Abstract
Exhaled breath research has been hindered by a lack of standardization in collection and analysis methodologies. Recently, the Respiration Collector for In Vitro Analysis (ReCIVA) sampling device has illustrated the potential to provide a consistent and convenient method for exhaled breath collection onto adsorbent media. However, the significant costs, compared to exhaled breath bags, associated with the standardized collector is believed to be the reason for limited widespread use by researchers in the exhaled breath field. For example, in addition to the sampling hardware, a single-use disposable silicon mask affixed with a filter is required for each exhaled breath collection. To reduce the financial burden, streamline device upkeep, reduce waste material, and ease the logistical burden associated with the single use masks, it is hypothesized that the consumable masks and filters could be sterilized by autoclaving for reuse. The masks were contaminated, autoclaved, and then tested for any surviving pathogens with spore strip standards and by measuring the optical density of cultures. The compound background collected when using the ReCIVA with new masks was compared to that collected with repeatedly autoclaved masks via thermal desorption gas chromatography mass spectrometry (TD-GC-MS). The capacity to block particulate matter of new filters was tested against that of autoclaved filters by introducing an aerosol and comparing pre-filter and post-filter particle counts. Finally, breath samplings were conducted with new masks and autoclaved masks to test for changes in measurements by TD-GC-MS of exogenous and endogenous compounds. The data illustrate the autoclave cycle sterilizes masks spiked with saliva to background levels (p = 0.2527). The results indicate that background levels of siloxane compounds are increased as masks are repetitively autoclaved. The data show that mask filters have significant breakthrough of 1 μm particles after five repetitive autoclaving cycles compared to new filters (p = 0.0219). Finally, exhaled breath results utilizing a peppermint ingestion protocol indicate two compounds associated with peppermint, menthone and 1-Methyl-4-(1-methylethyl)-cyclohexanol, and an endogenous exhaled breath compound, isoprene, show no significant difference if sampled with a new mask or a mask autoclaved five times (p > 0.1063). Collectively, the data indicate that ReCIVA masks and filters can be sterilized via autoclave and reused. The results suggest ReCIVA mask and filter reuse should be limited to three times to limit potentially problematic background contaminants and filter dysfunction.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Breath sampling has risen to the forefront of biomarker discovery as a fast, informative, and non-invasive biosampling procedure [1]. There are increasing reports within the literature of exhaled breath biomarkers and disease indicators, in a host of applications, as research continues to broaden in scope [2]. For instance, it has been shown that exhaled isoprene (2-methyl-1,3-butadiene) was inversely correlated with minimum oxygen saturation during controlled hypoxia exposures [3]. Additionally, Ibrahim et al illustrated 47 exhaled volatile compounds could stratify asthmatic from healthy with 86% accuracy [4]. For additional background information on exhaled breath and biomarker discovery applications please refer to Beauchamp et al, Davis et al and Pham et al [1, 2, 5]. Although discovery efforts have identified potential volatile biomarkers of interest, overall, the clinical integration of exhaled biomarkers has lagged, likely resulting from a deficiency of standardization in exhaled breath sampling and analysis.
The two primary methods of breath sampling are on-line sampling, where the breath is analyzed as it exhaled, and off-line sampling, where the breath volatiles are concentrated and stored for later analysis [2]. Although online has advantages such as immediate data collection, decreased opportunity for sample loss, and time-saving potential, off-line sampling is routinely utilized for the remote sample collection that is not easily applicable for on-line analysis [2]. The most frequent method of off-line breath sampling over the past decade has been collecting exhalations in breath bags; breath is sampled into a container remotely with volatiles concentrated onto adsorbent media and transferred to a lab for analysis [6]. However, proper sampling of lower airway tidal breath within a bag is often difficult, due to procedural requirements associated with collection. Often, exhaled breath bag sampling protocols require the subject to inhale, exhale normally for a period to exhale the upper airway breath, then exhale the remainder of the lung's capacity into the breath bag [6]. However, not all subjects are reliably able to provide adequate exhaled samples for a number of reasons, thereby introducing a potentially significant source of variability into the analysis. For example, individuals under respiratory stress, trauma, or incapacitation may not have the ability to perform the appropriate sampling maneuvers. As one possible approach to address exhaled breath sampling inconsistencies, the Respiration Collector for In Vitro Analysis (ReCIVA) sampling device was developed.
The ReCIVA, a disposable face mask and nondisposible hardware which is affixed over the mouth and nose of a test subject, allows for direct sampling of exhaled breath onto adsorbent media [7–10]. A disposable silicon mask/filter combination, with holes to allow thermal desorption (TD) tubes to be inserted, is placed within the ReCIVA hardware. TD tubes, which sit in-line with the subject's mouth within the mask, are inserted into up to four TD tube receptacles within the ReCIVA hardware. External breathing gas is provided to the subject by an inlet on the hardware. The hardware of the ReCIVA houses a dynamic pump, which is automatically activated based on real-time carbon dioxide measurements and pressure within the mask, drawing the subject's breath through the affixed TD tubes during exhalation. As a result, the ReCIVA device can be programed for versatile sampling volumes and distinct portions of exhalations (lower airway and/or upper airway) while allowing the subject to breathe normally.
The ReCIVA device has been shown to provide consistent exhaled breath data for off-line analysis [7, 8]. For example, data illustrate the ReCIVA device can provide results statistically similar to exhaled breath bags [8]. Additionally, results indicate consistency in sampling among different ReCIVA devices and with varying breathing rates [7]. Hence, there are significant benefits, analytically and functionally, to the use of the ReCIVA for exhaled breath sampling, especially with individuals that are unable to adhere to the proscribed breathing technique, such children, subjects with respiratory illness or distress, or those experiencing shortness of breath. Collectively, for these reasons, ReCIVA has been growing in popularity for research use, and has now been used to sample hundreds of subjects.
While a useful tool, there are drawbacks to the ReCIVA device, such as prolonged sampling time compared to breath bags, humidity issues with certain adsorbents from direct sampling on TD tubes, and overall cost [7–9]. Of those, the cost of the device and consumables has plausibly limited the ReCIVA's widespread adaptation by the breath research community. While hardware cost are often large but single purchases, the cost of consumables may be lower but more frequent, resulting in an increased overall operating cost. For example, the masks and filter combination specially manufactured for the ReCIVA device and have been recommended as single use due to risk of infection between subjects. Therefore, the consumable masks and filters present a potential barrier to large scale breath studies utilizing the ReCIVA device, as the costs of purchasing, shipping, storing, and disposing of thousands of masks for a study can be prohibitive, not only financially but also logistically. While simply reusing the masks, without proper sterilization, risks the spread of many diseases, such as influenza and coronavirus [11]. It is hypothesized that sterilization of the ReCIVA masks and filters through autoclaving would allow for their repeated use, easing the logistical burden while still providing adequate safety from communicable diseases.
2. Experimental methods
2.1. Participants
Two separate experiments (n = 9 for saliva, and n = 10 for peppermint) were performed. Both experiments were performed utilizing healthy male and female individuals. The studies were determined to be non-human use by the 711th Human Performance Wing Institutional Review Board (IRB # FWR20200094H, FWR20180006H). Therefore, participants were informed of the experimental design, free to stop at any time, but written informed consent was not required for this study.
2.2. TD tubes
The TD tubes utilized were biomonitoring Tenax/Carbograph (5TD, stainless steel, Markes International, South Wales, UK). Tubes were reconditioned in a Markes International TC-20 for 2 h at 320 °C with a backflush of 85 ml min−1 of 99.999% nitrogen. The tubes were stored at room temperature affixed with brass caps until needed.
2.3. Spore strip test
A spore strip test was developed for evaluating the effectiveness of autoclaving to sterilize the ReCIVA masks. Three Crosstex SporView CS020 test strips (Crosstex International Inc. Hauppauge, NY, USA), which hold spores of Geobacillus stearothermophilus, were affixed to new ReCIVA masks (Lot #: 539, Owlstone Medical, Cambridge, UK). The masks were steam autoclaved unwrapped at 134 °C with a 3 min sterilization time and a 20 min dry time, in a Tuttnauer 3870EA autoclave (Breda, Noord-Brabant, The Netherlands), as recommended by the United States Centers for Disease Control and Prevention [12]. Another three test strips were set aside and remained unautoclaved as a control. Each of the six test strips were cultured in SporView test tubes of dyed media and incubated for 7 d at 60 °C, as described by the manufacturer. Proliferation was determined by a visual color change from purple to yellow.
2.4. Pooled saliva test
A test was developed to evaluate the sterilization effectiveness performed using saliva obtained by passive drool from each of nine healthy volunteers and pooled into one source [13]. The pooled saliva was swabbed onto six new ReCIVA masks. Three of those six masks remained unautoclaved and were set aside as controls. The other three masks were autoclaved as described above for the test strips. All masks, autoclaved and non-autoclaved, were swabbed over the area of inoculation and swabs were cultured in vials of VWR general purpose nutrient broth (Radnor, PA, USA). Vials were incubated for 5 d at 37 °C, after which optical density (OD600) was recorded on a ThermoFisher NanoDrop One for cell count estimation (Waltham, MA, USA).
2.5. Glass head background test
The background volatiles associated with the ReCIVA masks were collected, as described previously, utilizing the Owlsone Medical ReCIVA sampler (Serial #065), 16 new ReCIVA masks and filters (Lot #: 539), the ReCIVA software (v1.48.0.0, Cambridge, UK), and a clean glass head [7, 8]. Briefly, scrubbed (Supelcarb HC Hydrocarbon Trap, Sigma-Aldrich, St. Louis, MO, USA) compressed air was provided to a ReCIVA sampler at 40 l min−1 through an AliCat Mass Flow Controller (AliCat, Tucson, AZ, USA). The ReCIVA device with mask and filters was affixed to a glass head evaluated to ensure no leaking, and the ReCIVA software was set to continuously pull 200 ml min−1 using the always-on feature through both the left and right pumps to a total volume of 550 ml per tube. Pump inlets proximal to the glass head were occluded with solid stainless-steel rods. Tenax/Carbograph 5TD tubes were inserted into the sampling ports distal to the mouth for sample collection. Refer to supplemental data 1 for an image of the glass head setup. Of the 16 new masks, one mask remained unautoclaved and considered the control. The remaining 15 masks were autoclaved, as described above, in succession, with glass head background testing following each autoclave cycle for up to 10 autoclave cycles. Please see supplemental data 2 for an illustration of the experimental design. Additional control samples were collected from the scrubbed air using an ALTEF 1 l breath bag, with 550 ml pulled through a 5TD tube using a calibrated MultiRae Pro pump (Rae Systems, Schaumburg, IL, USA). All TD tubes were capped and run within 7 d via thermal desorption gas chromatography-mass spectrometry (TD-GC-MS), as described in the TD-GC-MS section [14].
2.6. Filter test
For filter testing, a custom airtight chamber was constructed. See supplemental data 3 for an illustration and photo of the chamber setup. Briefly, an aerosol was created by injecting a solution of 1 µm Polystyrene Microspheres (FluoSpheres F13080, Thermo Fisher Scientific) at 0.25 ml min−1 through an aerosolizer supplied with 10 l min−1 of nitrogen. The aerosol was size-filtered to remove any contaminant particles before introduction into the airtight chamber. The particles on each side of the ReCIVA filter in the chamber were measured and counted with a TSI Optical Particle Sizer 3330 (Shoreview, MN, USA). Comparison of the particle counts from the pre-filter and post-filter portions of the chamber provides a measurement of the filtration action of the ReCIVA filter. The chamber was purged and disassembled; the ReCIVA filter was exchanged; and the setup was reassembled for further sampling. Utilizing this setup, filters from new masks, masks autoclaved five times, and masks autoclaved ten times were tested (n = 3 each).
2.7. Peppermint exposure test
Ten healthy volunteers, five male and five female, were provided an individually sealed Lifesaver® peppermint (Lot 109BQTNN02 34, Mars Wrigley, Chicago, IL, USA). Participants held the mint between their tongue and the roof of their mouth for 5 min, while sitting in a relaxed position. The mints were discarded and volunteers remained sitting for two additional minutes. Participants donned a ReCIVA device supplied with 40 l min−1 of medical grade air through an AliCat Master Flow Controller. The ReCIVA software was set to collect a total of 550 ml from lower airways on each pump, A and B, of the ReCIVA. One 5TD TD tube was collected on each pump, placed in the position distal to the mouth, with solid stainless-steel rods filling the proximal pump slots, as shown in supplemental data 1. Pristine masks and filters were used (pre-autoclave). Volunteers' breathing was guided utilizing Breathe+ iPhone app to a standard of 2 s inhalation and 4 s exhalation with no hold between [7]. Briefly, participants were instructed to inhale as a bar visually rose on the app, and exhale as the bar lowered. The masks and filters were autoclaved as described above once per day for five consecutive days. The ten subjects provided a second set of samples repeating the peppermint experiment described above with the post-autoclave masks. The samples were analyzed immediately after each batch was collected as described in the TD-GC-MS section.
2.8. TD gas-chromatography mass-spectrometry
All TD was conducted on a Markes TD-100xr with internal standards (1,4-diflurobenzene and chlorobenzene-d5, 1 ml of 1 ppm) automatically added to each TD tube. Following a 99.999% nitrogen dry purge for 1 min at 20 ml min−1, a 10 min 310 °C primary desorption onto an Air Toxics cold trap was performed. A trap purge of 1 min at 50 ml min−1 was conducted. A secondary trap desorption for 5 min at 315 °C (40 °C s−1) was performed with a flow path temperature of 180 °C. A 3.64:1 split ratio was applied to desorbed volatiles via the trap outlet prior to introduction onto a Rxi-624Sil 60 m × 0.32 mmID × 1.80 μm df GC column (Restek, Bellefonte, PA, USA). Volatiles were chromatographically separated following a 40 °C hold for 1 min using a linear gradient to 240 °C at a rate of 10 °C min−1 with a constant 2 ml min−1 helium carrier flow (99.9999%). The column was held at 240 °C for 20 min. Electron impact ionization (70 eV) was applied to the column eluent at a temperature of 275 °C in a Thermo Scientific Trace Ultra-ISQ GC-MS system. Ion filtering was performed using a single quadrupole over a 35–205 m z−1 range at 0.154 scans s−1. Prior to running samples, the instrument calibration was verified with an isoprene check standard [15, 16]. TD tubes were run and analyzed in a random order using Tracefinder EFS software (v. 3.2, Thermo Fischer Scientific). All GC-MS data were manually inspected and peak areas were determined utilizing the Thermo Scientific XCalibur software package (v.3.0.63) or the Tracefinder EFS software.
2.9. Statistical analysis
P-values for delta menthone, delta menthol, and delta isoprene were calculated in RStudio (R v. 3.6.3, Rstudio v. 1.2.1335) using the Wilcoxon signed-rank test, testing the null hypothesis that µ = 0. The p-values for testing pre against post for menthone, 1-methyl-4-(1-methylethyl)-cyclohexanol (MMECH), and isoprene were calculated in RStudio using the Wilcoxon signed-rank paired difference test [17]. All other p-values were calculated using Wilcoxon signed rank difference test, testing the null hypothesis that there was no difference between values. All p-values were labeled significant if less than 0.05.
3. Results & discussion
3.1. Spore strip test results
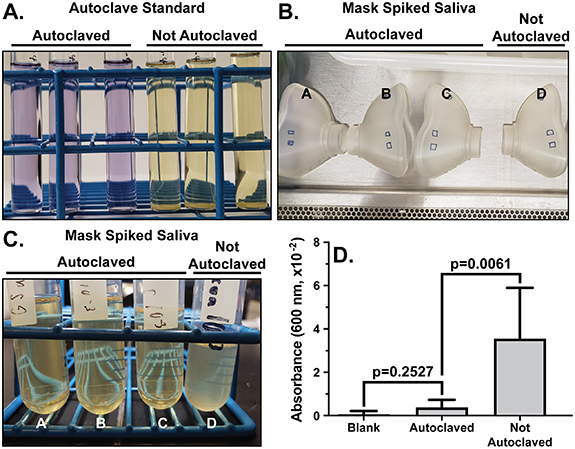
While the ReCIVA device has shown utility in the consistent collection of exhaled breath samples, the single use recommendation of the disposable ReCIVA mask/filter combination is plausibly both logistically and cost prohibitive to large scale studies utilizing the ReCIVA for collection [7, 8, 10]. This recommendation is hypothesized to be a result of a potential loss of sterility of masks and filters following breath collection during repeated use. To determine if an autoclave cycle could facilitate sterilization and ultimately reuse, an in vitro experiment utilizing an autoclave verification standard of G. stearothermophilus spores was performed. Figure 1(A) shows the cultures from the autoclave standard test strips that were not autoclaved and those that were autoclaved. The test strips that were not autoclaved changed in color from purple to yellow, evidencing proliferation. The cultures from the test strips that were autoclaved showed a lack of a proliferation; these strips remained purple. These data indicate that the autoclaved test strips show no proliferation of the test organism (G. stearothermophilus spores), with the autoclave settings, suggesting the spores were neutralized within the autoclave cycle (figure 1(A)).
Figure 1. The effectiveness of autoclaving for ReCIVA mask sterilization. (A) From the spore strip test, a representative photo of a standard of G. stearothermophilus growth in the test medium following autoclaving. Positive growth is indicated by change in color from purple to yellow. (B) From the pooled saliva test, a representative picture of masks spiked with human saliva samples (marked squares). n = 6 masks each autoclaved and not autoclaved. (C) A representative picture of cultures from human saliva spiked onto ReCIVA masks, autoclaved and not autoclaved. (D) The optical density (OD) at 600 nm absorbance measured from the human saliva samples, autoclaved and not autoclaved. Significance was determined by unpaired t-test and error bars represent the 95% confidence interval. The data illustrate autoclaving causes a significant reduction in bacterial growth from both standard and biological samples.
Download figure:
Standard image High-resolution image3.2. Pooled saliva test results
While standard tests strips provide information about autoclave cycle sterility, a more biologically relevant sample, such as human saliva, would be more representative of the potential contamination associated with the ReCIVA masks and filters in the field. Therefore, a pooled human saliva sample from nine healthy individuals was swabbed onto the ReCIVA masks, as illustrated in figure 1(B), and autoclaved. Figure 1(C) shows a representative photo of cultures from our control (not autoclaved) and those samples that were autoclaved. The control sample appears visually cloudy while those samples that were autoclaved remain clear indicating a proliferation of organisms within the control (not autoclaved) sample. Furthermore, the optical density (OD 600 nm) data provided in figure 1(D) shows a significant (p = 0.0061) increase in the OD for the samples that were not autoclaved compared to the autoclaved samples. Additionally, the autoclaved sample shows an insignificant (p = 0.2527) difference from the blank (media only) samples indicating no significant proliferation within the autoclaved samples (figure 1(D)). Collectively, the data from the spore strip and pooled saliva tests demonstrate the autoclave settings are effective for sterilization utilizing both a standard organism and human saliva.
While sterility was demonstrated with human saliva in figure 1, no specific microorganisms were represented or identified in this experiment but merely what was culturable in a rich broth from a pooled human saliva sample. Specific experimentation utilizing COVID-19, influenza, or a number of other contagious orally communicated organisms would be advantageous. However, our lab is not equipped to perform such experimentation. Therefore, a surrogate biological indicator was utilized. The verification standard test strips contain spores of G. stearothermophilus, a United States Centers for Disease Control and Prevention (US CDC) recommended sterility indicator. G stearothemophilus spores are considered extremely difficult to neutralize due to high heat resistance [12]. Therefore, neutralization of G. stearothermophilus spores, as shown in figure 1(A), likely indicates sterilization of nearly all organisms under the autoclave conditions.
Autoclaving has many appeals as a sterilization option. As opposed to chemical sterilization, autoclaving does not introduce potential interferents with volatile data collection or present an inhalation hazard to subjects [8]. Additionally, autoclave-based sterility offers a reliable method of sterilization that can be consistently repeated over many cycles with minor consumables and utilities. Furthermore, autoclaves are easily used and widely available for easy integration at many sampling sites. Therefore, introducing ReCIVA masks and filters into the autoclaving program and sterile storage would minimally impact current workload at these facilities. Based on the data and logistical considerations, autoclaving represents a viable path forward for ReCIVA mask sterility and potential reuse.
3.3. Glass head test background results
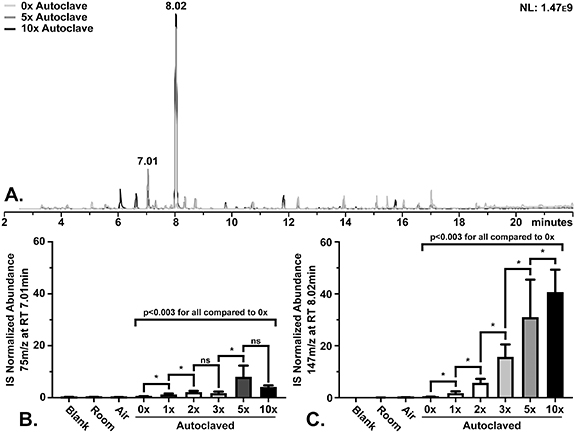
While the data presented in figure 1 suggest that autoclaving ReCIVA masks and filters can provide sterilization, previous data indicates background contamination within TD-GC-MS analysis resulting from siloxane compounds derived from the ReCIVA masks are present [7–10]. To evaluate if repeated autoclaving induces additional background in TD-GC-MS profiles, masks were repeatedly autoclaved as illustrated in supplemental data 2 and evaluated for discoloration and background contamination utilizing a glass head following each autoclave cycle. As shown in supplemental data 4, the repeated autoclaving of the masks and filters caused a gradual visually noticeable discoloration (yellowing), as the number of autoclave cycles increased compared to control (not autoclaved). However, no visible structural damage, cracking or hardening, was observed to either the mask or filter. While these results suggest a physical change in the color of the masks and filters, it was hypothesized that the effect of repeated autoclaving would not change the volatile background significantly. Figure 2(A) shows representative total ion chromatograms of background samples acquired following zero (new, 0×), five (5×), and ten (10×) times autoclaved masks and filters. The data show the primary contaminants detected were 75 m z−1 at 7.01 min and 147 m z−1 at 8.02 min overlapping with previous identifications of trimethyl silanol at RT 7.01 min and hexamethyl disiloxane at RT 8.02 min [7, 8, 10]. The contaminants will be here onward referred to as the compounds they were tentatively identified as, trimethyl silanol and hexamethyl disiloxane. Evaluation of these contaminants specifically through selected ion monitoring (SIM) illustrates a significant increase (p < 0.05) in the abundance of both contaminants after a single autoclave cycle (p = 0.0006 for trimethyl silanol and p < 0.0001 for hexamethyl disiloxane) (figures 2(B) and (C)). Hexamethyl disiloxane showed a significant stepwise increase in abundance with the addition of more autoclave cycles, whereas trimethyl silanol illustrated a non-significant difference between two, three, five, and ten autoclave cycles (figures 2(B) and (C)). These data illustrate that repetitive autoclaving of ReCIVA masks and filters induces both a physical and volatile profile change to the background of TD-GC-MS data.
Figure 2. Mask VOC background following repeated autoclaving from glass head test. (A) Representative TD-GC-MS profiles from masks autoclaved 0 (new), 5, and 10 times. The internal standard (IS) normalized abundances for (B) 75 m z−1 at RT 7.01 min (trimethyl silanol) and (C) 147 m z−1 at RT 8.02 min (hexamethyl disiloxane) detected from masks following repetitive autoclaving. Error bars signify the 95% confidence interval. * indicates statistical significance (p < 0.05) as determined by Wilcoxon signed rank test and ns signifies non-significance. The data illustrate a significant change in the background following repetitive autoclave cycles.
Download figure:
Standard image High-resolution imageThe foremost concern with background contaminants is that large amounts will cause significant peaks in the GC-MS data that could conceal potential peaks of interest. In previous studies, dry baking the masks and filters generally removed or reduced the volatile background contaminants associated with the masks [9]. However, possibly due to the moisture and/or pressure used in autoclaving, more contaminants were detected after autoclaving. For example, hexamethyl disiloxane illustrated an estimated 98.4% increase in abundance after five and an approximately 99.2% increase after ten autoclave cycles, compared to new masks. There is an approximate 50.8% increase in peak abundance between five and ten autoclave cycles from hexamethyl disiloxane. The increase in peak area, particularly from hexamethyl disiloxane, could obscure other peaks, such as trichloroethane (7.92 min), cyclohexane (8.03 min), carbon tetrachloride (8.11 min), and if large enough, benzene (8.31 min). In order to protect potential peaks of interest from masking, these results indicate that limiting each mask to three cycles of autoclaving and reuse would be ideal. However, if a targeted analysis is intended, it is possible that the masks could be reused more often through additional autoclave cycles. Additional experimentation would be required to confirm this hypothesis.
The background data illustrated in figure 2 demonstrate an increase in abundance of several siloxane compounds following repeated autoclaving cycles. These results represent a protocol where the masks were tested, autoclaved, allowed to stand overnight, and tested again. It is possible that there could be an accumulation of background emitted from a mask when autoclaved and stored, either open or under vacuum, for longer periods of time. While an experiment to test this scenario was not performed here, the data would add additional information pertaining to logistics, i.e. how long a mask could be stored following autoclaving prior to use. Therefore, while reuse via autoclaving is plausible, more research is required to fully utilize this approach.
3.4. Filter test results
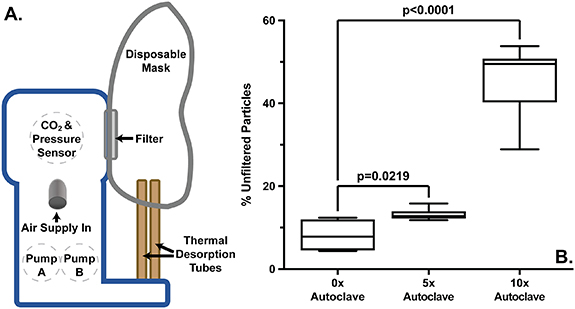
The discoloration of the ReCIVA filters shown in supplemental data 4 suggests a physical change as the number of repetitive autoclaving cycles increased. While mask discoloration was observed, no visible damage to the silicon masks was found. However, it is hypothesized that physical changes to the filters could impart reduced functionality for removing exhaled particulates. The filter rests between the subject's face and the ReCIVA hardware as shown in figure 3(A) and is hypothesized to trap pathogenic particles exhaled by the subject. To test for changes in the filter's functionality, a custom aerosol test chamber was constructed (supplemental data 3). A particle size of 1 μm was selected as the size of the smallest exhaled particles likely to contain and transmit communicable diseases, such as the influenza virus [18, 19]. The device generated and introduced 1 μm particles into one side of a chamber provided with N2 flow through a ReCIVA filter. Particles are counted on each side of the filter within the chamber to determine the filtering percentage of the filter. The data depicted in figure 3(B) show the new masks offer more filterability compared to the five-times-autoclaved filters (p = 0.0219) and the ten-times-autoclaved filters (p < 0.0001). These results demonstrate that autoclaving masks five times induces an approximate 5% decrease in the filters' removal of particles (0x μ = 8.1% ± 6.5%, 5x μ = 13.1% ± 1.5% unfiltered particles) whereas ten-times-autoclaving allowed approximately 45.0 ± 8.0% unfiltered particles. These data suggest that with repetitive autoclaving there is a gradual degradation in the ability of the ReCIVA filters to remove particulates.
Figure 3. The filter test for efficiency following repetitive autoclaving. (A) An illustration of the components of the ReCIVA device. (B) A box-plot of the percent unfiltered 1 μm particles allowed by filters following 0 (new), 5, and 10 times of autoclaving. Significance was determined by unpaired t-test and error bars represent the 95% confidence interval. The data show a significant increase in unfiltered particles following 5 and 10 times of autoclaving compared to new masks.
Download figure:
Standard image High-resolution imageAs our data indicate, the new masks' filters allow approximately 8.1% of 1 µm particles to pass (figure 3). As a result, such filtration may not be a reliable guard against microorganisms of this size projeced from the device. For example, it is estimated that an individual in close proximity to an infected person with influenza will inhale approximately 153 aerosolized viral particles of 1–4 µm in size per liter of air [20]. As the minimum infectious load of influenza is 1.95 × 103 viral particles, an individual breathing 6 l min−1 could be exposed to the minimum influenza infectious load, through the back of the ReCIVA, after 8–12 min using a new ReCIVA mask and filter [21]. A filter autoclaved 5×, with the reduced filterability, would decrease the time to potential infection to approximately 7–10 min of exposure. The time required to conduct samplings, mean ReCIVA breath collection is approximately 7 min 12 s, permits transmission even with new ReCIVA masks. Therefore, it is recommended that researchers wear PPE to adequately protect themselves from airborne pathogens and new or autoclaved ReCIVA masks are used to protect the participants [8]. It is hypothesized the filter's ultimate function, to protect the ReCIVA hardware from larger particles potentially exhaled during sampling, should not be impacted by a small loss of 1 µm particulate filtration function (∼5%) over the first five autoclave cycles.
3.5. Exhaled breath results from peppermint exposure test
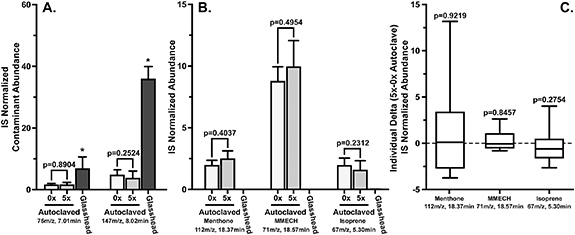
The data in figures 1–3 suggest both physical and functional changes in ReCIVA masks and filters resulting from repeated autoclave cycles. To evaluate the impact of repetitive autoclave cycles on exhaled breath collections, a peppermint experiment using a Lifesavers peppermint lozenge was performed. SIM analysis of trimethyl silanol (p = 0.8904) and hexamethyl disiloxane (p = 0.2524) show no statistical difference in abundance between new masks and masks autoclaved five times (figure 4(A)). Interestingly, the data show significantly lower abundances of the contaminant compounds when breath was sampled with the autoclaved masks than when the masks were evaluated with the glass head. These SIM results are similar to the abundances obtained in figures 2(B) and (C) for masks autoclaved five times. Evaluation of the headspace of the peppermint tentatively identified menthone and 1-methyl-4-(1-methylethyl)-cyclohexanol (MMECH) as the predominant volatile compounds emitted from the Lifesavers lozenges (supplemental data 5). SIM analysis of menthone (112 m z−1 at RT 18.37 min) and MMECH (71 m z−1 at RT 18.57 min) show an insignificant difference (p = 0.1454 and Δ p = 0.9219 menthone, p = 0.1743 and Δ p = 0.3570 MMECH) in the exhaled breath results obtained from new masks from those autoclaved five times (figures 4(B) and (C)). To more clearly visualize the variability among individuals' pre/post values, the delta values were calculated and displayed in figure 4(C). The large error bars in figure 4(C) highlight the variability of individual's day-to-day metabolism of exogenous, peppermint compounds, and the inherent variability of endogenous volatiles, such as isoprene. Overall, these data suggest that autoclaving does not impact exhaled breath results through monitoring exogenous peppermint compounds.
Figure 4. Comparison of the contaminant, peppermint, and breath compounds from new masks and masks autoclaved five (5×) times. (A) The abundances of contaminants (75 m z−1 at RT 7.01 min, 147 m z−1 at RT 8.02 min) from exhaled breath collections with repetitive autoclaving cycles. (B) The abundance of peppermint related compounds (menthone, 1-methyl-4-(1-methylethyl)-cyclohexanol (MMECH)) and isoprene (67 m z−1 at RT 5.30 min) from breath samples collected following a timed peppermint ingestion. (C) Box plots of delta abundances between peppermint related compounds and isoprene (5×–0× autoclaved) from exhaled breath collected following a timed peppermint ingestion. The data illustrate an insignificant difference in exogenous (peppermint) and endogenous (isoprene) compounds between new ReCIVA masks and filters and those autoclaved five times.
Download figure:
Standard image High-resolution imagePeppermint was chosen for this experiment due to its prior use as a benchmarking tool for sampling, including the ReCIVA, and TD-GC-MS analysis [22, 23]. It is possible for other volatiles to behave differently, due to changing surface interactions, within the ReCIVA masks. To further evaluate the likelihood of changing surface interactions affecting compound detection, isoprene was also measured from the samplings. SIM analysis of isoprene (67 m z−1 at RT 5.30 min), a representative endogenous compound, illustrates no significant difference in abundance from samples obtained from ReCIVA masks autoclaved repeatedly (5×, p = 0.3778 and Δp = 0.2754, figures 4(B) and (C)) [24–27]. Collectively, the data indicate both exogenous and endogenous exhaled breath compounds are statistically similar in breath samples obtained from new or masks autoclaved five times. However, it is possible that specific volatile compounds could have different surface interactions with the autoclaved masks. Therefore, it is recommended that researchers with specific compound(s) of interest evaluate the compound(s) within autoclaved masks prior to incorporating into a sampling protocol. The results suggest that implementation of autoclaving masks and filters into sampling protocols do not illustrate issues with data collection of breath compounds using the ReCIVA device.
These data indicate that glass head testing may not be representative of the abundance of the background contaminants that will be found in exhaled breath samples (figure 4(A), p > 0.05 for trimethyl silanol and hexamethyl disiloxane) [8]. It is hypothesized that there could be several factors contributing to the increased abundances of the background compounds trimethyl silanol and hexamethyl disiloxane when sampled using glass head experiments. For example, these compounds may be more likely to deposit on the subject's skin/nose or be inhaled during collection, thereby decreasing in abundance, while the compounds remain volatile on the glass head. Additionally, the change in airflow and/or humidity caused by a subject's breathing may decrease the amount of these compounds emitted from the masks. Additional research will be required to fully understand the dynamic background components between breath samples and the glass head experiments. For whatever reason, the decrease in detection of the primary background compounds when conducting trials with human subjects represents an encouraging sign for mask reuse after sterilization by autoclave. However, until the source of the difference in background contamination between exhaled breath and samples collected using the glass head is determined, it is advised to limit mask reuse to three autoclave cycles.
4. Conclusion
Since its release, the ReCIVA device has allowed for consistent, streamlined breath sampling and analysis from all individuals including those under respiratory distress. However additional logistical and operational costs of single use consumables is hypothesized to hinder the widespread use of the ReCIVA device. Autoclaving is a widely available sterilization technique that can allow for the reuse of ReCIVA consumable silicon masks, thus reducing issues around acquisition (supply chain) and disposal of large quantities of masks. The data illustrate autoclaving of masks and filters provides sterilization with an increase in background levels of siloxane compounds resulting from repeated autoclaving cycles. Additionally, data show that exhaled breath results utilizing a peppermint ingestion protocol show significant difference if sampled with a new mask versus a mask autoclaved five times. Collectively, the data indicate that ReCIVA masks and filters can be safely and successfully sterilized by autoclave for reuse up to a recommended three repetitions. However, organizational controls to manage and track masks would be required to ensure proper reuse. Especially where autoclaves are already easily accessible, implementation of ReCIVA mask and filter autoclave protocols into large scale research or diagnostic protocols could be feasible.
Data availability statement
The data cannot be made publicly available upon publication due to legal restrictions preventing unrestricted public distribution. The data that support the findings of this study are available upon reasonable request from the authors.
Disclaimer
The views expressed are those of the authors and do not reflect the official guidance or position of the United States Government, the Department of Defense or of the United States Air Force.
Supplementary data (0.5 MB PDF)