Plant trait-mediated drag forces on seedlings of four tidal marsh pioneer species
- 1Landscape Ecology and Environmental System Analysis, Institute of Geoecology, Technische Universität Braunschweig, Braunschweig, Germany
- 2Berlin-Brandenburg Institute of Advanced Biodiversity Research (BBIB), Berlin, Germany
- 3Ecosphere Research Group, University of Antwerp, Antwerp, Belgium
- 4Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Wadden Sea Research Station, List, Germany
- 5Biogeography and Biogeomorphology Group, University of Cambridge, Cambridge, United Kingdom
- 6Netherland Institute for Sea Research (NIOZ), Yerseke Spatial Ecology, Yerseke, Netherlands
- 7Faculty of Geosciences, Utrecht University, Utrecht, Netherlands
- 8Department of Geography, Trinity College Dublin, Dublin, Ireland
- 9Ludwig-Franzius-Institute for Hydraulic, Estuarine and Coastal Engineering, Leibniz University Hannover, Hannover, Germany
Salt marshes play an important role in coastal protection by reducing the impact of waves and shoreline erosion risks. While mature vegetation is responsible for the persistence and stability of marsh ecosystems, seedling survival of pioneer species is especially crucial for marsh propagation. Marsh seedlings, however, may be threatened by climate change induced increased coastal storm surge intensity and accompanying (extreme) wave conditions, imposing stronger drag forces on marsh seedlings. We test the hypothesis that drag forces experienced by seedlings increase with horizontal orbital velocity (Uw) in a species-specific manner, and that the drag forces experienced are individual-plant trait-mediated. To test our hypotheses, seedlings of four contrasting pioneer marsh species (Bolboschoenus maritimus, Schoenoplectus tabernaemontani, Spartina anglica, and Puccinellia maritima) were exposed to storm wave conditions in a flume, where Uw and experienced drag forces were measured. Linear mixed effect models demonstrated that seedling’s susceptibility to storm wave conditions is at least partly mediated by individual plant traits. Drag forces experienced by seedlings tended to increase with Uw, and with stem length and diameter. The interplay of both traits was complex, with increasing stem length being the most important trait accounting for increases in drag forces experienced at low to moderate Uw, while the stem diameter became more important with increasing Uw. Furthermore, experienced drag forces appeared to be affected by species-specific traits such as rigidity and leaf growth, being highest for Bolboschoenus maritimus and lowest for Puccinellia maritima. Our results provide important mechanistic insights into the drivers of tidal marsh seedling vulnerability to storm wave conditions due to experienced drag, both based on the traits of individual plants and species-specific ones. This type of knowledge is of key importance when modelling saltmarsh establishment and resilience under climate change.
1 Introduction
Intertidal wetlands are often characterized by the dynamic coexistence of a low-elevation non-vegetated tidal flat and a higher-elevation vegetated marsh (Fagherazzi et al., 2006), which have been identified as alternative stable ecosystem states resulting from positive feedbacks between sediment elevation and vegetation growth (van de Koppel et al., 2005; Marani et al., 2007; Wang and Temmerman, 2013). Vegetation can establish above a critical elevation threshold, as elevation determines, e.g., frequency and duration of tidal inundation, strength of hydrodynamic forces and sedimentary processes (see ‘windows of opportunity’ concept proposed by Balke et al., 2011; Balke et al., 2013). Once vegetation is established, it leads in turn to attenuation of waves and currents, and trapping of sediments, which further stimulates vegetation growth, hence creating a positive, so-called bio-geomorphic feedback (Bouma et al., 2009b; Bouma et al., 2014).
Shifts from the bare to vegetated ecosystem state are driven by pioneer marsh plant establishment, which may be limited by the hydrodynamic forces acting on the plants. Wave exposure generally increases with decreasing elevation in the intertidal zone (Mudd, 2011), and when wave energy exceeds a critical threshold, pioneer plant establishment and development is hampered (Friess et al., 2012; Cao et al., 2020; Zhu et al., 2020). Drag forces result from friction between the water flow and the plant shoot (e.g. Denny, 2021), and are known to be a function of both hydrodynamic parameters, such as horizontal orbital velocities (Uw; typically increasing with wave height), and plant traits (e.g. Paul et al., 2016; Vuik et al., 2018). After initial establishment, developing seedlings may suffer from strong mechanical stress caused by wave-induced drag forces (Paul et al., 2016; Rupprecht et al., 2017; Cao et al., 2020; Shao et al., 2020), potentially leading to breakage of shoots and uprooting (Rupprecht et al., 2017; Vuik et al., 2018). This can be exacerbated by sediment scouring around the stems as another consequence of wave exposure (Bouma et al., 2009a; Silinski et al., 2016).
In response to hydrodynamic stress, marsh pioneer plants have developed different morphological trait adaptations and growth strategies (e.g. Bornette and Puijalon, 2011; Puijalon et al., 2011; Silinski et al., 2018). Schoutens et al. (2021) suggested that a small stem diameter and a flexible plant morphology increase seedling survival under extreme wave conditions. That is, when simulating extreme wave events, the authors found a lower rate of seedling loss and a lower bending angle for the smaller individuals of the salt marsh pioneer species Spartina anglica and Puccinellia maritima, compared to the larger individuals of the brackish marsh pioneer species Schoenoplectus tabernaemontani and Bolboschoenus maritimus. Species-specific variation in how plants cope with similar environmental conditions can result in species sorting but also in intraspecific phenotypic responses (Silinski et al., 2018; Cao et al., 2020; Shao et al., 2020; Schoutens et al., 2022), ultimately, causing smaller and more flexible species to grow at wave-exposed locations, while larger and stiffer species may be found under more wave-sheltered conditions (Carus et al., 2017; Silinski et al., 2018; Schoutens et al., 2022). Cao et al. (2020) further indicated morphological plasticity of three marsh plant species: after wave exposure over seven weeks, the flexural rigidity of seedlings decreased, whereas the root/shoot biomass ratio increased, possibly to avoid high drag forces. Yet, the extent in which seedlings with low stature, small diameter, and/or low rigidity during storms indeed face significantly lower drag forces than the slightly larger and stiffer individuals, has rarely been explicitly investigated (e.g. Heuner et al., 2015; Paul et al., 2016).
As a consequence of climate change, we expect rising sea levels and increasing storm surge frequency and intensity in certain places, resulting in enhanced exposure of tidal marsh plants to waves, habitat loss and coastal squeeze under human impact (Spencer et al., 2016; Colombano et al., 2021; Hu et al., 2021). Since ecosystem services such as carbon storage, wave attenuation, and mitigation of shoreline erosion are particularly associated with vegetated tidal marsh areas (Shepard et al., 2011; Lau, 2013; Himes-Cornell et al., 2018), future protection and restoration of these marshes is an urgent task. Apart from generating favorable bed elevation, soil conditions and habitat connectivity (e.g., for the propagation of seeds and propagules; Wolters et al., 2008; Zhao et al., 2020), it is crucial to understand how pioneer species respond to wave induced stresses in order to ensure establishment and survival even under potentially altered wave climate (Zhu et al., 2020). However, knowledge about the drag forces acting on young marsh plants under high wave loads, and on how plant traits influence seedlings susceptibility to these forces, is scarce, thereby hampering modelling climate change scenarios.
In this study, we experimentally examined relations between horizontal orbital velocity (Uw) and drag forces (FD) acting on seedlings of four pioneer tidal marsh plant species, and how these relations were mediated by plant traits. Seedlings were exposed to different combinations of extreme wave heights and wave periods. We hypothesized first, that stem length and diameter control both interspecific and intraspecific variation in the experienced drag forces. Second, we expected that drag forces increase with Uw in a species-specific manner, indicating that further morphological traits such as rigidity or foliage additionally affect experienced drag forces.
2 Material and methods
2.1 Study species
To determine the effects of extreme wave conditions on the drag force exerted on pioneer marsh seedlings, stems of the two brackish marsh species Bolboschoenus maritimus (L.) and Schoenoplectus tabernaemontani (C.C. Gmel.) as well as of the two salt marsh species Spartina anglica (C.E. Hubb.) and Puccinellia maritima (Huds.) were exposed to different combinations of wave heights and wave periods under laboratory conditions. All four species reproduce either by clonal propagation or seed dispersal and colonize bare mudflats in NW European estuaries (Schoutens et al., 2021). While B. maritimus and S. tabernaemontani grow in the upper part of estuaries, S. anglica and P. maritima can be found in more seaward and often more exposed areas. Since (re-)colonization is pivotal for tidal marsh persistence and advance, it is, thus, important to understand the plant-flow-interaction of the four species, especially under potential harmful storm surge conditions and even when occurring as single plants in front of the dense vegetation stands.
The study species show distinct phenotypical properties that help them cope with environmental conditions. The leafy B. maritimus is characterized by thicker stems than S. tabernaemontani (e.g. Schoutens et al., 2021) and has triangular cross-sections (Carus et al., 2016). In contrast, S. tabernaemontani produces more flexible, almost circular and leafless stems. Its flexural stiffness of adult plants (0.013 Nm²) is distinctly lower than that of B. maritimus (0.047 Nm²; Schoutens et al., 2020). Commonly, both species form monospecific stands with a distinct zonation. Whereas the leafless S. tabernaemontani is growing in the seaward pioneer zone, B. maritimus grows more landward in a greater distance from the marsh edge (Heuner et al., 2019; Schulte Ostermann et al., 2021). S. anglica occurs primarily in low elevated, seaward pioneer zones of dynamic salt marshes (Adam, 1990). Shoots and leaves are stiff and upright growing (Bouma et al., 2005). P. maritima can be found in more sheltered pioneer salt marshes and shows flexible stems with leaves covering the sediment surface (Möller et al., 2014; Schoutens et al., 2021). Both species grow in dense tussocks (Bouma et al., 2009a) and can act as ecosystem engineers by enhancing the deposition of sediments (Sánchez et al., 2001; Langlois et al., 2003), and by altering the water flow (Bouma et al., 2013).
2.2 Preparation of experimental plants
Seedlings of all four species were grown from seeds on moist substrate following stratification (4°C during the night and room temperature during the day). In mid-June 2018, three weeks after stratification was initiated, seedlings were planted in fertilized (slow release Osmocote, Substral) sand from the Scheldt estuary (SW Netherlands) and grown under greenhouse conditions. After the transport to the laboratory facility in Hannover at the beginning of August 2018, seedlings were stored outside and irrigated with fresh water. During the experiment, the age of the seedlings ranged between 10 to 14 weeks.
2.3 Experimental setup
The study was conducted in the Large Wave Flume of the Forschungszentrum Küste (FZK) in Hannover, Germany and lasted for three weeks. We set up an experimental platform (40 m long) in the center of the flume (300 m long, 5 m wide, 7 m deep). The drag force experiment was installed at the front of the experimental platform (Figure 1) where four drag sensors were installed 20 cm apart and flush with the platform’s floor. Drag forces were measured using two sensors each developed by Deltares (custom-made device) and by Apex (ATI Gamma, North Carolina, USA; see ATI Industrial Automation, 2023). The Deltares sensors operate on the wheatstone bridge principle and were capable of recording forces in the direction, and counter to, wave propagation (Paul et al., 2016). The ATI Gamma is capable of recording forces that act on the sensor head in all three dimensions, but for sake of comparison on the data matching the setup of the Deltares sensors was used. Seedlings of all four species were equally distributed among the different sensors. Irregular waves were generated with a JONSWAP wave spectrum (Hasselmann et al., 1973) for a duration of 1000 waves with an inundation depth of 1.5 m to simulate storm flood conditions. Over a period of three weeks (August 14–31, 2018), we performed twelve different wave runs with five different combinations of significant wave height (Hs) and peak period (Tp) (Hs: 0.3-0.8 m; Tp: 2.5-5.8 s; see Table 1). Between wave runs, the flume was slowly drained to prevent currents during drainage that erode the soil surface.
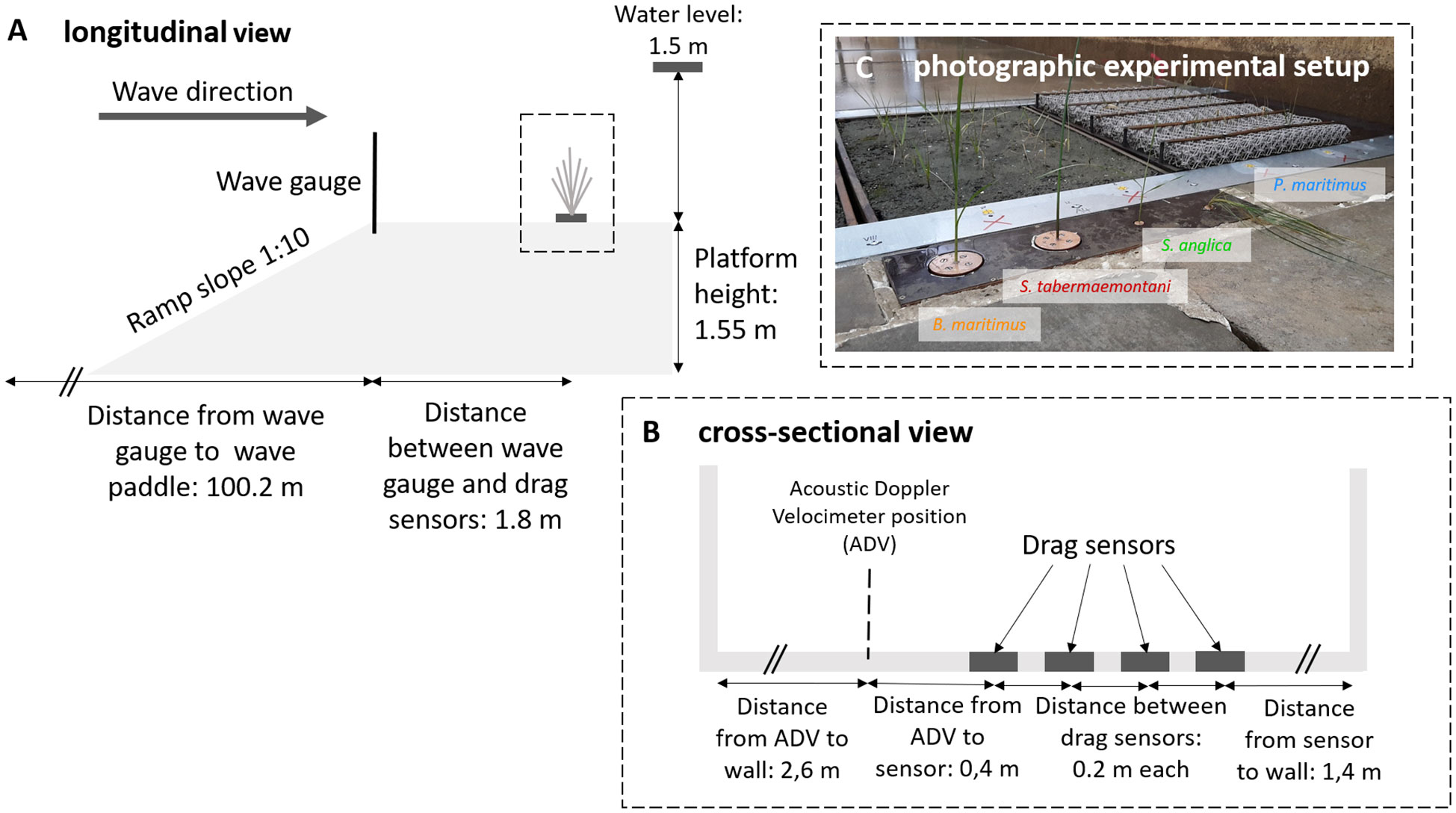
Figure 1 Schematic, not to scale, overview of instrument setup in the large wave flume (GWK, Hannover, Germany), indicating the instruments’ locations on the elevated platform (A), a cross-sectional view on the instrument setup in more detail (B) and a photographic plant setup (C).

Table 1 Significant wave height (Hs, m), wave peak period (Ts, s) and average horizontal orbital vlelocity (Uw) per wave run which consisted of 1000 waves. All wave runs included randomly generated waves of the JONSWAP spectrum, representing typical North Sea conditions.
Before each wave run, individual shoots of the study species were cut directly above the ground and individual seedling traits were measured. Afterwards, the single shoots were glued onto a wooden plate which was then screwed onto a drag sensor. This fitting transferred the forces imposed by the water onto the seedling’s aboveground biomass to the force sensor. It was thus possible to record forces acting on the individual shoots as it would be the case under natural conditions during (re-)colonization. In addition, control measurements were made with a wooden plate without any seedling attached to a drag sensor. Stem length was recorded with a ruler (resolution: 0.1 cm) and stem diameter was obtained with a caliper gauge (resolution: 0.01 cm).
Wave heights were recorded using a wave gauge array, installed above the platform fronting the drag sensors. An Acoustic Doppler Velocimeter (ADV, Nortek Vector) was placed in line with the drag sensors on the central axis of the flume. The ADV measured horizontal orbital velocities (Uw) at 5 cm above the bed at 25 Hz, whereas wave gauges and drag sensors recorded time series at 100 Hz.
2.4 Hydrodynamic background of measured drag forces
In this flume study, profile drag, i.e. the drag force produced by a surface standing perpendicular to the water flow (Bouma et al., 2005), was experimentally measured. The horizontal drag force FD is theoretically described as
where ρ is the fluid’s density, Ap is the frontal area, CD is the drag coefficient and U is the mean horizontal fluid velocity (Stone and Shen, 2002). Plants are able to reduce or avoid drag (e.g. Bornette and Puijalon, 2011), by, for example, reconfiguration (Bouma et al., 2005), or to increase their tolerance to drag by investments in tissue rigidity or anchoring (e.g. Puijalon et al., 2008; avoidance/tolerance strategies in Silinski et al., 2018).
2.5 Data processing
Time series data of horizontal orbital velocity Uw measured by the ADV and recorded drag force data were used to quantify how much energy of the wave is absorbed by the aboveground biomass of the tested pioneer seedlings. To eliminate noise from the time series signals, a low pass (5 Hz) filter was applied to the wave gauge and ATI drag sensor data (ATI Industrial Automation, 2023). In the latter case, an additional high pass (0.2 Hz) filter was applied, and values with a correlation< 85% were removed from the ADV time series. Consecutively, the despiking algorithm of Mori et al. (2007) was applied to data from all drag sensors and the ADV to smooth data and close gaps. Subsequently, the absolute values for maximum drag forces were matched with the maximum orbital velocities for each wave period. While the ADV recorded the velocity components in three dimensions (along-flume, across-flume and vertical) only the along-flume component is considered in this study as it matches the direction in which the forces were recorded.
2.6 Statistical analysis
Species-specific differences in the recorded stem diameter and stem length were assessed with a non-parametric Kruskal-Wallis test, followed by a non-parametric pairwise comparison using Wilcoxon rank sum test with Holm correction. We used linear mixed-effect models (LMM) to investigate relations between Uw and experienced drag forces, and how these were mediated by individual traits. Therefore, species was treated as random factor. Fixed factors varied depending on the tested hypothesis.
LMM 1: First, to analyze in which way drag forces generally increase with flow velocity, we fitted a LMM including Uw as fixed factor. We tested whether including second- and third-degree polynomial terms improved the model. To investigate whether species significantly varied in drag forces, random-effect species terms were reduced, and likelihood ratio tests of model reductions were applied. The model (excluding any traits) had the following structure, with fixed coefficients in Roman and random coefficients in Greek:
where αj[i] is the random intercept varying by species (i.e. groups, j = 4 species). Including a varying slope did not further improve the model.
LMM 2: Second, to test whether drag forces were mediated by seedling’s traits, we fitted LMMs additionally including stem diameter and length as fixed effects. We considered models of different complexity, including one of the two traits at a time (LMM 2.1 = Length, LMM 2.2 = Diameter), and including both traits (LMM 2.3 = Length + Diameter). We also explored interactions between all fixed factors. Again, we applied likelihood ratio tests to test whether species random effects were still significant after accounting for differences in stem diameter and length among individuals. The final, full model (LMM 2.3) had the following structure:
where again αj[i] is the random intercept varying by species, and bi (Uwi) and zi (Traits) are the coefficients for the fixed effects. Note that the traits varied by shoot. Quadratic effects for Length and Diameter were not significant, and therefore not included in the final model.
Both the response variable drag force, and the predictor variable Uw were left-skewed and had to be transformed prior to the analysis. Drag force was log transformed; for Uw we instead applied a square root transformation, because the log transformation was too strong and yielded a right-skewed data distribution. All other variables were standardized (subtracting the mean, dividing by standard deviation). We also explored fitting generalized linear mixed-effect models (GLMM) with a log link to the untransformed response variable, (a) with a Gaussian error distribution, (b) with an Inverse Gaussian error distribution, and (c) with a Gamma error distribution; these models, however, gave an inferior fit.
We calculated Akaike’s Information Criterion (AIC, Akaike, 1974) for the null model excluding any fixed effects, but including the random effect for species, and the full models including all fixed and random effects. ΔAICNull is the difference in AIC between the null model and the full model. If ΔAIC > 2, the empirical support for the full model is considerably higher than for the simpler model (Burnham and Anderson, 2010). We further calculated the difference between the full models and simpler models excluding species traits, but still including Uw as a fixed effect.
We used R version 4.2.1 (R Core Team, 2022) for model fitting, with the LMMs fitted using the add-on library lme4 version 1.1-30 (Bates et al., 2015). P-values for fixed effects were approximated using Satterthwaite’s method as implemented in the package lmerTest version 3.1-3 (Kuznetsova et al., 2017). Marginal and conditional R²-Values for the LMMs were calculated using the function rsquared.glmm() in the package gabtool (Pigeon, 2016). 3D-Plots were generated using lattice version 0.20-45 (Sarkar, 2008).
3 Results
The JONSWAP spectra of the individual wave runs differed in wave height and period (see Table 1) which resulted in different Uw increased with the tested wave height scenarios (β = 0.64 ± 0.004 (SE), p< 0.001, R² =0.39); at the same time, the variation in Uw increased (Figure 2).
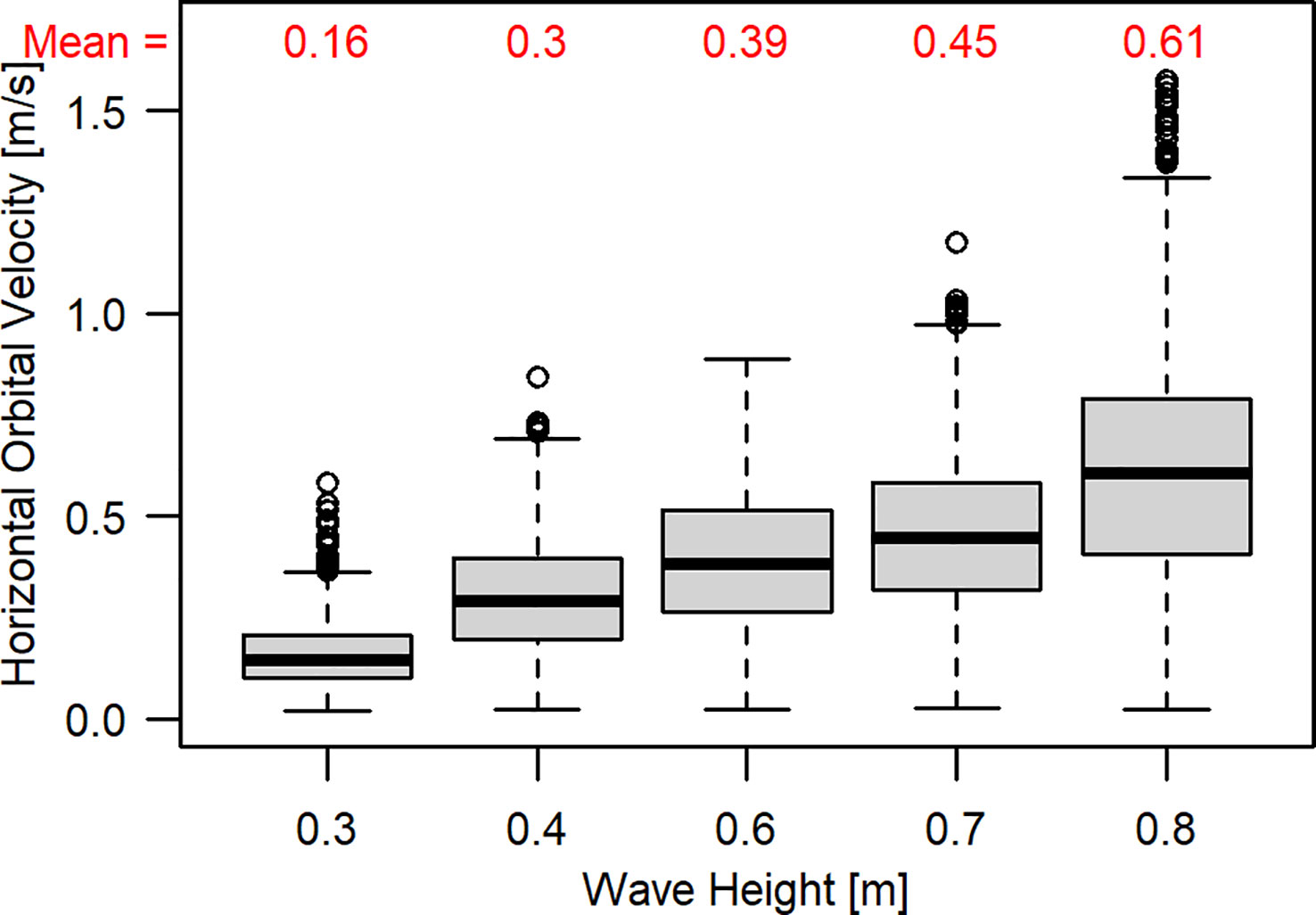
Figure 2 Differences in measured horizontal orbital velocity (Uw) between experimental runs with varying wave heights (m).
3.1 Interspecific variation in plant traits
Both stem length (Figure 3A) and stem diameter (Figure 3B) of individual seedlings differed significantly between species (Kruskal-Wallis test; p< 0.01). Stems of S. tabernaemontani were on average longest, and those of S. anglica shortest. P. maritima had a significantly smaller stem diameter than the other species (Kruskal-Wallis χ2 = 23532, df = 3, p< 0.001).
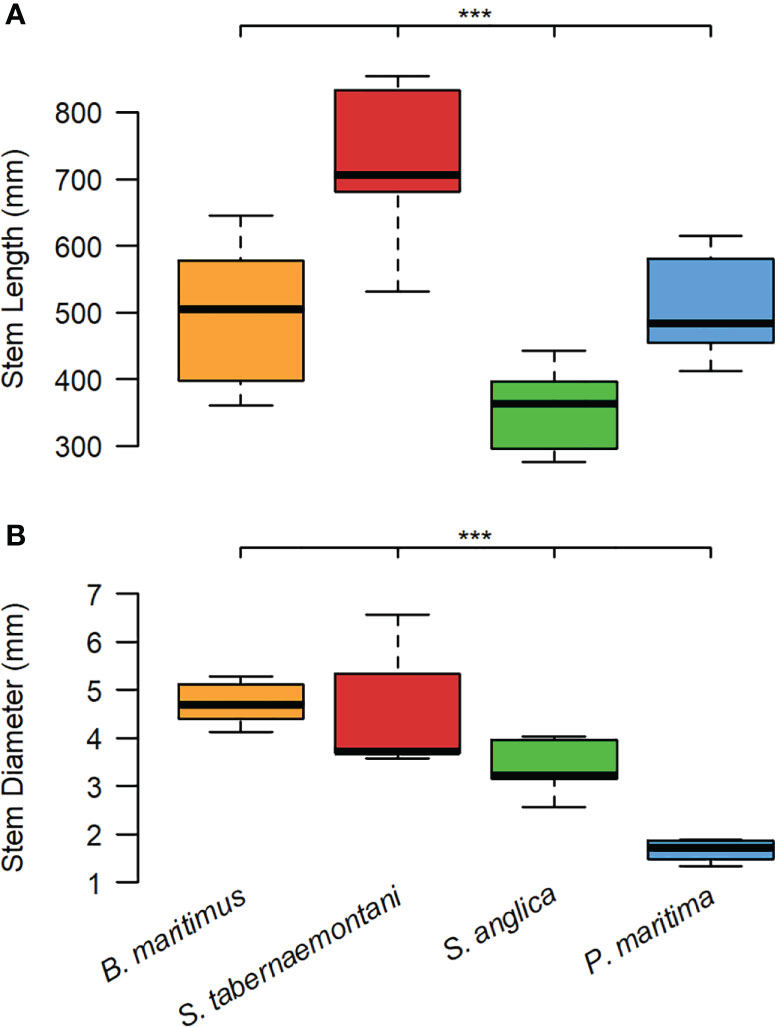
Figure 3 Interspecific differences in plant traits: (A) stem length (B. maritimus: Mean = 497 mm, SD = 119, n = 5; S. tabernaemontani: Mean = 721 mm, SD = 131, n = 5; S. anglica: Mean = 358 mm, SD = 59, n = 9; P. maritima: Mean = 509 mm, SD = 85, n = 5; Kruskal-Wallis χ2 = 23532, df = 3, p< 0.001 (***)), and (B) stem diameter (B. maritimus: Mean = 4.72 mm, SD = 0.48, n = 5; S. tabernaemontani: Mean = 4.58 mm, SD = 1.33, n = 5; S. anglica: Mean = 3.4 mm, SD = 0.55, n = 9; P. maritima: Mean = 1.66 mm, SD = 79, n = 5; Kruskal-Wallis χ2 = 26840, df = 3, p< 0.001 (***)).
3.2 Interspecific variation in drag forces in relation to Uw and plant traits
Drag forces generally increased with Uw (Figure 4A; Figures 5A–D). Overall, B. maritimus experienced the highest drag forces, and P. maritima the lowest. This could be partly explained by differences in stem length between the species, but also by other species traits that were not quantified in our study (e.g. leaf area and stiffness).
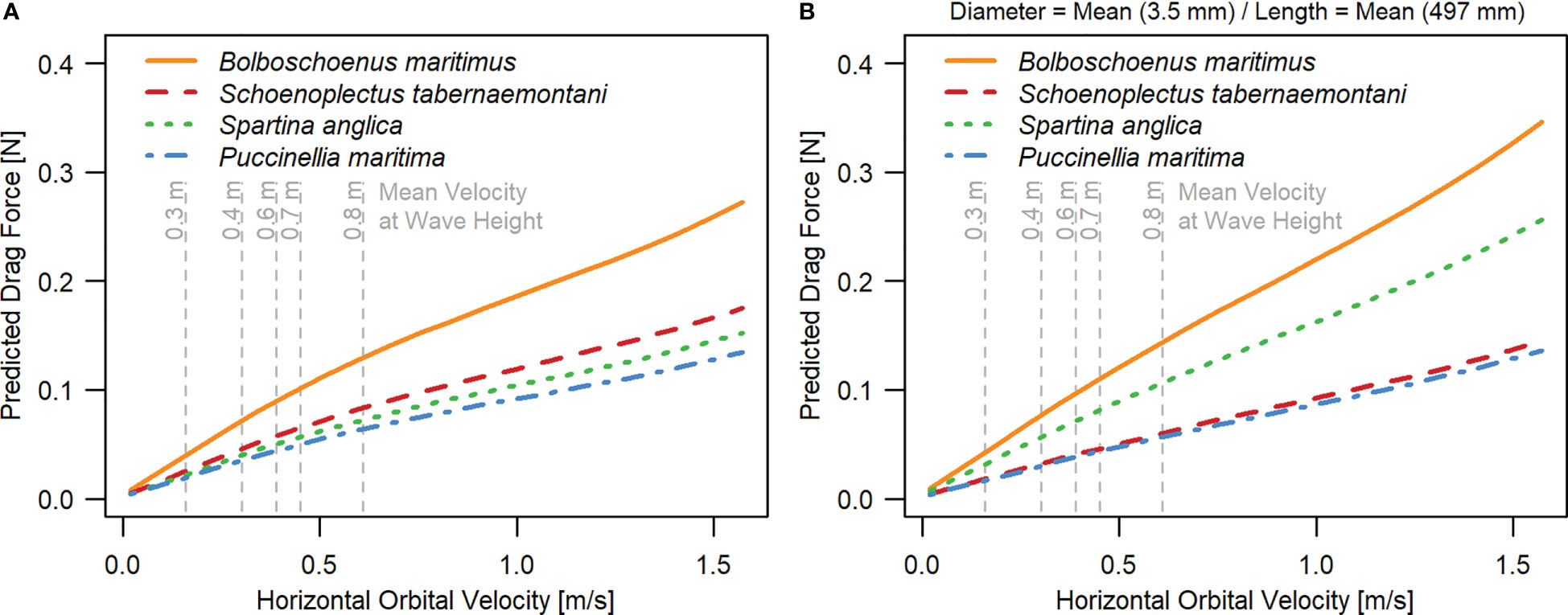
Figure 4 Predicted species-specific drag forces in relation to horizontal orbital velocity (Uw), (A) based on LMM 1 not accounting for varying stem lengths and diameters, and (B) based on LMM 2.3, additionally including stem length and diameter as predictors (see Material & Methods – Statistical Analysis). In case of (B) predictions were made assuming the same average stem length and diameter for all seedlings. Parameter estimates of LMM 1 and LMM 2.3 are given in Table S1 and Table S2C in Supplementary Material. Similar figures for models LMM2.1 (including stem length only) and LMM2.2 (including stem diameter only) as well as their parameter estimates are given in Supplementary Material (Figures 1A, B; Table S2A, B).
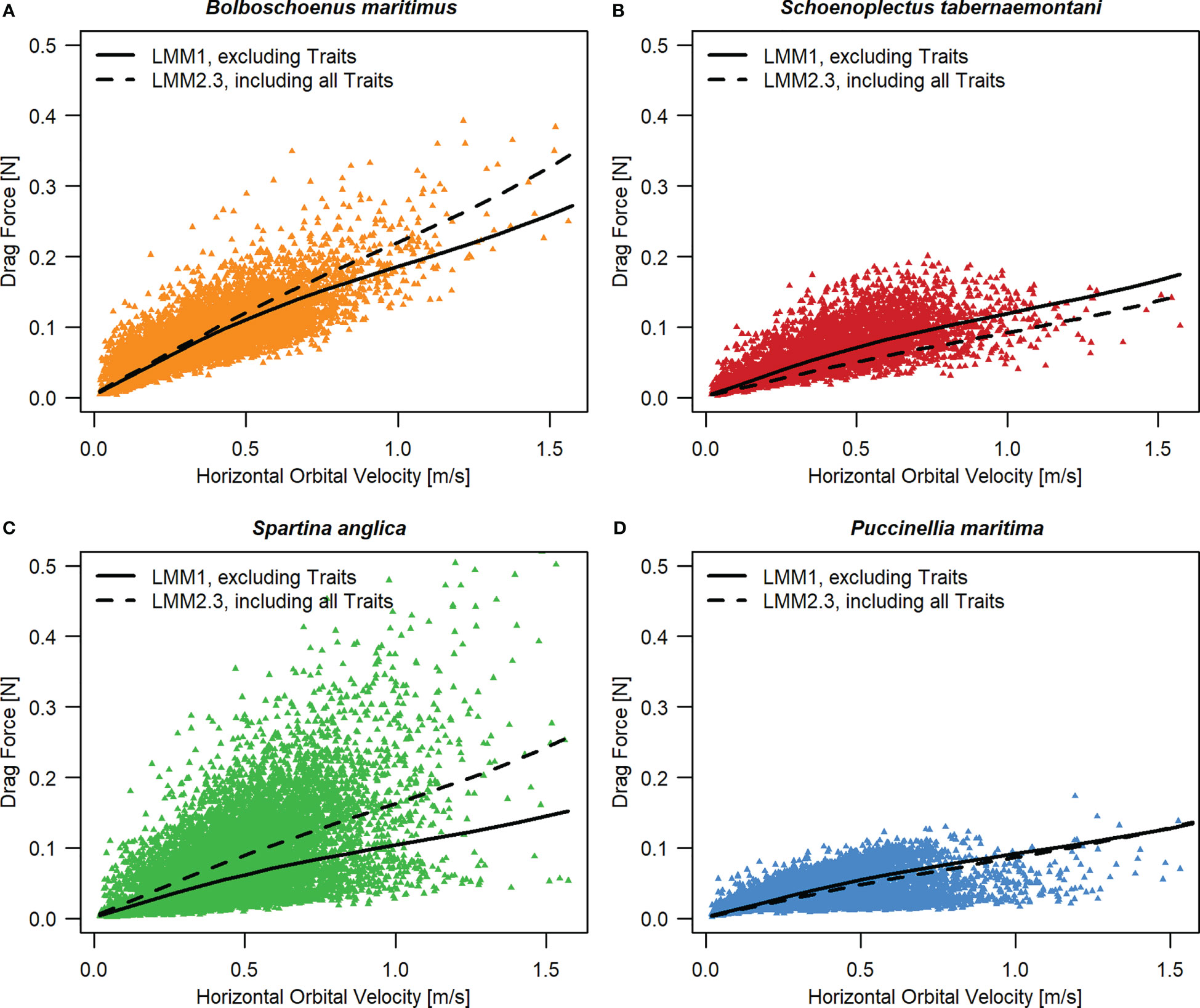
Figure 5 Original data for drag forces in relation to horizontal orbital velocity (Uw) for B. maritimus (A), S. tabernaemontani (B), S. anglica (C), and P. maritima (D), including the regression lines for LMM1 (Figure 4A) and LMM2.3 (Figure 4B).
Linear mixed effect models including stem length (Figure 6A; Table S1A, marginal R2 = 0.55, conditional R2 = 0.70) and stem diameter (Figure 6B; Table S1B, marginal R2 = 0.54, conditional R2 = 0.62) as predictors, respectively, showed that drag forces significantly increased with stem length and with stem diameter. Overall, differences in experienced drag forces among individuals with varying stem lengths and diameters increased with increasing Uw, especially in the case of stem diameter.
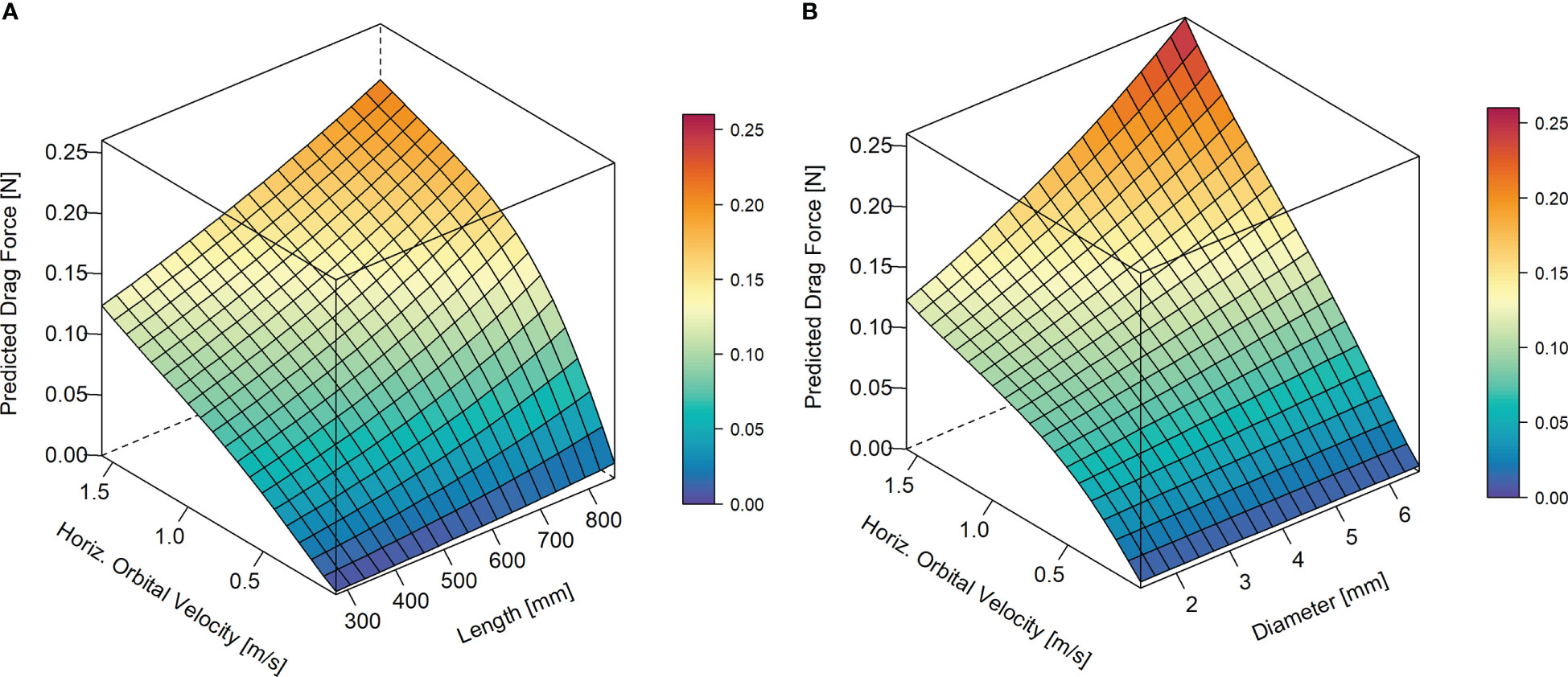
Figure 6 Effects of horizontal orbital velocity (Uw; m/s), and stem length (A) and diameter (B), respectively, on predicted mean drag force (N). Response surfaces were calculated based on the coefficients of the fitted linear mixed effects models (LMM 2.1 & LMM 2.2) with Uw and stem length (A) and stem diameter (B), respectively, as predictors. In addition, models included species-specific random intercepts. Parameter estimates of LMM 2.1 and LMM 2.2 are given in Tables S2A, B in Supplementary Material.
The model including both stem length and stem diameter revealed interactions between these two traits (Figure 7, marginal R2 = 0.57, conditional R2 = 0.75). Seedlings with long and thin shoots generally experienced the highest drag forces, and seedlings with short and thin shoots the lowest drag force. For short shoots, the drag force increased with diameter, especially at high Uw, whereas the opposite was true for individuals with long shoots.
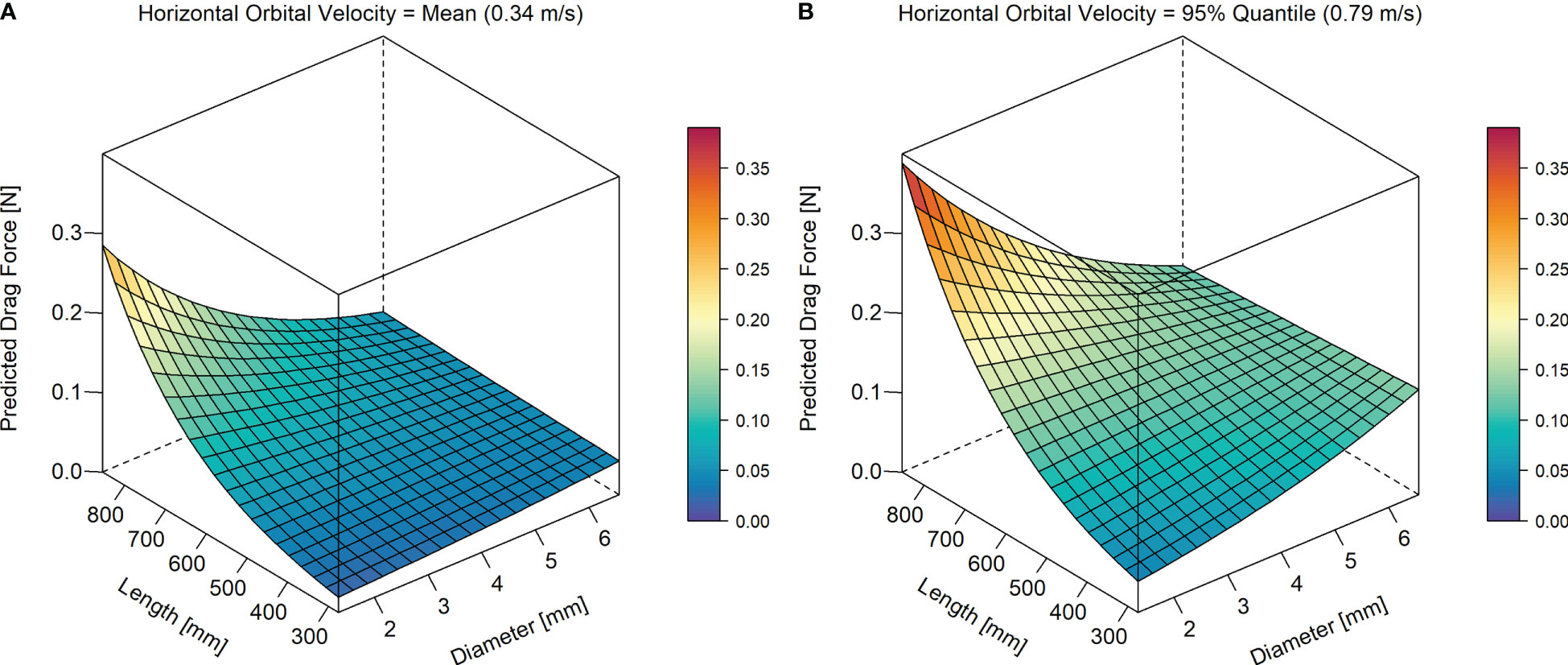
Figure 7 Predicted mean drag force (N), in relation to stem length (mm) and diameter (mm) at (A) mean measured horizontal orbital velocity (Uw: 0.34 m/s), and at (B) the 95% quantile of measured Uw (0.79 m/s). Response surfaces were calculated based on the partial regression coefficients of the fitted linear mixed effects models (LMM 2.3) with velocity and both stem length and diameter as predictors. The response surface for the 5% quantile of Uw is shown in Figure S2 in Supplementary Material. Models included species-specific random intercepts (see Figure 4B above for the species-specific effects). Parameter estimates of LMM 2.3 are given in Table S2C in Supplementary Material.
Comparing random species effects of models including both plant traits showed that – whilst accounting for individual variation in shoot length and diameter – B. maritimus, followed by S. anglica, experienced the highest drag forces, and P. maritima and S. tabernaemontani the lowest (Figure 4B).
4 Discussion
The results suggest that seedling’s susceptibility to storm wave conditions is individual-plant rather than species trait-mediated. That is, irrespective of species identity, the drag forces experienced by individual seedlings increased with Uw, and with increasing stem length and diameter. However, the interplay of these parameters was complex: at low to moderate Uw, stem length was distinctly the most important trait, determining the exerted drag forces on seedlings. With increasing velocity, the stem diameter gained in importance. Considering the individual-plant trait combination of stem length and stem diameter challenges the previous assumption that long and thick stems probably lead to increased drag forces. This discrepancy is probably due to the fact that other traits such as leaf growth and flexibility, which were not directly measured in this experiment, also play a decisive role for seedling’s vulnerability to drag forces. That is, when assuming similar stem length and diameter across species, the highest drag forces were predicted for B. maritimus, followed by S. anglica (Figure 4B), which both are known to have stiffer shoots than P. maritima (S. anglica vs P. maritima: Bouma et al., 2009a) and the leafless S. tabernaemontani (B. maritimus vs S. tabernaemontani: Schoutens et al., 2020). Our results, thus, indicate that investigating plant trait combinations rather than individual traits alone is important for assessing vulnerability of tidal marsh seedlings to storm surge conditions.
4.1 Inter- and intraspecific variation in drag forces in relation to plant traits
As hypothesized, stem length and diameter both control interspecific and intraspecific variation in the experienced drag forces, but stem length best explained drag forces. This is in agreement with Albayrak et al. (2014), who showed that long stems are correlated with higher drag forces, coupled with a high risk for breakage. Likewise, in another flume experiment, seedlings of B. maritimus and S. tabernaemontani experienced higher breakage than the smaller seedlings of S. anglica and P. maritima (Schoutens et al., 2021). Since external mechanical forces, e.g. drag, can cause disturbance for vegetation (Ennos, 1997; Read and Stokes, 2006), plants can adapt to their environment to evade potential mechanical failure (Puijalon et al., 2011). Commonly, they either avoid external forcing by preventive traits (e.g. increased flexibility to minimize drag forces), or show traits that help the plant tolerate external forcing (e.g. increased plant resistance to breakage) (Puijalon et al., 2008; Puijalon et al., 2011; Silinski et al., 2018). Hence, minimizing shoot size can be a potential strategy to avoid drag forces. Indeed, for Spartina maritima tussocks Sánchez et al. (2001) observed increasing shoot sizes with distance from the exposed edge to the tussock center (and an inferred decrease in flow velocities). Similarly, shoot lengths of B. maritimus increased with increasing distance from exposed marsh edges and decreasing wave height (Silinski et al., 2015; Silinski et al., 2018).
We also found a positive relation between stem diameter and the experienced drag force. Moreover, the importance of stem diameter was intensified with increasing Uw (Figure 6B). This supports the hypothesis of Temple et al. (2021) that observed decreases in stem diameter with rising wave heights of the tidal marsh species Juncus roemerianus and Spartina alterniflora reflect an adaptation strategy to minimize drag forces. Additionally, a smaller stem diameter might be advantageous as it lowers scouring events induced by high wave energy. Schoutens et al. (2021) found deeper scouring holes around the basal parts of B. maritimus and S. tabernaemontani for individuals with thick stems than with thin stems. These species also suffered the most from shoot loss and shoot breakage presumably due to wave-induced increased drag forces. Besides typical species-specific variations in shoot traits and potential trait adaptations, seedling’s shoot length and diameter may also vary as a function of growth. Therefore, vulnerability of seedlings may change during their growing season until a mature vegetation is established. Both annual (new development every year) or perennial (new development or regrowing aboveground shoots) species, thus, need a disturbance-free period – following the Windows of Opportunity (Balke et al., 2011; Balke et al., 2014) – to develop and adapt sufficient shoots and roots that allows the plant to withstand rough abiotic circumstances. The increased vulnerability of young plants to their surrounding compared to mature vegetation makes it even more urgent, especially for future restoration projects, to understand the needs of young plants and incorporate them into conservation measures to ensure their survival.
The study of Schoutens et al. (2021) further indicated that thicker stems show higher flexural stiffness, and thus lower capacity to bend over with the flow. Vulnerability of tidal marsh seedlings is thus likely determined by different traits or trait combinations, rather than by single traits alone. So far, most studies focused on single traits (cf. Schwarz et al., 2015; Silinski et al., 2015; Cao et al., 2018), whereas complex interactions were widely neglected. In our study, including both stem length and diameter, as well as their interaction, into one model, leads to different conclusions as compared to analyzing the effects of these traits independently. Surprisingly, highest drag forces were predicted for long and thin, instead of for long and thick stems (Figure 7). This deviates from the assumption that a small stem length, combined with a small stem diameter, is optimal to prevent high drag forces (see e.g. Silinski et al., 2018). Furthermore, a small stem diameter may prevent scouring, whereas increasing stem lengths did not affect the degree of scouring (Bouma et al., 2009b). Experienced drag forces and scouring both are known bottlenecks in seedling development (Friess et al., 2012). A possible explanation is that it is not the combination of a high stem length and a thin diameter itself that caused the high experienced drag forces in our experiment, but the interaction with some other, not measured, traits. Alternatively, the found effects may be due to a model artifact driven by the correlation between stem length and diameter, which, however, was with a Pearson’s correlation coefficient of 0.34 still moderate.
Flexural stiffness and the presence of leaves have been identified as two other crucial traits contributing to variances in drag forces (Bouma et al., 2005; Heuner et al., 2015). This is indirectly supported by our study: assuming similar stem lengths and diameters across species, our models predicted highest experienced drag forces for the stiff B. maritimus, and lowest for S. tabernaemontani and P. maritima, having rather flexible stems (Bouma et al., 2009a; Schoutens et al., 2020). Likewise, Heuner et al. (2015) observed stiffness as well as associated drag forces being twice as high for B. maritimus when compared to S. tabernaemontani, possibly due to the detected higher lignin content of the former species. Similar differences in the flexural stiffness and drag forces between both species were observed by Schoutens et al. (2020). In contrast, the study of Schoutens et al. (2021) indicated a slightly higher flexural stiffness of S. tabernaemontani than of B. maritimus seedlings. Inconsistence in the reported flexural stiffness may be evoked by effects of other individual plant traits such as stem diameter that were not measured in the studies, or by plant age. While Heuner et al. (2015) and Schoutens et al. (2020) examined adult plants, Schoutens et al. (2021) used seedlings of the four test species. It is not apparent whether the differences in the stiffness is due to species-specific trait variations or to adaptations during wave conditions in the experiments. However, plant stiffness is often assigned to the tolerance strategy as an adaptation to environmental conditions such as increased wave exposure (Puijalon et al., 2008; Puijalon et al., 2011; Silinski et al., 2018). In mature vegetation, Heuner et al. (2015), thus, found B. maritimus with its tolerating traits to have a greater ecosystem engineering effect than S. tabernaemontani which follows more an avoidance strategy. This could also be an explanation for the typical zonation of both species along the elevation gradient (Schoutens et al., 2020). The opposing result of Schoutens et al. (2021) indicates that other traits than stem diameter and length may additionally vary during growing season without being a function of seedling growth but influencing the vulnerability of plants. The question which traits are essential for seedling survival, if their relevance varies between species, and if they change - due to growth or adaptation - should be aspects of future research. It is also necessary to observe whether changes in traits also matter in already developed vegetation.
Observed higher drag forces experienced by B. maritimus than by S. tabernaemontani, were attributed to leaf growth: whereas B. maritimus forms leaves along the entire stem, S. tabernaemontani follows an avoidance strategy not only by developing a flexible shoot basis (Heuner et al., 2015; Schoutens et al., 2020), but also by a photosynthetically active but leafless stem (Heuner et al., 2015; Heuner et al., 2019). Although leaves typically exhibit higher flexibility and hence higher capacity for reconfiguration than stems, studies showed that they contribute measurably to drag forces (Zhang and Nepf, 2021: more than 70%; Bal et al., 2011: up to 60%). In contrast, Paul et al. (2016) and Vuik et al. (2018) suggested that leaves of S. anglica and P. maritima contribute only little to exerted drag forces. However, our study challenges this view: S. anglica, having stiff leaves, experienced the second highest drag force whilst accounting for varying stem length and diameter among seedlings. Therefore, our findings illustrate that the consideration of stem geometry is not sufficient and future studies should also include biomechanical plant traits and overall plant shape in the inter- and intraspecific trait combinations when considering the vulnerability of tidal marsh seedlings albeit their assessment is more labor-intensive and destructive.
4.2 Transferability of laboratory results to natural conditions
The experimental setup made it possible to test the mechanical effects of drag forces under varying wave conditions acting on different plant individuals, and the impact of individual plant traits on the exerted drag forces under extreme wave conditions. Study seedlings, however, grew under artificial conditions until their exposure in the flume, which might have caused trait differences between shoots used here, and shoots growing in the field under exposed and unpredictable conditions. In the study of Cao et al. (2020), after seven weeks of wave treatment, seedlings of S. anglica, B. maritimus, and Phragmites australis showed significantly reduced growth and flexural stiffness, as well as increased root/shoot ratio, possibly in order to cope with wave forces. Similar intra-specific variations in plant plasticity were related to different wave and current exposures in the field (Sánchez et al., 2001; Carus et al., 2017; Silinski et al., 2018; Schoutens et al., 2022). Our plants grown under greenhouse conditions were not exposed to waves or currents during their growth phase and thus had no opportunity to adapt. Still, even under natural conditions, seedlings are not permanently exposed to (high) wave conditions, especially when growing in a closed vegetation cover as they normally do (tussock growth of S. anglica and P. maritima or monospecific stands of B. maritimus and S. tabernaemontani). Studies showed that flow velocity, turbulence intensity, and turbulent kinetic energy are significantly reduced within a vegetated canopy, all being inversely related to the amount of biomass or the stem density present in the water column, respectively (Leonard and Reed, 2002; Leonard and Croft, 2006). Our focus here was on the mechanics of exerted drag forces in relation to trait measures and Uw, rather than on trait adaptations to high wave conditions, and slight deviations from expressed traits of seedlings in nature should not have an impact on the conclusions drawn.
The hydrodynamic conditions applied in this flume study exceeded or at least corresponded to natural storm surge conditions in the tidal marsh pioneer zone (cf. Schoutens et al., 2021). However, wave exposure was shorter in duration than would be likely to occur naturally, and over tidal cycles, water depths may be less than as applied in our flume experiment. In addition, while the applied JONSWAP wave spectrum is representative of North Sea wave conditions, it does not reflect conditions at any specific location (Hasselmann et al., 1973). Conclusions on potential changes in the drag forces due to different water depths or wave breaking, especially in a specific location, can therefore not be drawn from this experiment. Nevertheless, both aspects should be further addressed in future research. Heuner et al. (2015) found significant differences between drag forces acting on B. maritimus and those acting on S. tabernaemontani at a water depth of 20 cm, while differences already disappeared at a water depth of 35 cm, presumably due to submergence of leaves and thus increased frontal area.
4.3 Seedling vulnerability and attenuation capacity under storm surge conditions
Increased storminess (Donat et al., 2011) and rising sea levels under climate change are expected to potentially lead to longer flooding durations and increasing wave loads on tidal marsh pioneer plants, associated with higher Uw (Hu et al., 2021). Plant morphological adaptations and growth strategies, e.g. to avoid uprooting or breakage by drag forces or to increase a plant’s tolerance to wave forcing (Bornette and Puijalon, 2011; Puijalon et al., 2011; Silinski et al., 2018), affect the ability of a plant to cope with non-optimal conditions and to persist in a certain environment.
Species-dependent adaptations may in turn modify wave regimes and Uw (Rupprecht et al., 2017). This requires attention if vegetation is used for nature based coastal protection (e.g. wave attenuation and shoreline stabilization; Morris et al., 2018). Our study indicated that seedlings with longer stems led to higher drag forces than seedlings with a short stature, but they potentially attenuate also more wave energy. Generally, the higher the exerted drag forces are, the larger is the loss of wave energy and the lower is the distance over which the wave energy is dissipated (Bouma et al., 2010; Heuner et al., 2015; Schoutens et al., 2020). For example, the stiff S. anglica is known to dissipate more hydrodynamic energy than the flexible seagrass Zostera noltii (Bouma et al., 2005) or than the short-statured P. maritima (Rupprecht et al., 2015), but the plants themselves yielded large drag forces, possibly reducing the tolerance of S. anglica to increased wave loads (Bouma et al., 2005). S. anglica is expected to dissipate more wave energy than P. maritima (Rupprecht et al., 2015), implying that twice the stem density of P. maritima is required to achieve the same level of wave attenuation as for S. anglica (Maza et al., 2015). Morris (2007) even assumed that plants with high drag at their preferred elevation improve sedimentation and, therefore, contribute to marsh resilience in spite of SLR.
Several studies showed that the positive attenuation effect on waves (see equation e.g. in Mendez and Losada, 2004) is capped by a species- and possibly even individual-dependent resistance threshold to stem breakage which can be expressed by a mean critical Uw (Silinski et al., 2015; Silinski et al., 2016; Silinski et al., 2018). Vuik et al. (2018), for example, found that B. maritimus has a lower resistance to stem breakage (critical mean orbital velocity: 0.3 - 1.0 m/s) compared to S. anglica (critical mean orbital velocity: 0.5 - 1.2 m/s), questioning the derivation of species-specific wave attenuation capacity simply from drag forces, especially since plant resistance to stem breakage or stem folding by wave forces may also be individual-dependent.
Individual responses of seedlings to extreme wave events, and their effects on tidal marsh resilience, thus constitute an uncertainty when predicting marsh establishment, which is often not adequately considered in marsh evolution models (e.g. Mariotti and Fagherazzi, 2010; Mariotti and Fagherazzi, 2013; Poppema et al., 2019; Hu et al., 2021; Gourgue et al., 2022). In our study, we demonstrated that stem lengths and diameters, and possibly even flexural stiffness and leaves, all affect drag forces acting on individuals, and should be thus incorporated into models, together with species’ critical thresholds with respect to the local hydrodynamic conditions. However, other traits such as growth form (e.g., tussock growth or vegetation density) likely also impact the response of plant individuals and may lead to scale-dependent feedbacks (van Wesenbeeck et al., 2008b). Schwarz et al. (2011) observed a positive tussock growth of Scirpus mariqueter and S. anglica in a transplantation experiment in the Yangtze River Estuary, China, while single seedlings were not able to survive under natural wave conditions. Furthermore, B. maritimus and S. tabernaemontani showed the longest and thickest stems in our experiment, with potentially adverse effects under increased wave energies, but in nature, both species grow as monospecific stands along estuary shorelines, and are thus typically less exposed to high wave loads (van Wesenbeeck et al., 2008a; Callaghan et al., 2010; Yang et al., 2012). Higher stem length may be advantageous in denser vegetation, where competition for light may become increasingly important (Carus et al., 2017). Such trade-offs in traits may have evolved as a consequence of a whole suite of selection pressures imposed by the habitat – wave action being only one of them.
To conclude, large scale flume experiments can help to close fundamental knowledge gaps about plant behavior under increased wave exposure and to reveal related processes and parameters that need to be included in future marsh restoration projects and models, but they still need to be embedded in mesocosm and field studies. Especially the vulnerability of young plants to their abiotic environment compared to mature vegetation makes the investigation of intraspecific and interspecific traits and their interaction, also under the aspect of adaptation strategies, an important issue and should be the basis for restoration of tidal marshes. Building scientific knowledge on the conditions favoring seedling germination, establishment and growth, based on both laboratory and field studies, is a prerequisite for preserving tidal marsh resilience, and for successful implementation of restoration projects.
Data availability statement
Original datasets analyzed in this study are publicly available from the TU Braunschweig repository: doi: 10.24355/dbbs.084-202305021335-0
Author contributions
CS and SL analyzed and interpreted the data, discussed the results and wrote the manuscript. MP and BS discussed the results and edited the manuscript. MP, IM, ST, TB, KS, BE and SR designed the study and gathered the data. All authors contributed to the article and approved the submitted version.
Funding
The work described in this study was supported by the European Community’s Horizon 2020 Research and Innovation Programme through the grant to HYDRALAB-PLUS (grant no. 654110). CSS is funded by the Ministry for Science and Culture of Lower Saxony and the Volkswagen-Foundation Niedersächsiches Vorab through Gute Küste Niedersachsen (grant no. ZN3722).
Acknowledgments
We would like to thank the team from the Forschungszentrum Küste (FZK) as well as the RESIST UK to provide the data set. We thank our colleagues of the Institute of Geoecology, Technische Universität Braunschweig for the fruitful discussions and statistical advices.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1172492/full#supplementary-material
References
Akaike H. (1974). A new look at the statistical model identification. IEEE Trans Autom Contr 19, 716–723.
Albayrak I., Nikora V., Miler O., O’Hare M. T. (2014). Flow–plant interactions at leaf, stem and shoot scales: drag, turbulence, and biomechanics. Aquat Sci. 76, 269–294. doi: 10.1007/s00027-013-0335-2
ATI Industrial Automation (2023) F/T sensor: gamma. Available at: https://www.ati-ia.com/products/ft/ft_models.aspx?id=gamma (Accessed February 1, 2023).
Bal K. D., Bouma T. J., Buis K., Struyf E., Jonas S., Backx H., et al. (2011). Trade-off between drag reduction and light interception of macrophytes: comparing five aquatic plants with contrasting morphology. Funct. Ecol. 25, 1197–1205. doi: 10.1111/j.1365-2435.2011.01909.x
Balke T., Bouma T. J., Horstman E. M., Webb E. L., Erftemeijer P. L., Herman P. M. (2011). Windows of opportunity: thresholds to mangrove seedling establishment on tidal flats. Mar. Ecol. Prog. Ser. 440, 1–9. doi: 10.3354/meps09364
Balke T., Herman P. M. J., Bouma T. J. (2014). Critical transitions in disturbance-driven ecosystems: identifying windows of opportunity for recovery. J. Ecol. 102, 700–708. doi: 10.1111/1365-2745.12241
Balke T., Webb E. L., van den Elzen E., Galli D., Herman P. M. J., Bouma T. J. (2013). Seedling establishment in a dynamic sedimentary environment: a conceptual framework using mangroves. J. Appl. Ecol. 50, 740–747. doi: 10.1111/1365-2664.12067
Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67 (1), 1–48. doi: 10.18637/jss.v067.i01
Bornette G., Puijalon S. (2011). Response of aquatic plants to abiotic factors: a review. Aquat Sci. 73, 1–14. doi: 10.1007/s00027-010-0162-7
Bouma T. J., de Vries M. B., Herman P. M. J. (2010). Comparing ecosystem engineering efficiency of two plant species with contrasting growth strategies. ECOLOGY 91, 2696–2704. doi: 10.1890/09-0690.1
Bouma T. J., Friedrichs M., Klaassen P., van Wesenbeeck B. K., Brun F. G., Temmerman S., et al. (2009a). Effects of shoot stiffness, shoot size and current velocity on scouring sediment from around seedlings and propagules. Mar. Ecol. Prog. Ser. 388, 293–297. doi: 10.3354/meps08130
Bouma T. J., Friedrichs M., van Wesenbeeck B. K., Temmerman S., Graf G., Herman P. M. J. (2009b). Density-dependent linkage of scale-dependent feedbacks: a flume study on the intertidal macrophyte spartina anglica. Oikos 118, 260–268. doi: 10.1111/j.1600-0706.2008.16892.x
Bouma T. J., Temmerman S., van Duren L. A., Martini E., Vandenbruwaene W., Callaghan D. P., et al. (2013). Organism traits determine the strength of scale-dependent bio-geomorphic feedbacks: a flume study on three intertidal plant species. Geomorphology 180–181, 57–65. doi: 10.1016/j.geomorph.2012.09.005
Bouma T. J., van Belzen J., Balke T., Zhu Z., Airoldi L., Blight A. J., et al. (2014). Identifying knowledge gaps hampering application of intertidal habitats in coastal protection: opportunities & steps to take. Coast. Eng. 87, 147–157. doi: 10.1016/j.coastaleng.2013.11.014
Bouma T. J., Vries M. B., Low E., Peralta G., Tánczos I. C., van de Koppel J., et al. (2005). Trade-offs related to ecosystem engineering: a case study on stiffness of emerging macrophytes. ECOLOGY 86, 2187–2199. doi: 10.1890/04-1588
Burnham K. P., Anderson D. R. (2010). Model selection and multimodel inference: a practical information-theoretic approach (New York, NY: Springer).
Callaghan D. P., Bouma T. J., Klaassen P., van der Wal D., Stive M., Herman P. (2010). Hydrodynamic forcing on salt-marsh development: distinguishing the relative importance of waves and tidal flows. Estuarine Coast. Shelf Sci. 89, 73–88. doi: 10.1016/j.ecss.2010.05.013
Cao H., Zhu Z., Balke T., Zhang L., Bouma T. J. (2018). Effects of sediment disturbance regimes on spartina seedling establishment: implications for salt marsh creation and restoration. Limnol. Oceanogr. 63, 647–659. doi: 10.1002/lno.10657
Cao H., Zhu Z., James R., Herman P. M. J., Zhang L., Yuan L., et al. (2020). Wave effects on seedling establishment of three pioneer marsh species: survival, morphology and biomechanics. Ann. Bot. 125, 345–352. doi: 10.1093/aob/mcz136
Carus J., Heuner M., Paul M., Schröder B. (2017). Plant distribution and stand characteristics in brackish marshes: unravelling the roles of abiotic factors and interspecific competition. Estuar. Coast. Shelf Sci. 196, 237–247. doi: 10.1016/j.ecss.2017.06.038
Carus J., Paul M., Schröder B. (2016). Vegetation as self-adaptive coastal protection: reduction of current velocity and morphologic plasticity of a brackish marsh pioneer. Ecol. Evol. 6, 1579–1589. doi: 10.1002/ece3.1904
Colombano D. D., Litvin S. Y., Ziegler S. L., Alford S. B., Baker R., Barbeau M. A., et al. (2021). Climate change implications for tidal marshes and food web linkages to estuarine and coastal nekton. Estuaries Coasts 44, 1637–1648. doi: 10.1007/s12237-020-00891-1
Denny M. (2021). Wave-energy dissipation: seaweeds and marine plants are ecosystem engineers. Fluids 6, 151. doi: 10.3390/fluids6040151
Donat M. G., Renggli D., Wild S., Alexander L. V., Leckebusch G. C., Ulbrich U. (2011). Reanalysis suggests long-term upward trends in European storminess since 1871. Geophys. Res. Lett. 38, L14703. doi: 10.1029/2011GL047995
Ennos A. (1997). Wind as an ecological factor. Trends Ecol. Evol. 12, 108–111. doi: 10.1016/S0169-5347(96)10066-5
Fagherazzi S., Carniello L., D'Alpaos L., Defina A. (2006). Critical bifurcation of shallow microtidal landforms in tidal flats and salt marshes. Proc. Natl. Acad. Sci. U.S.A. 103, 8337–8341. doi: 10.1073/pnas.0508379103
Friess D. A., Krauss K. W., Horstman E. M., Balke T., Bouma T. J., Galli D., et al. (2012). Are all intertidal wetlands naturally created equal? bottlenecks, thresholds and knowledge gaps to mangrove and saltmarsh ecosystems. Biol. Rev. 87, 346–366. doi: 10.1111/j.1469-185X.2011.00198.x
Gourgue O., van Belzen J., Schwarz C., Vandenbruwaene W., Vanlede J., Belliard J.-P., et al. (2022). Biogeomorphic modeling to assess the resilience of tidal-marsh restoration to sea level rise and sediment supply. Earth Surf. Dynam. 10, 531–553. doi: 10.5194/esurf-10-531-2022
Hasselmann K., Barnett T. P., Bouws E., Carlson H., Cartwright D. E., Enke K., et al. (1973). Measurements of wind-wave growth and swell decay during the joint north Sea wave project (JONSWAP). Ergänzungsheft zur Deutschen Hydrographischen. Zeitschrift. Reihe. A Nr. 12, 1–95.
Heuner M., Schröder B., Schröder U., Kleinschmit B. (2019). Contrasting elevational responses of regularly flooded marsh plants in navigable estuaries. Ecohydrology Hydrobiology 19, 38–53. doi: 10.1016/j.ecohyd.2018.06.002
Heuner M., Silinski A., Schoelynck J., Bouma T. J., Puijalon S., Troch P., et al. (2015). Ecosystem engineering by plants on wave-exposed intertidal flats is governed by relationships between effect and response traits. PLoS One 10, e0138086. doi: 10.1371/journal.pone.0138086
Himes-Cornell A., Pendleton L., Atiyah P. (2018). Valuing ecosystem services from blue forests: a systematic review of the valuation of salt marshes, sea grass beds and mangrove forests. Ecosystem Serv. 30, 36–48. doi: 10.1016/j.ecoser.2018.01.006
Hu Z., Borsje B. W., Belzen J., Willemsen P. W. J. M., Wang H., Peng Y., et al. (2021). Mechanistic modeling of marsh seedling establishment provides a positive outlook for coastal wetland restoration under global climate change. Geophysical Res. Lett. 48, e2021GL095596. doi: 10.1029/2021GL095596
Kuznetsova A., Brockhoff P. B., Christensen R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Soft. 82 (13), 1–26. doi: 10.18637/jss.v082.i13
Langlois E., Bonis A., Bouzillé J. (2003). Sediment and plant dynamics in saltmarshes pioneer zone: puccinellia maritima as a key species? Estuar. Coast. Shelf Sci. 56, 239–249. doi: 10.1016/S0272-7714(02)00185-3
Lau W. W. (2013). Beyond carbon: conceptualizing payments for ecosystem services in blue forests on carbon and other marine and coastal ecosystem services. Ocean Coast. Manage. 83, 5–14. doi: 10.1016/j.ocecoaman.2012.03.011
Leonard L. A., Croft A. L. (2006). The effect of standing biomass on flow velocity and turbulence in spartina alterniflora canopies. Estuarine Coast. Shelf Sci. 69, 325–336. doi: 10.1016/j.ecss.2006.05.004
Leonard L. A., Reed D. J. (2002). Hydrodynamics and sediment transport through tidal marsh canopies. J. Coast. Res. 36, 459–469. doi: 10.2112/1551-5036-36.sp1.459
Marani M., D'Alpaos A., Lanzoni S., Carniello L., Rinaldo A. (2007). Biologically-controlled multiple equilibria of tidal landforms and the fate of the Venice lagoon. Geophys. Res. Lett. 34, L11402. doi: 10.1029/2007GL030178
Mariotti G., Fagherazzi S. (2010). A numerical model for the coupled long-term evolution of salt marshes and tidal flats. J. Geophys. Res. 115, F01004. doi: 10.1029/2009JF001326
Mariotti G., Fagherazzi S. (2013). Critical width of tidal flats triggers marsh collapse in the absence of sea-level rise. PNAS 110, 5353–5356. doi: 10.1073/pnas.1219600110
Maza M., Lara J. L., Losada I. J., Ondiviela B., Trinogga J., Bouma T. J. (2015). Large-Scale 3-d experiments of wave and current interaction with real vegetation. part 2: experimental analysis. Coast. Eng. 106, 73–86. doi: 10.1016/j.coastaleng.2015.09.010
Mendez F. J., Losada I. J. (2004). An empirical model to estimate the propagation of random breaking and nonbreaking waves over vegetation fields. Coast. Eng. 51, 103–118. doi: 10.1016/j.coastaleng.2003.11.003
Möller I., Kudella M., Rupprecht F., Spencer T., Paul M., van Wesenbeeck B. K., et al. (2014). Wave attenuation over coastal salt marshes under storm surge conditions. Nat. Geosci 7, 727–731. doi: 10.1038/ngeo2251
Mori N., Suzuki T., Kakuno S. (2007). Noise of acoustic Doppler velocimeter data in bubbly flows. J. Eng. Mech. 133, 122–125. doi: 10.1061/(ASCE)0733-9399(2007)133:1(122
Morris J. T. (2007). “Ecological engineering in intertidial saltmarshes,” in Lagoons and coastal wetlands in the global change context: impact and management issues: selected papers of the international conference "CoastWetChange", Venice 26-28 April 2004. Eds. Viaroli P., Lasserre P., Campostrini P. (Dordrecht, Cham: Springer Netherlands; Springer International Publishing AG), 161–168.
Morris R. L., Konlechner T. M., Ghisalberti M., Swearer S. E. (2018). From grey to green: efficacy of eco-engineering solutions for nature-based coastal defence. Glob Chang Biol. 24, 1827–1842. doi: 10.1111/gcb.14063
Mudd S. M. (2011). The life and death of salt marshes in response to anthropogenic disturbance of sediment supply. Geol 39, 511–512. doi: 10.1130/focus052011.1
Paul M., Rupprecht F., Möller I., Bouma T. J., Spencer T., Kudella M., et al. (2016). Plant stiffness and biomass as drivers for drag forces under extreme wave loading: a flume study on mimics. Coast. Eng. 117, 70–78. doi: 10.1016/j.coastaleng.2016.07.004
Pigeon G. (2016). Gabtool: package with gab's personnal function (Vienna, Austria: R Foundation for Statistical Computing).
Poppema D. W., Willemsen P. W., Vries M. B., Zhu Z., Borsje B. W., Hulscher S. J. (2019). Experiment-supported modelling of salt marsh establishment. Ocean Coast. Manage. 168, 238–250. doi: 10.1016/j.ocecoaman.2018.10.039
Puijalon S., Bouma T. J., Douady C. J., van Groenendael J., Anten N. P. R., Martel E., et al. (2011). Plant resistance to mechanical stress: evidence of an avoidance-tolerance trade-off. New Phytol. 191, 1141–1149. doi: 10.1111/j.1469-8137.2011.03763.x
Puijalon S., Léna J.-P., Rivière N., Champagne J.-Y., Rostan J.-C., Bornette G. (2008). Phenotypic plasticity in response to mechanical stress: hydrodynamic performance and fitness of four aquatic plant species. New Phytol. 177, 907–917. doi: 10.1111/j.1469-8137.2007.02314.x
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/.
Read J., Stokes A. (2006). Plant biomechanics in an ecological context. Am. J. Bot. 93, 1546–1565. doi: 10.3732/ajb.93.10.1546
Rupprecht F., Möller I., Evans B., Spencer T., Jensen K. (2015). Biophysical properties of salt marsh canopies [[/amp]]mdash; quantifying plant stem flexibility and above ground biomass. Coast. Eng. 100, 48–57. doi: 10.1016/j.coastaleng.2015.03.009
Rupprecht F., Möller I., Paul M., Kudella M., Spencer T., van Wesenbeeck B. K., et al. (2017). Vegetation-wave interactions in salt marshes under storm surge conditions. Ecol. Eng. 100, 301–315. doi: 10.1016/j.ecoleng.2016.12.030
Sánchez J., SanLeon D., Izco J. (2001). Primary colonisation of mudflat estuaries by spartina maritima (Curtis) fernald in Northwest Spain: vegetation structure and sediment accretion. Aquat. Bot. 69, 15–25. doi: 10.1016/S0304-3770(00)00139-X
Schoutens K., Heuner M., Fuchs E., Minden V., Schulte-Ostermann T., Belliard J.-P., et al. (2020). Nature-based shoreline protection by tidal marsh plants depends on trade-offs between avoidance and attenuation of hydrodynamic forces. Estuar. Coast. Shelf Sci. 236, 106645. doi: 10.1016/j.ecss.2020.106645
Schoutens K., Luys P., Heuner M., Fuchs E., Minden V., Schulte Ostermann T., et al. (2022). Traits of tidal marsh plants determine survival and growth response to hydrodynamic forcing: implications for nature-based shoreline protection. Mar. Ecol. Prog. Ser. 693, 107–124. doi: 10.3354/meps14091
Schoutens K., Reents S., Nolte S., Evans B., Paul M., Kudella M., et al. (2021). Survival of the thickest? impacts of extreme wave-forcing on marsh seedlings are mediated by species morphology. Limnology Oceanography 66, 2936–2951. doi: 10.1002/lno.11850
Schulte Ostermann T., Kleyer M., Heuner M., Fuchs E., Temmerman S., Schoutens K., et al. (2021). Hydrodynamics affect plant traits in estuarine ecotones with impact on carbon sequestration potentials. Estuar. Coast. Shelf Sci. 259, 107464. doi: 10.1016/j.ecss.2021.107464
Schwarz C., Bouma T. J., Zhang L. Q., Temmerman S., Ysebaert T., Herman P. (2015). Interactions between plant traits and sediment characteristics influencing species establishment and scale-dependent feedbacks in salt marsh ecosystems. Geomorphology 250, 298–307. doi: 10.1016/j.geomorph.2015.09.013
Schwarz C., Ysebaert T., Zhu Z., Zhang L., Bouma T. J., Herman P. M. J. (2011). Abiotic factors governing the establishment and expansion of two salt marsh plants in the Yangtze estuary, China. Wetlands 31, 1011–1021. doi: 10.1007/s13157-011-0212-5
Shao D., Zhou W., Bouma T. J., Asaeda T., Wang Z. B., Liu X., et al. (2020). Physiological and biochemical responses of the salt-marsh plant spartina alterniflora to long-term wave exposure. Ann. Bot. 125, 291–300. doi: 10.1093/aob/mcz067
Shepard C. C., Crain C. M., Beck M. W. (2011). The protective role of coastal marshes: a systematic review and meta-analysis. PLoS One 6, e27374. doi: 10.1371/journal.pone.0027374
Silinski A., Heuner M., Schoelynck J., Puijalon S., Schröder U., Fuchs E., et al. (2015). Effects of wind waves versus ship waves on tidal marsh plants: a flume study on different life stages of scirpus maritimus. PLoS One 10, e0118687. doi: 10.1371/journal.pone.0118687
Silinski A., Schoutens K., Puijalon S., Schoelynck J., Luyckx D., Troch P., et al. (2018). Coping with waves: plasticity in tidal marsh plants as self-adapting coastal ecosystem engineers. Limnol. Oceanogr. 63, 799–815. doi: 10.1002/lno.10671
Silinski A., van Belzen J., Fransen E., Bouma T. J., Troch P., Meire P., et al. (2016). Quantifying critical conditions for seaward expansion of tidal marshes: a transplantation experiment. Estuar. Coast. Shelf Sci. 169, 227–237. doi: 10.1016/j.ecss.2015.12.012
Spencer T., Schuerch M., Nicholls R. J., Hinkel J., Lincke D., Vafeidis A. T., et al. (2016). Global coastal wetland change under sea-level rise and related stresses: the DIVA wetland change model. Global Planetary Change 139, 15–30. doi: 10.1016/j.gloplacha.2015.12.018
Stone B. M., Shen H. T. (2002). Hydraulic resistance of flow in channels with cylindrical roughness. J. Hydraul. Eng. 128, 500–506. doi: 10.1061/(ASCE)0733-9429(2002)128:5(500
Temple N. A., Sparks E. L., Webb B. M., Cebrian J., Virden M. F., Lucore A. E., et al. (2021). Responses of two fringing salt marsh plant species along a wave climate gradient. Mar. Ecol. Prog. Ser. 675, 53–66. doi: 10.3354/meps13843
van de Koppel J., van der Wal D., Bakker J. P., Herman P. M. J. (2005). Self-organization and vegetation collapse in salt marsh ecosystems. Am. Nat. 165, E1–12. doi: 10.1086/426602
van Wesenbeeck B. K., van de Koppel J., Herman P. M. J., BERTNESS M. D., van der Wal D., Bakker J. P., et al. (2008a). Potential for sudden shifts in transient systems: distinguishing between local and landscape-scale processes. Ecosystems 11, 1133–1141. doi: 10.1007/s10021-008-9184-6
van Wesenbeeck B. K., van de Koppel J., Herman P. M. J., Bouma T. J. (2008b). Does scale-dependent feedback explain spatial complexity in salt-marsh ecosystems? Oikos 117, 152–159. doi: 10.1111/j.2007.0030-1299.16245.x
Vuik V., Suh Heo H. Y., Zhu Z., Borsje B. W., Jonkman S. N. (2018). Stem breakage of salt marsh vegetation under wave forcing: a field and model study. Estuar. Coast. Shelf Sci. 200, 41–58. doi: 10.1016/j.ecss.2017.09.028
Wang C., Temmerman S. (2013). Does biogeomorphic feedback lead to abrupt shifts between alternative landscape states? an empirical study on intertidal flats and marshes. J. Geophys. Res. Earth Surf. 118, 229–240. doi: 10.1029/2012JF002474
Wolters M., Garbutt A., Bekker R. M., Bakker J. P., Carey P. D. (2008). Restoration of salt-marsh vegetation in relation to site suitability, species pool and dispersal traits. J. Appl. Ecol. 45, 904–912. doi: 10.1111/j.1365-2664.2008.01453.x
Yang S. L., Shi B. W., Bouma T. J., Ysebaert T., Luo X. X. (2012). Wave attenuation at a salt marsh margin: a case study of an exposed coast on the Yangtze estuary. Estuaries Coasts 35, 169–182. doi: 10.1007/s12237-011-9424-4
Zhang X., Nepf H. (2021). Wave-induced reconfiguration of and drag on marsh plants. J. Fluids Structures 100, 103192. doi: 10.1016/j.jfluidstructs.2020.103192
Zhao Z., Yuan L., Li W., Tian B., Zhang L. (2020). Re-invasion of spartina alterniflora in restored saltmarshes: seed arrival, retention, germination, and establishment. J. Environ. Manage 266, 110631. doi: 10.1016/j.jenvman.2020.110631
Keywords: tidal marshes, flume experiment, seedlings, drag forces, plant traits
Citation: Steinigeweg CS, Löbel S, Schröder B, Schoutens K, Reents S, Evans BR, Temmerman S, Bouma TJ, Möller I and Paul M (2023) Plant trait-mediated drag forces on seedlings of four tidal marsh pioneer species. Front. Mar. Sci. 10:1172492. doi: 10.3389/fmars.2023.1172492
Received: 23 February 2023; Accepted: 15 May 2023;
Published: 30 May 2023.
Edited by:
Luzhen Chen, Xiamen University, ChinaReviewed by:
James Morris, University of South Carolina, United StatesYining Chen, Ministry of Natural Resources, China
Copyright © 2023 Steinigeweg, Löbel, Schröder, Schoutens, Reents, Evans, Temmerman, Bouma, Möller and Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlotte S. Steinigeweg, c.steinigeweg@tu-braunschweig.de
 Charlotte S. Steinigeweg
Charlotte S. Steinigeweg Swantje Löbel
Swantje Löbel Boris Schröder
Boris Schröder Ken Schoutens
Ken Schoutens Svenja Reents
Svenja Reents Ben R. Evans
Ben R. Evans Stijn Temmerman
Stijn Temmerman Tjeerd J. Bouma
Tjeerd J. Bouma Iris Möller
Iris Möller Maike Paul
Maike Paul