Ford CE (2007) The application of olivine geothermometry to infer crystallization temperatures of parental liquids: implications for the temperature of MORB magmas. Chem Geol 241:207–233. https://doi.org/10.1016/j.chemgeo.2007.01.015
Faramarzi NS, Jamshidibadr M, Heuss-Assbichler S, Borg G (2019) Mineral chemistry and fluid inclusion composition as petrogenetic tracers of iron oxide-apatite ores from Hormuz Island, Iran. J Afr Earth Sci 155:90–108
Feng M, Song W, Kynicky J, Smith M, Cox C, Kotlanova M, Brtnicky M, Fu W, Wei C (2020) Primary rare earth element enrichment in carbonatites: evidence from melt inclusions in Ulgii Khiid carbonatite, Mongolia. Ore Geol Rev 117:103294. https://doi.org/10.1016/j.oregeorev.2019.103294
Foose MP, McLelland JM (1995) Proterozoic low-Ti iron-oxide deposits in New York and New Jersey: relation to Fe-oxide (Cu–U–Au–rare earth element) deposits and tectonic implications. Geology 23:665–668
Förster H, Jafarzadeh A (1994) The Bafq mining district in Central Iran – a highly mineralized infracambrian volcanic field. Econ Geol 89:1697–1721
Fourie PJ (2000) The Vergenoeg fayalite iron-oxide fluorite deposit, South Africa: some new aspects. Hydrothermal Iron Oxide Copper-Gold Relat Depos: Glob Perspect: PGC Publ Adelaide 1:309–320
Frutos J, Oyarzun J (1975) Tectonic and geochemical evidence concerning the genesis of El Laco magnetite lava flow deposits, Chile. Econ Geol 70:988–990. https://doi.org/10.2113/gsecongeo.70.5.988
Gaskell DR (2007) The determination of phase diagrams for slag systems. In: Zhao JC (ed) Methods for Phase Diagram Determination. Elsevier Science Ltd, Oxford, pp 442–458
Geijer P (1910) Geology of the Kiruna district, Vetenskapliga och praktiska undersökningar i Lappland. PA Norstedt Stockholm Paper III:26
Ghiorso MS, Evans BW (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. Am J Sci 308:957–1039
Giebel RJ, Marks MA, Gauert CD, Markl G (2019) A model for the formation of carbonatite-phoscorite assemblages based on the compositional variations of mica and apatite from the Palabora Carbonatite Complex, South Africa. Lithos 324:89–104
Giggenbach WF (1987) Redox processes governing the chemistry of fumarolic gas discharges from White Island, New Zealand. Apl Geochem 2:143–161. https://doi.org/10.1016/0883-2927(87)90030-8
Gilbert CD, Williams-Jones AE (2008) Vapour transport of rare earth elements (REE) in volcanic gas: evidence from encrustations at Oldoinyo Lengai. J Volcanol Geotherm Res 176:519–528. https://doi.org/10.1016/j.jvolgeores.2008.05.003
Goff BH, Weinberg R, Groves DI, Vielreicher NM, Fourie PJ (2004) The giant Vergenoeg fluorite deposit in a magnetite–fluorite–fayalite REE pipe: a hydrothermally-altered carbonatite-related pegmatoid? Miner Petrol 80:173–199. https://doi.org/10.1007/s00710-003-0012-6
Groves DI, Vielreicher NM (2001) The Phalabowra (Palabora) carbonatite-hosted magnetite-copper sulfide deposit, South Africa: an end-member of the iron-oxide-copper-gold-rare earth element deposit group? Min Dep 36:189–194
Groves DI, Bierlein FP, Meinert LD, Hitzman MW (2010) Iron Oxide Copper-Gold (IOCG) deposits through Earth history: implications for origin, lithospheric setting, and distinction from other epigenetic iron oxide Deposits. Econ Geol 105:641–654
Guzmics T, Mitchell RH, Szabó C, Berkesi M, Milke R, Ratter K (2012) Liquid immiscibility between silicate, carbonate and sulfide melts in melt inclusions hosted in co-precipitated minerals from Kerimasi volcano (Tanzania): evolution of carbonated nephelinitic magma. Contr Miner Petrol 164:101–122
Harlov DE, Andersson UB, Förster H-J, Nyström JO, Dulski P, Broman C (2002) Apatite–monazite relations in the Kiirunavaara magnetite–apatite ore, northern Sweden. Chem Geol 191:47–72
Harlov DE, Meighan CJ, Kerr ID, Samson IM (2016) Mineralogy, chemistry, and fluid-aided evolution of the Pea Ridge Fe oxide-(Y + REE) Deposit, Southeast Missouri, USA. Econ Geol 111:1963–1984. https://doi.org/10.2113/econgeo.111.8.1963
Hayes B, Ashwal LD, Webb SJ, Bybee GM (2017) Large-scale magmatic layering in the Main Zone of the Bushveld Complex and episodic downward magma infiltration. Contr Miner Petrol 172:1–16
He H-L, Yu S-Y, Song X-Y, Du Z-S, Dai Z-H, Zhou T, Xie W (2016) Origin of nelsonite and Fe–Ti oxides ore of the Damiao anorthosite complex, NE China: evidence from trace element geochemistry of apatite, plagioclase, magnetite and ilmenite. Ore Geol Rev 79:367–381. https://doi.org/10.1016/j.oregeorev.2016.05.028
Heidarian H, Lentz D, Alirezaei S, Peighambari S, Hall D (2016) Using the chemical analysis of magnetite to constrain various stages in the formation and genesis of the Kiruna-type Chadormalu magnetite-apatite deposit, Bafq district, Central Iran. Miner Petrol 110:927–942
Heidarian H, Alirezaei S, Lentz DR (2017) Chadormalu Kiruna-type magnetite-apatite deposit, Bafq district, Iran: insights into hydrothermal alteration and petrogenesis from geochemical, fluid inclusion, and sulfur isotope data. Ore Geol Rev 83:43–62. https://doi.org/10.1016/j.oregeorev.2016.11.031
Helvaci C (1984) Apatite-rich iron deposits of the Avnik (Bingoel) region, southeastern Turkey. Econ Geol 79:354–371. https://doi.org/10.2113/gsecongeo.79.2.354
Henriquez F, Martin RF (1978) Crystal growth textures in magnetite flows and feeder dykes, El Laco, Chile. Canad Miner 16:581–589
Henríquez F, Nyström JO (1998) Magnetite bombs at El Laco volcano, Chile. GFF 120:269–271
Hildebrand RS (1986) Kiruna-type deposits; their origin and relationship to intermediate subvolcanic plutons in the Great Bear magmatic zone, Northwest Canada. Econ Geol 81:640–659. https://doi.org/10.2113/gsecongeo.81.3.640
Hitzman MW, Oreskes N, Einaudi MT (1992) Geological characteristics and tectonic setting of Proterozoic iron-oxide (Cu-U-Au-REE) deposits. Precam Res 58:241–287
Hofstra AH, Meighan CJ, Song X, Samson I, Marsh EE, Lowers HA, ..., Hunt AG (2016) Mineral thermometry and fluid inclusion studies of the Pea Ridge iron oxide-apatite–rare earth element deposit, Mesoproterozoic St. Francois Mountains terrane, southeast Missouri, USA. Econ Geol 111(8):1985–2016.
Honour VC, Holness MB, Partridge JL, Charlier B (2019) Microstructural evolution of silicate immiscible liquids in ferrobasalts. Contr Miner Petrol 174:77. https://doi.org/10.1007/s00410-019-1610-6
Hou T, Zhang Z, Kusky T (2011) Gushan magnetite–apatite deposit in the Ningwu basin, Lower Yangtze River Valley, SE China: hydrothermal or Kiruna-type? Ore Geol Rev 43:333–346. https://doi.org/10.1016/j.oregeorev.2011.09.014
Hou T, Zhang Z, Encarnacion J, Huang H, Wang M (2012) Geochronology/geochemistry of the Washan dioritic porphyry associated with Kiruna-type iron ores, Middle-Lower Yangtze River Valley, eastern China: implications for petrogenesis/mineralization. Int Geol Rev 54:1332–1352. https://doi.org/10.1080/00206814.2012.659109
Hou T, Charlier B, Holtz F, Veksler I, Zhang Z, Thomas R, Namur O (2018) Immiscible hydrous Fe–Ca–P melt and the origin of iron oxide-apatite ore deposits. Nat Comm 9:1415. https://doi.org/10.1038/s41467-018-03761-4
Huang X-W, Beaudoin G (2019) Textures and chemical compositions of magnetite from Iron Oxide Copper-Gold (IOCG) and Kiruna-type iron oxide-apatite (IOA) deposits and their implications for ore genesis and magnetite classification schemes. Econ Geol 114:953–979. https://doi.org/10.5382/econgeo.4651
Hunt JA, Baker T, Thorkelson DJ (2010) Wernecke Breccia: Proterozoic IOCG mineralised breccia system, Yukon, Canada. In: Porter TM (ed) Hydrothermal iron oxide copper-gold and related deposits: A global perspective. PGC Publishing, Adelaide, V. 4, pp 345–356
Hurai V, Simon K, Wiechert U, Hoefs J, Konečný P, Huraiová M, Pironon J, Lipka J (1998) Immiscible separation of metalliferous Fe/ Ti-oxide melts from fractionating alkali basalt: P-T-fO2 conditions and two-liquid elemental partitioning. Contr Mineral Petrol 133:12–29. https://doi.org/10.1007/s004100050433
Hurai V, Paquette J-L, Huraiová M, Slobodník M, Hvožďara P, Siegfried P, Gajdošová M, Milovská S (2017) New insights into the origin of the Evate apatite-iron oxide-carbonate deposit, Northeastern Mozambique, constrained by mineralogy, textures, thermochronometry, and fluid inclusions. Ore Geol Rev 80:1072–1091. https://doi.org/10.1016/j.oregeorev.2016.09.017
Hynek BM, Arvidson RE, Phillips RJ (2002) Geologic setting and origin of Terra Meridiani hematite deposit on Mars. J Geophys Res: Planet 107:18-11–18-14
Injoque J (2002) Fe oxide-Cu-Au deposits in Peru: An integrated view. In: Porter TM (ed) Hydrothermal iron oxide copper-gold and related deposits: A global perspective. PGC Publishing, Adelaide, v 2, pp 97–113
Jakobsen JK, Veksler IV, Tegner C, Brooks CK (2005) Immiscible iron- and silica-rich melts in basalt petrogenesis documented in the Skaergaard intrusion. Geology 33:885–888. https://doi.org/10.1130/g21724.1
Jami M (2005) Geology, geochemistry and evolution of the Esfordi phosphate: Iron deposit, Bafq area, Central Iran. PhD. Thesis. University of New South Wales
Jonsson E, Troll VR, Högdahl K, Harris C, Weis F, Nilsson KP, Skelton A (2013) Magmatic origin of giant “Kiruna-type” apatite-iron-oxide ores in Central Sweden. Sci Rep 3:1644. https://doi.org/10.1038/srep01644
Kamenetsky VS, Charlier B, Zhitova L, Sharygin V, Davidson P, Feig S (2013) Magma chamber–scale liquid immiscibility in the Siberian Traps represented by melt pools in native iron. Geology 41:1091–1094. https://doi.org/10.1130/G34638.1
Kamenetsky VS, Doroshkevich AG, Elliott HA, Zaitsev AN (2021) Carbonatites: contrasting, complex, and controversial. Elements 17:307–314
Keller T, Tornos F, Hanchar JM, Pietruszka DK, Soldati A, Dingwell DB, Suckale J (2022) Genetic model of the El Laco magnetite-apatite deposits by extrusion of iron-rich melt. Nat Comm 13:6114. https://doi.org/10.1038/s41467-022-33302-z
Kjarsgaard B, Hamilton D (1988) Liquid immiscibility and the origin of alkali-poor carbonatites. Miner Mag 52:43–55
Knipping JL, Bilenker LD, Simon AC, Reich M, Barra F, Deditius AP, Lundstrom C, Bindeman I, Munizaga R (2015) Giant Kiruna-type deposits form by efficient flotation of magmatic magnetite suspensions. Geology 43:591–594. https://doi.org/10.1130/g36650.1
Kodosky LG, Keith TEC (1995) Further insights into the geochemical evolution of fumarolic alteration, Valley of Ten Thousand Smokes, Alaska. J Volcan Geother Res 65:181–190. https://doi.org/10.1016/0377-0273(94)00117-Y
Kolker A (1982) Mineralogy and geochemistry of Fe-Ti oxide and apatite (nelsonite) deposits and evaluation on the liquid inmiscibility hipothesis. Econ Geol 77:1146–1158
Krasnova N, Petrov T, Balaganskaya E, Garcia D, Moutte D, Zaitsev A, Wall F (2004) Introduction to phoscorites: Occurrence, composition, nomenclature and petrogenesis. In: Wall F, Zaitev AN (eds) Phoscorites and carbonatites from mantle to mine: The key example of the Kola alkaline province. Mineralogical Society of Great Britain and Ireland, London, pp 45–74
La Cruz NL, Ovalle JT, Simon AC, Konecke BA, Barra F, Reich M, Leisen M, Childress TM (2020) The geochemistry of magnetite and apatite from the El Laco iron oxide-apatite deposit, Chile: implications for ore genesis. Econ Geol 115:1461–1491. https://doi.org/10.5382/econgeo.4753
Lamy-Chappuis B, Heinrich CA, Driesner T, Weis P (2020) Mechanisms and patterns of magmatic fluid transport in cooling hydrous intrusions. Earth Planet Sci Lett 535:116111
Lan C, Long X, Zhao T, Zhai M (2022) Mineralization and magmatic origin of the 2.1 Ga Zhaoanzhuang magnetite-apatite deposit, North China Craton: constraints on the formation of the Paleoproterozoic iron oxide-apatite deposits. Precambr Res 380:106839. https://doi.org/10.1016/j.precamres.2022.106839
Lee M, Garcia D, Moutte J, Williams C, Wall F (2004) Carbonatites and phoscorites from the Sokli Complex, Finland. In: Wall F, Zaitev AN (eds) Phoscorites and carbonatites from mantle to mine: The key example of the Kola Alkaline Province. Mineralogical Society of Great Britain and Ireland, London, pp 133–162
Lester GW, Clark AH, Kyser TK, Naslund HR (2013a) Experiments on liquid immiscibility in silicate melts with H2O, P, S, F and Cl: implications for natural magmas. Contr Mineral Petrol 166:329–349. https://doi.org/10.1007/s00410-013-0878-1
Lester GW, Kyser TK, Clark AH, Layton-Matthews D (2013b) Trace element partitioning between immiscible silicate melts with H2O, P, S, F, and Cl. Chem Geol 357:178–185. https://doi.org/10.1016/j.chemgeo.2013.08.021
Levresse G, Tornos F, Velasco F, Corona-Esquivel R (2020) Subaerial explosive deposition of magnetite-apatite mineralization: the Artillero deposit, Peña Colorada district, Colima, Mexico. Ore Geol Rev 126:103736. https://doi.org/10.1016/j.oregeorev.2020.103736
Li W, Audétat A, Zhang J (2015) The role of evaporites in the formation of magnetite–apatite deposits along the Middle and Lower Yangtze River, China: evidence from LA-ICP-MS analysis of fluid inclusions. Ore Geol Rev 67:264–278. https://doi.org/10.1016/j.oregeorev.2014.12.003
Lindsley DM (1983) Pyroxene thermometry. Amer Mineral 68:477–493
Lindsley DH (1991) Experimental studies of oxide minerals. Rev Miner Geochem 25:69–106
Liu Y, Fan Y, Zhou T, Zhang L, White NC, Hong H, Zhang W (2018) LA-ICP-MS titanite U-Pb dating and mineral chemistry of the Luohe magnetite-apatite (MA)-type deposit in the Lu-Zong volcanic basin, Eastern China. Ore Geol Rev 92:284–296. https://doi.org/10.1016/j.oregeorev.2017.11.018
Lledo HL, Naslund HR, Jenkins DM (2020) Experiments on phosphate–silicate liquid immiscibility with potential links to iron oxide apatite and nelsonite deposits. Contr Miner Petrol 175:1–33
Locock AJ (2008) An Excel spreadsheet to recast analyses of garnet into end-member components, and a synopsis of the crystal chemistry of natural silicate garnets. Comp Geosc 34:1769–1780
Loucks RR (2014) Distinctive composition of copper-ore-forming arc magmas. Aust J Earth Sci 61:5–16. https://doi.org/10.1080/08120099.2013.865676
Luo G, Zhang Z, Du Y, Pang Z, Zhang Y, Jiang Y (2015) Origin and evolution of ore-forming fluids in the Hemushan magnetite–apatite deposit, Anhui Province, Eastern China, and their metallogenic significance. J Asian Earth Sci 113:1100–1116. https://doi.org/10.1016/j.jseaes.2014.08.018
Lupulescu MV, Hughes JM, Chiarenzelli JR, Bailey DG (2017) Texture, crystal structure, and composition of fluorapatites from iron oxide-apatite deposits, Eastern Adirondack Mountains, New York. Can Miner 55:399–417. https://doi.org/10.3749/canmin.1600057
Lyons JI (1988) Volcanogenic iron oxide deposits, Cerro de Mercado and vicinity Durango, Mexico. Econ Geol 83–8:1886–1906
Marschik R, Akker B, Rieger AA, Chiaradia M, Spangenberg JE (2011) The Cerro Negro Norte magnetite(-apatite) deposit, northern Chile: Sulphur, lead, and strontium isotope characteristics. In: Proceedings 10th Biennial SGA Meeting. Townsville, pp 640–643
Martinsson O (2015) Genesis of the Per Geijer apatite iron ores, Kiruna area, Northern Sweden. In: Proceedings 13th SGA Biennal Meeting, Nancy. pp 24–27
Martinsson O, Billström K, Broman C, Weihed P, Wanhainen C (2016) Metallogeny of the Northern Norrbotten Ore Province, northern Fennoscandian Shield with emphasis on IOCG and apatite-iron ore deposits. Ore Geol Rev 78:447–492. https://doi.org/10.1016/j.oregeorev.2016.02.011
Mateo L, Tornos F, Hanchar JM, Villa IM, Stein HJ, Delgado A (2023) The Montecristo mining district, northern Chile: the relationship between vein-like magnetite-(apatite) and iron oxide-copper–gold deposits. Min Dep. https://doi.org/10.1007/s00126-023-01172-0
Matthews SJ, Marquillas RA, Kemp AJ, Grange FK, Gardeweg MC (1996) Active skarn formation beneath Lascar Volcano, northern Chile: a petrographic and geochemical study of xenoliths in eruption products. J Metam Geol 14:509–530. https://doi.org/10.1046/j.1525-1314.1996.00359.x
Matthews S, Sparks S, Gardeweg M (1995) The relationships between magma mixing and volatile behaviour at Lascar Volcano (23 22'S-67 44'W), Northern Chile: Significance for the formation of copper sulphide and magnetite-apatite orebodies: Giant Ore Deposits II. Queen's University, pp 146–181
McBirney AR, Nakamura Y (1974) Immiscibility in late-stage magmas of the Skaergaard intrusion. Carnegie Inst Wash Yearb 73:348–352
Mercer CN, Watts KE, Gross J (2020) Apatite trace element geochemistry and cathodoluminescent textures—a comparison between regional magmatism and the Pea Ridge IOA-REE and Boss IOCG deposits, southeastern Missouri iron metallogenic province, USA. Ore Geol Rev 116:103129. https://doi.org/10.1016/j.oregeorev.2019.103129
Meyer C (1988) Ore Deposits as Guides to Geologic History of the Earth. Ann Rev Earth Planet Sci 16:147–171. https://doi.org/10.1146/annurev.ea.16.050188.001051
Mikhailova JA, Kalashnikov AO, Sokharev VA, Pakhomovsky YA, Konopleva NG, Yakovenchuk VN, Bazai AV, Goryainov PM, Ivanyuk GY (2016) 3D mineralogical mapping of the Kovdor phoscorite–carbonatite complex (Russia). Min Dep 51:131–149. https://doi.org/10.1007/s00126-015-0594-z
Monteiro LVS, Xavier RP, de Carvalho ER, Hitzman MW, Johnson CA, de Souza CR, Torresi I (2008) Spatial and temporal zoning of hydrothermal alteration and mineralization in the Sossego iron oxide-copper-gold deposit, Carajas Mineral Province, Brazil: paragenesis and stable isotope constraints. Min Dep 43:129–159
Moore F, Modabberi S (2003) Origin of Choghart iron oxide deposit, Bafq mining district, Central Iran: new isotopic and geochemical evidence. J Sci Rep Iran 14:259–270
Mungall J, Long K, Brenan J, Smythe D, Naslund H (2018) Immiscible shoshonitic and Fe-P-oxide melts preserved in unconsolidated tephra at El Laco volcano, Chile. Geology 46:255–258. https://doi.org/10.1130/G39707.1
Mungall JE, Long K, Brenan JR, Naslund HR (2011) Formation of iron oxide and phosphate tephra of the El Laco volcano, Chile from an Fe-P-O-S magma. In: Proceedings V Symposium on volcanism and associated ore deposits, Goias, pp 1–3
Munizaga R, Lagos M (2015) Antecedentes geologicos del yacimiento de magnetita-apatito Los Colorados, provincia del Huasco, Tercera Region de Atacama, Chile. In: Proccedings XIV Congreso Geológico Chileno. La Serena, pp 102–105
Nabatian G, Ghaderi M, Corfu F, Neubauer F, Bernroider M, Prokofiev V, Honarmand M (2014) Geology, alteration, age, and origin of iron oxide–apatite deposits in Upper Eocene quartz monzonite, Zanjan district, NW Iran. Miner Dep 49:217–234. https://doi.org/10.1007/s00126-013-0484-1
Nabelek PI, Whittington AG, Sirbescu MLC (2010) The role of H2O in rapid emplacement and crystallization of granite pegmatites: resolving the paradox of large crystals in highly undercooled melts. Contr Miner Petrol 160:313–325. https://doi.org/10.1007/s00410-009-0479-1
Nadoll P, Angerer T, Mauk JL, French D, Walshe J (2014) The chemistry of hydrothermal magnetite: a review. Ore Geol Rev 61:1–32. https://doi.org/10.1016/j.oregeorev.2013.12.013
Naldrett AJ (1989) Magmatic sulfide deposits. Oxford University Press, London
Naranjo JA, Henríquez F, Nyström JO (2010) Subvolcanic contact metasomatism at El Laco Volcanic Complex, Central Andes. Andean Geol 37:110–120
Naslund HR, Henriquez F, N JO, Vivallo W, Dobbs FM (2002) Magmatic iron ores and associated mineralisation; examples from the Chilean High Andes and Coastal Cordillera. In: Porter TM (ed) Hydrothermal iron oxide copper-gold & related deposits: a global perspective, vol 2. PGC Publishing, Adelaide, pp 207–226
Naslund HR, Mungall JE, Henriquez F, Nyström JO, Lledó H, Lester GW, Aguirre R (2009) Melt inclusions in silicate lavas and iron-oxide tephra of the El Laco volcano. Abstracts XII Congreso Geológico Chileno, Santiago, Chile
Neumann E-R, Svensen HH, Polozov AG, Hammer Ø (2017) Formation of Si-Al-Mg-Ca-rich zoned magnetite in an end-Permian phreatomagmatic pipe in the Tunguska Basin, East Siberia. Min Dep 52:1205–1222. https://doi.org/10.1007/s00126-017-0717-9
Nie L, Zhou T, Fan Y, Zhang L, Cooke D, White N (2017) Geology, geochemistry and genesis of the Makou magnetite-apatite deposit in the Luzong volcanic basin, Middle-Lower Yangtze River Valley Metallogenic Belt, Eastern China. Ore Geol Rev 91:264–277. https://doi.org/10.1016/j.oregeorev.2017.09.022
Niiranen K (2006) The deep parts of the Kiirunavaara apatite-magnetite ore body in Kiruna, Northern Sweden and the impact of future mining on the township of Kiruna. In: Proceedings 6th International Mining Geology Conference (AusIMM), Darwin 2006, pp 57–67
Nikolenko AM, Redina AA, Doroshkevich AG, Prokopyev IR, Ragozin AL, Vladykin NV (2018) The origin of magnetite-apatite rocks of Mushgai-Khudag Complex, South Mongolia: mineral chemistry and studies of melt and fluid inclusions. Lithos 320–321:567–582. https://doi.org/10.1016/j.lithos.2018.08.030
Nold JL, Davidson P, Dudley MA (2013) The Pilot Knob magnetite deposit in the Proterozoic St. Francois Mountains Terrane, southeast Missouri, USA: a magmatic and hydrothermal replacement iron deposit. Ore Geol Rev 53:446–469. https://doi.org/10.1016/j.oregeorev.2013.02.007
Nold JL, Dudley MA, Davidson P (2014) The Southeast Missouri (USA) Proterozoic iron metallogenic province types of deposits and genetic relationships to magnetite-apatite and iron oxide-copper-gold deposits. Ore Geol Rev 57:154–171. https://doi.org/10.1016/j.oregeorev.2013.10.002
Nordstrand J, Andersson UB (2013) Mineral chemistry of gangue minerals of the Kiirunavaara iron ore, evidence for a transition from magmatic to hydrothermal conditions. In: Proceedings 12th SGA Biennial Metting, Uppsala, pp 1663–1666
Nyström JO, Henriquez F (1994) Magmatic features of iron ores of the Kiruna type in Chile and Sweden: ore textures and magnetite geochemistry. Econ Geol 89:820–839
Nyström JO, Billström K, Henríquez F, Fallick AE, Naslund HR (2008) Oxygen isotope composition of magnetite in iron ores of the Kiruna type in Chile and Sweden. GFF 130:177–188. https://doi.org/10.1080/11035890809452771
Nyström JO, Henríquez F, Naranjo JA, Naslund HR (2016) Magnetite spherules in pyroclastic iron ore at El Laco, Chile. Am Mineral 101:587–595. https://doi.org/10.2138/am-2016-5505
Okamoto H (1990) The Fe-P (iron-phosphorous) system. Bull Alloy Phase Diagr 11:404–412. https://doi.org/10.1007/BF02843320
Oreskes N, Einaudi MT (1992) Origin of hydrothermal fluids at Olympic Dam: preliminary results from fluid inclusions and stable isotopes. Econ Geol 87:64–90
Ovalle JT, La Cruz NL, Reich M, Barra F, Simon AC, Konecke BA, Rodriguez-Mustafa MA, Deditius AP, Childress TM, Morata D (2018) Formation of massive iron deposits linked to explosive volcanic eruptions. Sci Rep 8:14855. https://doi.org/10.1038/s41598-018-33206-3
Ovalle JT, Reich M, Barra F, Simon AC, Deditius AP, Le Vaillant M, Neill OK, Palma G, Romero R, Román N (2022) Magmatic-hydrothermal evolution of the El Laco iron deposit revealed by trace element geochemistry and high-resolution chemical mapping of magnetite assemblages. Geochim Cosmochim Acta 230:330–357
Palma G, Barra F, Reich M, Simon AC, Romero R (2020) A review of magnetite geochemistry of Chilean iron oxide-apatite (IOA) deposits and its implications for ore-forming processes. Ore Geol Rev 126:103748. https://doi.org/10.1016/j.oregeorev.2020.103748
Palmieri M, Brod JA, Cordeiro P, Gaspar JC, Barbosa PAR, de Assis LC, Junqueira-Brod TC, Silva SE, Milanezi BP, Machado SA, Jácomo MH (2022) The Carbonatite-related Morro do Padre Niobium Deposit, Catalão II Complex Central Brazil. Econ Geol 117:1497–1520. https://doi.org/10.5382/econgeo.4951
Panno SV, Hood WC (1983) Volcanic stratigraphy of the Pilot Knob iron deposits, Iron County, Missouri. Econ Geol 78:972–982. https://doi.org/10.2113/gsecongeo.78.5.972
Parada M, López-Escobar L, Oliveros V, Fuentes F, Morata D, Calderón M, Aguirre L, Féraud G, Espinoza F, Moreno H (2007) Andean magmatism. In: Moreno T, Gibbons W (eds) The Geology of Chile. Geological Society London, pp 115–146
Parak T (1991) Volcanic sedimentary rock related metallogenesis in the Kiruna Skellefte belt of Northern Sweden. In: Hutchinson (ed) Historical perspectives of genetic concepts and case histories of famous discoveries. Economic Geology Monograph v 8, pp 20–50
Park CF (1961) A magnetite “flow” in northern Chile. Econ Geol 56:431–436
Parmigiani A, Faroughi S, Huber C, Bachmann O, Su Y (2016) Bubble accumulation and its role in the evolution of magma reservoirs in the upper crust. Nature 532:492–495
Peters ST, Alibabaie N, Pack A, McKibbin SJ, Raeisi D, Nayebi N, Torab F, Ireland T, Lehmann B (2019) Triple oxygen isotope variations in magnetite from iron-oxide deposits, central Iran, record magmatic fluid interaction with evaporite and carbonate host rocks. Geology 48:211–215
Peters STM, Feng D, T, Troll V, Pack A, Andersson U, Tornos F, Lehmann B, Di Rocco T (2021) A triple oxygen isotope search for evaporite-derived oxygen in magnetite apatite deposits. Geological Society of America Abstracts with Programs, Portland, v 53, n 6
Philpotts AR (1967) Origin of certain iron-titanium oxide and apatite rocks. Econ Geol 62:303–315. https://doi.org/10.2113/gsecongeo.62.3.303
Philpotts AR (1978) Textural evidence for liquid immiscibility in tholeiites. Miner Mag 42:417–425. https://doi.org/10.1180/minmag.1978.042.324.02
Philpotts AR, Doyle CD (1983) Effect of magma oxidation state on the extent of silicate liquid immiscibility in a tholeiitic basalt. Am J Sci 283:967–986. https://doi.org/10.2475/ajs.283.9.967
Pietruszka DK, Hanchar JM, Tornos F, Wirth R, Graham N, Severin K, Velasco F, Steele-MacInnis M, Bain WM (2023a) Magmatic immiscibility and the origin of magnetite-(apatite) iron deposits. Res Square. https://doi.org/10.21203/rs.3.rs-2156064/v1
Pietruszka DK, Hanchar JM, Tornos F, Whitehouse MJ, Velasco F (2023) Tracking isotopic sources of immiscible melts at the enigmatic magnetite-(apatite) deposit at El Laco, Chile, using Pb isotopes. GSA Bulletin. https://doi.org/10.1130/B36506.1
Pleše P, Higgins MD, Mancini L, Lanzafame G, Brun F, Fife JL, Casselman J, Baker DR (2018) Dynamic observations of vesiculation reveal the role of silicate crystals in bubble nucleation and growth in andesitic magmas. Lithos 296–299:532–546. https://doi.org/10.1016/j.lithos.2017.11.024
Pleše P, Higgins MD, Baker DR, Lanzafame G, Kudrna Prašek M, Mancini L, Rooyakkers SM (2019) Production and detachment of oxide crystal shells on bubble walls during experimental vesiculation of andesitic magmas. Contr Mineral Petrol 174:21. https://doi.org/10.1007/s00410-019-1556-8
Polozov AG, Svensen HH, Planke S, Grishina SN, Fristad KE, Jerram DA (2016) The basalt pipes of the Tunguska Basin (Siberia, Russia): high temperature processes and volatile degassing into the end-Permian atmosphere. Palaeogeogr Palaeoclimatol Palaeoecol 441:51–64
Raab AK (2001) Geology of the Cerro Negro Norte Fe-Oxide (Cu-Au) District, Coastal Cordillera, Northern Chile. MSc Thesis, University of Oregon
Redmond PB, Einaudi MT, Inan EE, Landtwing MR, Heinrich CA (2004) Copper deposition by fluid cooling in intrusion centered systems: new insights from the Bingham porphyry ore deposit, Utah. Geology 32:217–220
Reeve J (1990) Olympic Dam copper-uranium-gold-silver deposit. In: Hughes F (ed) Geology of the mineral deposits of Australia and Papua New Guinea. AusIMM, pp 1009. https://doi.org/10.1130/B36506.11035
Rhodes AL, Oreskes N (1994) The magnetite "lava flows(?)", El Laco, Chile: New evidence for formation by vapor transport. In: Proceedings 7th Congreso Geologico Chileno, Concepción, pp 1501–1505
Rhodes AL, Oreskes N (1999) Oxygen isotope composition of magnetite deposits at El Laco, Chile: Evidence of formation from isotopically heavy fluids. In: Skinner BJ (ed) Economic Geology Special Publication 7, pp 333–351
Rhodes AL, Oreskes N, Sheets S (1999) Geology and REE geochemistry of the magnetite deposits at El Laco, Chile. In: Skinner BJ (ed) Economic Geology Special Publication 7, pp 299–332
Riehl W, Cabral AR (2018) Meta-evaporite in the Carajás mineral province, northern Brazil. Miner Dep 53:895–902. https://doi.org/10.1007/s00126-018-0810-8
Ripp GS, Khodyreva EV, Izbrodin IA, Rampilov MO, Lastochkin EI, Posokhov VF (2017) Genetic nature of apatite–magnetite ore in North Gurvunur deposit, western Transbaikal region. Geol Ore Dep 59:407–420. https://doi.org/10.1134/s1075701517050051
Roedder E, Weiblen PW (1971) Petrology of silicate melt inclusions, Apollo 11 and Apollo 12 and terrestrial equivalents. In: Proceedings 2nd Lunar Science Conference, Houston, pp 507–528
Rojas PA, Barra F, Reich M, Deditius A, Simon A, Uribe F, Romero R, Rojo M (2018) A genetic link between magnetite mineralization and diorite intrusion at the El Romeral iron oxide-apatite deposit, northern Chile. Miner Dep 53:947–966. https://doi.org/10.1007/s00126-017-0777-x
Ryerson F, Hess P (1978) Implications of liquid-liquid distribution coefficients to mineral-liquid partitioning. Geochim Cosmochim Acta 42:921–932
Scheuber E, Mertmann D, Ege H, Silva-González P, Heubeck C, Reutter K-J, Jacobshagen V (2006) Exhumation and basin development related to formation of the Central Andean Plateau, 21° S. In: Oncken O, Chong G, Franz G, Giese P, Götze H-J, Ramos VA, Strecker MR, Wigger P (eds) The Andes: active subduction orogeny. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 285–301
Schwarzenbach EM, Steele-MacInnis M (2020) Fluids in submarine mid-ocean ridge hydrothermal settings. Elements 16:389–394
Sial A, Ferreira V, Toselli AJ, Parada MA, Aceñolaza FG, Pimentel M, Alonso RN (2001) Carbon and oxygen isotope compositions of some Upper Cretaceous-Paleocene sequences in Argentina and Chile. Int Geol Rev 43:892–909
Sillitoe RH (2003) Iron oxide-copper-gold deposits: an Andean view. Min Dep 38:787–812
Sillitoe RH (2010) Porphyry Copper Systems. Econ Geol 105:3–41. https://doi.org/10.2113/gsecongeo.105.1.3
Sillitoe RH, Burrows DR (2002) New field evidence on the origin on the El Laco magnetite deposit, Northern Chile. Econ Geol 97:1101–1109
Skirrow RG (2022) Iron oxide copper-gold (IOCG) deposits–a review (part 1): settings, mineralogy, ore geochemistry and classification. Ore Geol Rev 140:104569
Soldati A, Farrell J, Wysocki R, Karson J (2021) Imagining and constraining ferrovolcanic eruptions and landscapes through large-scale experiments. Nat Comm 12:1711
Soloviev S (2010) Iron oxide copper-gold and related mineralisation of the Siberian craton, Russia: 1—iron oxide deposits in the Angara and Ilim river basins, south-central Siberia. In: Porter TM (ed) Hydrothermal iron oxide copper-gold and related deposits: a global perspective. PGC Publishing, Adelaide, pp 495–514
Spencer KJ, Lindsley DH (1981) A solution model for coexisting iron-titanium oxides. Am Miner 66:1189–1201
Stagno V, Fei Y (2020) The redox boundaries of Earth’s interior. Elements 16:167–172
Steele-MacInnis M, Bain WM, Tornos F, Hanchar JM, Pietruszka DK, Lehmann B (2021) Carbonate-sulfate melts and their role in magnetite-apatite formation worldwide. Geological Society of America Abstracts with Programs, Portland, v 53, pp 6
Stoughton B (2018) The metallurgy of iron and steel. Forgotten books ed
Symonds RB, Rose WI, Reed MH, Lichte FE, Finnegan DL (1987) Volatilization, transport and sublimation of metallic and non-metallic elements in high temperature gases at Merapi Volcano, Indonesia. Geochim Cosmo Acta 51:2083–2101. https://doi.org/10.1016/0016-7037(87)90258-4
Taghipour S, Kananian A, Harlov D, Oberhänsli R (2015a) Kiruna-type iron oxide-apatite deposits, Bafq district, central Iran: fluid-aided genesis of fluorapatite-monazite-xenotime assemblages. Can Mineral 53:479–496. https://doi.org/10.3749/canmin.4344
Taghipour S, Kananian A, Mackizadeh MA, Somarin AK (2015b) Skarn mineral assemblages in the Esfordi iron oxide–apatite deposit, Bafq district, Central Iran. Arab J Geosc 8:2967–2981
Thorkelson DJ, Mortensen JK, Davidson GJ, Creaser RA, Perez WA, Abbott JG (2001) Early mesoproterozoic intrusive breccias in Yukon, Canada: the role of hydrothermal systems in reconstructions of North America and Australia. Precambr Res 111:31–55
Torab FM, Lehmann B (2007) Magnetite-apatite deposits of the Bafq District, Central Iran: Apatite geochemistry and monazite geochronology. Miner Mag 71:347–366
Tornos F, Velasco F, Hanchar J (2016) Iron-rich melts, magmatic magnetite and superheated magmatic-hydrothermal systems: the El Laco deposit, Chile. Geology 44:427–430. https://doi.org/10.1130/G37705.1
Tornos F, Hanchar JM, Velasco F, Muñizaga R, Levresse G (2017a) The roots and tops of magnetite-apatite mineralization: Evolving magmatic-hydrothermal systems. In: Proceedings 14th SGA Biennial Meeting, Quebec, pp 831–834
Tornos F, Velasco F, Hanchar JM (2017b) The magmatic to magmatic-hydrothermal evolution of the El Laco deposit (Chile) and its implications for the genesis of magnetite-apatite deposits. Econ Geol 112:1595–1628. https://doi.org/10.5382/econgeo.2017.4523
Tornos F, Hanchar JM, Munizaga R, Velasco F, Galindo C (2021) The role of the subducting slab and melt crystallization in the formation of magnetite-(apatite) systems, Coastal Cordillera of Chile. Miner Dep 56:253–278. https://doi.org/10.1007/s00126-020-00959-9
Tornos F, Steele-MacInnis M, Hanchar JM (2023) Limitations in using magnetite and actinolite chemistry as tracers of temperature and origin in IOA-IOCG systems. In: Proceedings GAC-MAC Annual Meeting, Sudbury, pp P09 SS18
Tritlla J, Sole J, Camprubí A, Centeno-García E, Corona-Esquivel R, Iriondo A, Sanchez Martinez S, Gasca Durán A, Cienfuegos Alvarado E, Morales Puente P (2003) Estructura y edad del depósito de hierro de Peña Colorada (Colima): un posible equivalente fanerozoico de los depósitos de tipo IOCG. Rev Mex Ciencias Geol 20:182–201
Troll VR, Weis FA, Jonsson E, Andersson UB, Majidi SA, Högdahl K, Harris C, Millet M-A, Chinnasamy SS, Kooijman E (2019) Global Fe–O isotope correlation reveals magmatic origin of Kiruna-type apatite-iron-oxide ores. Nat Com 10:1712
Tuttle O, Bowen N (1950) High-temperature albite and contiguous feldspars. J Geol 58:572–583
Valley PM, Hanchar JM, Whitehouse MJ (2011) New insights on the evolution of the Lyon Mountain Granite and associated Kiruna-type magnetite-apatite deposits, Adirondack Mountains, New York State. Geosphere 7:357–389. https://doi.org/10.1130/ges00624.1
Vartiainen H, Paarma H (1979) Geological characteristics of the Sokli carbonatite complex, Finland. Econ Geol 74:1296–1306
Veksler IV, Lentz D (2006) Parental magmas of plutonic carbonatites, carbonate-silicate immiscibility and decarbonation reactions: Evidence from melt and fluid inclusions. In: Webster JD (ed) Melt inclusions in plutonic rocks. Short course series / Mineralogical Association of Canada, pp 123–149
Velasco F, Tornos F (2009) Pegmatite-like magnetite-apatite deposits of northern Chile: a place in the evolution of immiscible iron oxide melts? In: Williams P et al (eds) Smart Science for Exploration and Mining. James Cook University, Townsville, Economic Geology Research Unit, pp 665–667
Velasco F, Tornos F, Hanchar JM (2016) Immiscible iron- and silica-rich melts and magnetite geochemistry at the El Laco volcano (northern Chile): evidence for a magmatic origin for the magnetite deposits. Ore Geol Rev 79:346–366. https://doi.org/10.1016/j.oregeorev.2016.06.007
Velasco F, de la Pinta N, Tornos F, Briezewski T, Larrañaga A (2020) The relationship between destinezite to acid sulfate alteration at the El Laco magnetite deposit, Chile. Am Mineral 105:860–872. https://doi.org/10.2138/am-2020-7122
Veloso E, Cembrano J, Arancibia G, Heuser G, Neira S, Siña A, Garrido I, Vermeesch P, Selby D (2017) Tectono-metallogenetic evolution of the Fe–Cu deposit of Dominga, northern Chile. Miner Dep 52:595–620. https://doi.org/10.1007/s00126-016-0682-8
Vidal CE, Injoque-Espinoza J, Sidder GB, Mukasa SB (1990) Amphibolitic Cu-Fe skarn deposits in Central Coast of Perú. Econ Geol 85:1447–1461
Visser W, Koster Van Groos AF (1979) Effects of P2O5 and TiO2 on liquid-liquid equilibria in the system K2O-FeO-Al2O3-SiO2. Am J Sci 279:970–988
Von der Flaass GS (1992) Magmatic stage in evolution of the Angara-Ilim type ore-forming system. Russ Geol Geophys 33:67–72
Von der Flaass G, Naumov VA (1995) Cup-shaped structures of iron-ore deposits in the south of the Siberian platform (Russia). Geol Ore Depos 37:340–350
Wallace PJ, Plank T, Edmonds M, Hauri EH (2015) Chapter 7 - Volatiles in Magmas. In: Sigurdsson H (ed) The Encyclopedia of Volcanoes, 2nd edn. Academic Press, Amsterdam, pp 163–183
Walter BF, Giebel RJ, Steele-MacInnis M, Marks MAW, Kolb J, Markl G (2021) Fluids associated with carbonatitic magmatism: a critical review and implications for carbonatite magma ascent. Earth-Sci Rev 215:103509
Warr LN (2021) IMA–CNMNC approved mineral symbols. Min Mag 85:291–320. https://doi.org/10.1180/mgm.2021.43
Weis F, Troll VR, Jonsson E, Högdahl K, Harris C, Sun W, Nilsson KP, Dahrén B (2021) Absence of hydrothermal oxygen isotope variations in host rocks supports magmatic origin of the giant Grängesberg iron oxide–apatite (IOA) deposit, Central Sweden. Int J Earth Sci. https://doi.org/10.1007/s00531-021-02122-9
Wells PRA (1977) Pyroxene thermometry in simple and complex systems. Contr Mineral Petrol 62:129–139. https://doi.org/10.1007/BF00372872
Williams P, Barton MD, Johnson DA, Fontboté L, Haller Ad, Mark G, Oliver NHS, Marschik R (2005) Iron oxide copper-gold deposits: geology, space-time distribution, and possible modes of origin. In: Hedenquist JW, Thompson JFH, Goldfarb RJ, Richards JP (eds) Economic Geology - 100 ann volume. Society of Economic Geologists, Littleton, pp 371–406
Wilson L, Head JW III (1981) Ascent and eruption of basaltic magma on the Earth and Moon. J Geophys Res: Solid Earth 86:2971–3001. https://doi.org/10.1029/JB086iB04p02971
Woods AW, Cardoso SSS (1997) Triggering basaltic volcanic eruptions by bubble-melt separation. Nature 385:518–520
Xavier RP, Soares Monteiro LV, Moreto CPN, Pestilho ALS, Coelho de Melo GH, Delinardo da Silva MA, Aires B, Ribeiro C, Freitas e Silva FH (2012) The Iron Oxide Copper-Gold Systems of the Carajás Mineral Province, Brazil. In: Hedenquist JW, Harris M, Camus F (eds) Geology and Genesis of Major Copper Deposits and Districts of the World: A Tribute to Richard H. Sillitoe. Society of Economic Geologists, pp 433–454
Xie Q, Zhang Z, Campos E, Deng J, Cheng Z, Fei X, Ke S (2021) Constraints of Fe-O isotopes on the origin of magnetite in the El Laco Kiruna-type iron deposit, Chile. Ore Geol Rev 130:103967. https://doi.org/10.1016/j.oregeorev.2020.103967
Yan S, Liu W (2022) Rare earth elements in the iron-oxide apatite (IOA) deposit: Insights from apatite. Int Geol Rev. https://doi.org/10.1080/00206814.2022.2028198
Yao Z, Mungall JE (2022) Magnetite layer formation in the Bushveld Complex of South Africa. Nat Comm 13:416
Yu J, Chen Y, Mao J, Pirajno F, Duan C (2011) Review of geology, alteration and origin of iron oxide–apatite deposits in the Cretaceous Ningwu basin, Lower Yangtze River Valley, eastern China: implications for ore genesis and geodynamic setting. Ore Geol Rev 43:170–181. https://doi.org/10.1016/j.oregeorev.2011.07.008
Zeng L-P, Zhao X-F, Spandler C, Hu H, Hu B, Li J-W, Hu Y (2022) Origin of high-Ti magnetite in magmatic-hydrothermal systems: evidence from iron oxide-apatite (IOA) deposits of eastern china. Econ Geol 117:923–942
Zevallos RA (1966) Geology of the Acari iron mining district Arequipa. Thesis Missouri University Science Technology, Peru, p 186
Zhang L, Schlesinger ME, Brow RK (2011) Phase equilibria in the Fe2O3–P2O5 system. J Am Ceram Soc 94:1605–1610. https://doi.org/10.1111/j.1551-2916.2010.04287.x
Zhang Z, Wu B, Wang T, Hui H (2020) Settling of immiscible droplets: A theoretical model for the missing link between microscopic and outcrop observations. J Geophys Res: Solid Earth 125. https://doi.org/10.1029/2019JB018829
Zhou T, Fan Y, Yuan F, Zhang L, Qian B, Ma L, Yang X (2013) Geology and geochronology of magnetite–apatite deposits in the Ning-Wu volcanic basin, eastern China. J Asian Earth Sci 66:90–107. https://doi.org/10.1016/j.jseaes.2012.12.030
Zimmer K, Zhang Y, Lu P, Chen Y, Zhang G, Dalkilic M, Zhu C (2016) SUPCRTBL: a revised and extended thermodynamic dataset and software package of SUPCRT92. Comp Geosc 90:97–111. https://doi.org/10.1016/j.cageo.2016.02.013
Acknowledgements
Compañía Minera del Pacífico (CMP) granted access to their mines and core shack (2006–2022), gave logistic support, and provided abundant information on El Laco. We especially acknowledge Rodrigo Munizaga, Boris Castillo and Paulina Salgado as well as the guards of the El Laco camp. Thanks are extended to Flor Colquehuanca (Shougang Peru-Marcona), Percy Sandoval (MINSUR-Mina Justa), Luis Villa and Hennie Terblanche (MINERSA-Vergenoeg), Kirsten Holme (LKAB-Kiruna), Kathy Ehrig (BHP-Olympic Dam), Yu Fan and Taofa Zheng (Lower-Middle Yangtze), Gilles Levresse (Mexico), Warren Day (Missouri district), and Samaneh Kansar Zamin and Ali Sholeh (Bafq district) for logistic support and discussions at the field. We thank Ulf Andersson, Wyatt Bain, Tobias Bauer, James Brenan, Fernando Henriquez, Tobias Keller, Corey Meighan, Steve Piercey, Dorota Pietruzska, Jean Luc Pilotte, Henrikki Rutanen, Roger Skirrow, John Slack, Jenny Suckale, Francisco Velasco, Xinyue Xu, and Noel White for the discussions on the topic. We also acknowledge Candela Pita for helping with the treatment of the analytical data, John Fournelle and Pavel Izbekov for sharing their knowledge on the Valley of the Ten Thousand Smokes, Stuart Simmons on the transport of iron by gas, Ignacio Moreno Ventas for sharing his knowledge on slag-matte separation, and Monica Alvarez del Buergo and Guillem Gisbert for the XRF analyses. The original manuscript was reviewed by Robert Martin and an anonymous reviewer and edited by Bernd Lehmann. We appreciate their insightful comments that improved the quality of the original work.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by the project NANOMET PID2022-138768OB-I00 (Spain) and the project Exploration Information Systems (EIS) of Horizon Europe (Contract 101057357) to FT, NSERC Discovery Grants (Canada) to JMH and MSM and grants PID2020-117332GB-C21 and PID2020-117332GB-C22 (MCIN/AEI/10.13039/501100011033) to EC. The contribution of VK is supported by a Visiting Scientist Award by the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Fernando Tornos and John Hanchar conceived and developed the project. Material preparation, data collection, and analysis were performed by Elena Crespo, Vadim Kamenetsky, Fernando Tornos, and John Hanchar. The thermodynamic approach and genetic models were discussed between Matthew Steele-MacInnis, Fernando Tornos, and Cesar Casquet. The first draft of the manuscript was written by Fernando Tornos, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editorial handling: B. Lehmann
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tornos, F., Hanchar, J.M., Steele-MacInnis, M. et al. Formation of magnetite-(apatite) systems by crystallizing ultrabasic iron-rich melts and slag separation. Miner Deposita 59, 189–225 (2024). https://doi.org/10.1007/s00126-023-01203-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-023-01203-w