Abstract
The development of hyperpolarized bioresponsive probes for magnetic resonance imaging (MRI) applications is an emerging and rapidly growing topic in chemistry. A wide range of hyperpolarized molecular biosensors for functional MRI have been developed in recent years. These probes comprise many different types of small-molecule reporters that can be hyperpolarized using dissolution dynamic nuclear polarization and parahydrogen-induced polarization or xenon-chelated macromolecular conjugates hyperpolarized using spin-exchange optical pumping. In this Perspective, we discuss how the amplified magnetic resonance signals of these agents are responsive to biologically relevant stimuli such as target proteins, reactive oxygen species, pH or metal ions. We examine how functional MRI using these systems allows a great number of biological processes to be monitored rapidly. Consequently, hyperpolarized bioresponsive probes may play a critical role in functional molecular imaging for observing physiology and pathology in real time.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dale, B. M., Brown, M. A., Semelka, R. C. & Brown, M. A. MRI: Basic Principles and Applications 5th edn (John Wiley & Sons, 2015).
Nikolaou, P., Goodson, B. M. & Chekmenev, E. Y. NMR hyperpolarization techniques for biomedicine. Chem. Eur. J. 21, 3156–3166 (2015).
Keshari, K. R. & Wilson, D. M. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 43, 1627–1659 (2014).
Meersmann, T & Brunner, E. Hyperpolarized Xenon-129 Magnetic Resonance: Concepts, Production, Techniques and Applications (Royal Society of Chemistry, 2015).
Schröder, L. in Hyperpolarized and Inert Gas MRI (eds Albert, M. S. & Hane, F. T.) 263–277 (Academic Press, 2017).
Hövener, J.-B. et al. Parahydrogen-based hyperpolarization for biomedicine. Angew. Chem. Int. Ed. 57, 11140–11162 (2018).
Sherry, A. D. & Woods, M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu. Rev. Biomed. Eng. 10, 391–411 (2008).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Angelovski, G. Heading toward macromolecular and nanosized bioresponsive MRI probes for successful functional imaging. Acc. Chem. Res. 50, 2215–2224 (2017).
Li, H. & Meade, T. J. Molecular magnetic resonance imaging with Gd(iii)-based contrast agents: challenges and key advances. J. Am. Chem. Soc. 141, 17025–17041 (2019).
Wei, H., Frey, A. M. & Jasanoff, A. Molecular fMRI of neurochemical signaling. J. Neurosci. Meth. 364, 109372 (2021).
Kondo, Y., Nonaka, H., Takakusagi, Y. & Sando, S. Design of nuclear magnetic resonance molecular probes for hyperpolarized bioimaging. Angew. Chem. Int. Ed. 60, 14779–14799 (2021).
The top 10 causes of death. World Health Organization https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
Nelson, S. J. et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci. Transl. Med. 5, 198ra108 (2013).
Ardenkjær-Larsen, J. H. et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl Acad. Sci. USA 100, 10158–10163 (2003).
Doura, T., Hata, R., Nonaka, H., Ichikawa, K. & Sando, S. Design of a 13C magnetic resonance probe using a deuterated methoxy group as a long-lived hyperpolarization unit. Angew. Chem. Int. Ed. 51, 10114–10117 (2012).
Lippert, A. R., Keshari, K. R., Kurhanewicz, J. & Chang, C. J. A hydrogen peroxide-responsive hyperpolarized 13C MRI contrast agent. J. Am. Chem. Soc. 133, 3776–3779 (2011).
Wibowo, A., Park, J. M., Liu, S.-C., Khosla, C. & Spielman, D. M. Real-time in vivo detection of H2O2 using hyperpolarized 13C-thiourea. ACS Chem. Biol. 12, 1737–1742 (2017).
Jindal, A. K. et al. Hyperpolarized 89Y complexes as pH sensitive NMR probes. J. Am. Chem. Soc. 132, 1784–1785 (2010).
Hata, R., Nonaka, H., Takakusagi, Y., Ichikawa, K. & Sando, S. Design of a hyperpolarized 15N NMR probe that induces a large chemical-shift change upon binding of calcium ions. Chem. Commun. 51, 12290–12292 (2015).
Mishra, A., Pariani, G., Oerther, T., Schwaiger, M. & Westmeyer, G. G. Hyperpolarized multi-metal 13C-sensors for magnetic resonance imaging. Anal. Chem. 88, 10790–10794 (2016).
Nonaka, H. et al. A platform for designing hyperpolarized magnetic resonance chemical probes. Nat. Commun. 4, 2441 (2013).
Hundshammer, C. et al. Hyperpolarized amino acid derivatives as multivalent magnetic resonance pH sensor molecules. Sensors 18, 600 (2018).
Nishihara, T. et al. A strategy to design hyperpolarized 13C magnetic resonance probes using [1-13C]α-amino acid as a scaffold structure. Chem. Asian J. 12, 949–953 (2017).
Düwel, S. et al. Imaging of pH in vivo using hyperpolarized 13C-labelled zymonic acid. Nat. Commun. 8, 15126 (2017).
Gallagher, F. A. et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 453, 940–943 (2008).
Hundshammer, C. et al. Deuteration of hyperpolarized 13C-labeled zymonic acid enables sensitivity-enhanced dynamic MRI of pH. ChemPhysChem 18, 2422–2425 (2017).
Korenchan, D. E. et al. Dicarboxylic acids as pH sensors for hyperpolarized 13C magnetic resonance spectroscopic imaging. Analyst 142, 1429–1433 (2017).
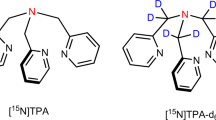
Suh, E. H., Park, J. M., Lumata, L., Sherry, A. D. & Kovacs, Z. Hyperpolarized 15N-labeled, deuterated tris(2-pyridylmethyl)amine as an MRI sensor of freely available Zn2+. Commun. Chem. 3, 185 (2020).
Wang, S. et al. Amino acid-derived sensors for specific Zn2+ detection using hyperpolarized 13C magnetic resonance spectroscopy. Chem. Eur. J. 25, 11842–11846 (2019).
Lee, Y., Zeng, H., Ruedisser, S., Gossert, A. D. & Hilty, C. Nuclear magnetic resonance of hyperpolarized fluorine for characterization of protein–ligand interactions. J. Am. Chem. Soc. 134, 17448–17451 (2012).
Pinon, A. C., Capozzi, A. & Ardenkjær-Larsen, J. H. Hyperpolarization via dissolution dynamic nuclear polarization: new technological and methodological advances. Magn. Reson. Mater. Phys. Biol. Med. 34, 5–23 (2021).
Khan, A. S. et al. Enabling clinical technologies for hyperpolarized 129xenon magnetic resonance imaging and spectroscopy. Angew. Chem. Int. Ed. 60, 22126–22147 (2021).
Shapiro, M. G. et al. Genetically encoded reporters for hyperpolarized xenon magnetic resonance imaging. Nat. Chem. 6, 630–635 (2014).
Zemerov, S. D. & Dmochowski, I. J. Cryptophane–xenon complexes for 129Xe MRI applications. RSC Adv. 11, 7693–7703 (2021).
Riggle, B. A., Wang, Y. & Dmochowski, I. J. A. A “smart” 129Xe NMR biosensor for pH-dependent cell labeling. J. Am. Chem. Soc. 137, 5542–5548 (2015).
Jayapaul, J. & Schröder, L. Probing reversible guest binding with hyperpolarized 129Xe-NMR: characteristics and applications for cucurbit[n]urils. Molecules 25, 957 (2020).
Kotera, N. et al. A sensitive zinc-activated 129Xe MRI probe. Angew. Chem. Int. Ed. 51, 4100–4103 (2012).
Schröder, L., Lowery, T. J., Hilty, C., Wemmer, D. E. & Pines, A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science 314, 446–449 (2006).
Chambers, J. M. et al. Cryptophane zenon-129 nuclear magnetic resonance biosensors targeting human carbonic anhydrase. J. Am. Chem. Soc. 131, 563–569 (2009).
Witte, C. et al. Live-cell MRI with xenon hyper-CEST biosensors targeted to metabolically labeled cell-surface glycans. Angew. Chem. Int. Ed. 54, 2806–2810 (2015).
Schlundt, A. et al. A xenon-129 biosensor for monitoring MHC–peptide interactions. Angew. Chem. Int. Ed. 48, 4142–4145 (2009).
Klass, S. H. et al. Rotaxane probes for the detection of hydrogen peroxide by 129Xe hyperCEST NMR spectroscopy. Angew. Chem. Int. Ed. 58, 9948–9953 (2019).
Adams, R. W. et al. Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science 323, 1708–1711 (2009).
Cavallari, E. et al. The 13C hyperpolarized pyruvate generated by ParaHydrogen detects the response of the heart to altered metabolism in real time. Sci. Rep. 8, 8366 (2018).
Bhattacharya, P. et al. Parahydrogen-induced polarization (PHIP) hyperpolarized MR receptor imaging in vivo: a pilot study of 13C imaging of atheroma in mice. NMR Biomed. 24, 1023–1028 (2011).
Shchepin, R. V. et al. 15N hyperpolarization of imidazole-15N2 for magnetic resonance pH sensing via SABRE-SHEATH. ACS Sens. 1, 640–644 (2016).
Olaru, A. M., Burns, M. J., Green, G. G. R. & Duckett, S. B. SABRE hyperpolarisation of vitamin B3 as a function of pH. Chem. Sci. 8, 2257–2266 (2017).
Iali, W. et al. Hyperpolarising pyruvate through signal amplification by reversible exchange (SABRE). Angew. Chem. Int. Ed. 58, 10271–10275 (2019).
Iali, W., Rayner, P. J. & Duckett, S. B. Using parahydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates. Sci. Adv. 4, eaao6250 (2018).
Acknowledgements
G.A. gratefully acknowledges financial support from the Shanghai Municipal Science and Technology Major Project (grant no. 2019SHZDZX02) and National Natural Science Foundation of China (grant nos. 22174154 and T2250710181). G.W. gratefully acknowledges financial support from Jiangsu University (grant no. 5501310019), the Natural Science Foundation of Jiangsu Province (grant no. BK20220528) and the National Natural Science Foundation of China (grant no. 22207046).
Author information
Authors and Affiliations
Contributions
G.A. conceived the idea for this Perspective. G.A., B.J.T. and G.W. contributed to discussions and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks George Lu, Mor-Miri Mishkovsky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1 and Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Angelovski, G., Tickner, B.J. & Wang, G. Opportunities and challenges with hyperpolarized bioresponsive probes for functional imaging using magnetic resonance. Nat. Chem. 15, 755–763 (2023). https://doi.org/10.1038/s41557-023-01211-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01211-3