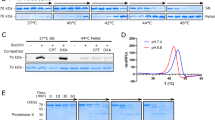
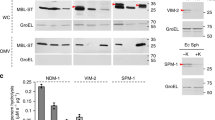
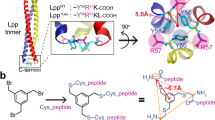
Abstract
Protein stability is an essential property for biological function. In contrast to the vast knowledge on protein stability in vitro, little is known about the factors governing in-cell stability. Here we show that the metallo-β-lactamase (MBL) New Delhi MBL-1 (NDM-1) is a kinetically unstable protein on metal restriction that has evolved by acquiring different biochemical traits that optimize its in-cell stability. The nonmetalated (apo) NDM-1 is degraded by the periplasmic protease Prc that recognizes its partially unstructured C-terminal domain. Zn(II) binding renders the protein refractory to degradation by quenching the flexibility of this region. Membrane anchoring makes apo-NDM-1 less accessible to Prc and protects it from DegP, a cellular protease degrading misfolded, nonmetalated NDM-1 precursors. NDM variants accumulate substitutions at the C terminus that quench its flexibility, enhancing their kinetic stability and bypassing proteolysis. These observations link MBL-mediated resistance with the essential periplasmic metabolism, highlighting the importance of the cellular protein homeostasis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files. The following protein structures were downloaded from the Research Collaboratory for Structural Bioinformatics PDB: apo-rNDM-1 (PDB 3SPU and PDB 3SBL), Prc (PDB 5WQL), holo-rNDM-1 (PDB 5ZGX), apo-rNDM-1 (PDB 3SBL), holo-rBcII (PDB 3I13), apo-rBcII (PDB 3I0V) and Prc K477A (PDB: 5WQL). Source data are provided with this paper.
References
DePristo, M. A., Weinreich, D. M. & Hartl, D. L. Missense meanderings in sequence space: a biophysical view of protein evolution. Nat. Rev. Genet. 6, 678–687 (2005).
Weinreich, D. M., Delaney, N. F., Depristo, M. A. & Hartl, D. L. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312, 111–114 (2006).
Thomas, V. L., McReynolds, A. C. & Shoichet, B. K. Structural bases for stability-function tradeoffs in antibiotic resistance. J. Mol. Biol. 396, 47–59 (2010).
Socha, R. D. & Tokuriki, N. Modulating protein stability—directed evolution strategies for improved protein function. FEBS J. 280, 5582–5595 (2013).
Sanchez-Ruiz, J. M. Protein kinetic stability. Biophys. Chem. 148, 1–15 (2010).
Monteith, W. B., Cohen, R. D., Smith, A. E., Guzman-Cisneros, E. & Pielak, G. J. Quinary structure modulates protein stability in cells. Proc. Natl Acad. Sci. USA 112, 1739–1742 (2015).
Colón, W. et al. Biological roles of protein kinetic stability. Biochemistry 56, 6179–6186 (2017).
Jaswal, S. S., Sohl, J. L., Davis, J. H. & Agard, D. A. Energetic landscape of α-lytic protease optimizes longevity through kinetic stability. Nature 415, 343–346 (2002).
Kelch, B. A. et al. Structural and mechanistic exploration of acid resistance: kinetic stability facilitates evolution of extremophilic behavior. J. Mol. Biol. 368, 870–883 (2007).
López, C., Delmonti, J., Bonomo, R. A. & Vila, A. J. Deciphering the evolution of metallo-β-lactamases: a journey from the test tube to the bacterial periplasm. J. Biol. Chem. 298, 101665 (2022).
Meini, M. R., Tomatis, P. E., Weinreich, D. M. & Vila, A. J. Quantitative description of a protein fitness landscape based on molecular features. Mol. Biol. Evol. 32, 1774–1787 (2015).
Bershtein, S., Segal, M., Bekerman, R., Tokuriki, N. & Tawfik, D. S. Robustness–epistasis link shapes the fitness landscape of a randomly drifting protein. Nature 444, 929–932 (2006).
Bahr, G., Gonzalez, L. J. & Vila, A. J. Metallo-beta-lactamases in the age of multidrug resistance: from structure and mechanism to evolution, dissemination, and inhibitor design. Chem. Rev. 121, 7957–8094 (2021).
Gonzalez, L. J. et al. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-beta-lactamase. Nat. Chem. Biol. 12, 516–522 (2016).
Bahr, G. et al. Clinical evolution of New Delhi metallo-beta-lactamase (NDM) optimizes resistance under Zn(II) deprivation. Antimicrob. Agents Chemother. 62, e01849–17 (2018).
Lonergan, Z. R. & Skaar, E. P. Nutrient zinc at the host-pathogen interface. Trends Biochem. Sci. 44, 1041–1056 (2019).
Zygiel, E. M. & Nolan, E. M. Transition metal sequestration by the host-defense protein calprotectin. Annu. Rev. Biochem. 87, 621–643 (2018).
Moran-Barrio, J., Limansky, A. S. & Viale, A. M. Secretion of GOB metallo-beta-lactamase in Escherichia coli depends strictly on the cooperation between the cytoplasmic DnaK chaperone system and the Sec machinery: completion of folding and Zn(II) ion acquisition occur in the bacterial periplasm. Antimicrob. Agents Chemother. 53, 2908–2917 (2009).
Cheng, Z. et al. Evolution of New Delhi metallo-beta-lactamase (NDM) in the clinic: iffects of NDM mutations on stability, zinc affinity, and mono-zinc activity. J. Biol. Chem. 293, 12606–12618 (2018).
King, D. & Strynadka, N. Crystal structure of New Delhi metallo-beta-lactamase reveals molecular basis for antibiotic resistance. Protein Sci. 20, 1484–1491 (2011).
Prunotto, A., Bahr, G., Gonzalez, L. J., Vila, A. J. & Dal Peraro, M. Molecular bases of the membrane association mechanism potentiating antibiotic resistance by New Delhi metallo-beta-lactamase 1. ACS Infect. Dis. 6, 2719–2731 (2020).
Su, M. Y. et al. Structural basis of adaptor-mediated protein degradation by the tail-specific PDZ-protease Prc. Nat. Commun. 8, 1516 (2017).
Singh, S. K., Parveen, S., SaiSree, L. & Reddy, M. Regulated proteolysis of a cross-link–specific peptidoglycan hydrolase contributes to bacterial morphogenesis. Proc. Natl Acad. Sci. USA 112, 10956–10961 (2015).
Silber, K. R., Keiler, K. C. & Sauer, R. T. Tsp: a tail-specific protease that selectively degrades proteins with nonpolar C termini. Proc. Natl Acad. Sci. USA 89, 295–299 (1992).
Krojer, T. et al. Structural basis for the regulated protease and chaperone function of DegP. Nature 453, 885–890 (2008).
Gonzalez, L. J., Moreno, D. M., Bonomo, R. A. & Vila, A. J. Host-specific enzyme-substrate interactions in SPM-1 metallo-beta-lactamase are modulated by second sphere residues. PLoS Path. 10, e1003817 (2014).
Schwechheimer, C. & Kuehn, M. J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619 (2015).
Fontana, A., Polverino de Laureto, P., De Filippis, V., Scaramella, E. & Zambonin, M. Probing the partly folded states of proteins by limited proteolysis. Fold. Des. 2, R17–R26 (1997).
Rivière, G. et al. NMR characterization of the influence of zinc(II) ions on the structural and dynamic behavior of the New Delhi metallo-β-lactamase-1 and on the binding with flavonols as inhibitors. ACS Omega 5, 10466–10480 (2020).
Shen, Y. & Bax, A. Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J. Biomol. NMR 56, 227–241 (2013).
Narita, S. I. & Tokuda, H. Bacterial lipoproteins; biogenesis, sorting and quality control. Biochim. Biophys. Acta 1862, 1414–1423 (2017).
Brambilla, L., Moran-Barrio, J. & Viale, A. M. Low-molecular-mass penicillin binding protein 6b (DacD) is required for efficient GOB-18 metallo-beta-lactamase biogenesis in Salmonella enterica and Escherichia coli. Antimicrob. Agents Chemother. 58, 205–211 (2014).
Sawa, J. et al. Molecular adaptation of the DegQ protease to exert protein quality control in the bacterial cell envelope. J. Biol. Chem. 286, 30680–30690 (2011).
Lopez, C., Ayala, J. A., Bonomo, R. A., Gonzalez, L. J. & Vila, A. J. Protein determinants of dissemination and host specificity of metallo-beta-lactamases. Nat. Commun. 10, 3617 (2019).
Iwanczyk, J. et al. Role of the PDZ domains in Escherichia coli DegP protein. J. Bacteriol. 189, 3176–3186 (2007).
Chueh, C.-K. et al. Structural basis for the differential regulatory roles of the PDZ domain in C-terminal processing proteases. mBio 10, e01129–19 (2019).
Keiler, K. C. & Sauer, R. T. Identification of active site residues of the Tsp protease. J. Biol. Chem. 270, 28864–28868 (1995).
Pierce, B. G. et al. ZDOCK server: interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 30, 1771–1773 (2014).
Banzhaf, M. et al. Outer membrane lipoprotein NlpI scaffolds peptidoglycan hydrolases within multi‐enzyme complexes in Escherichia coli. EMBO J. 39, e102246 (2020).
Makena, A. et al. Biochemical characterization of New Delhi metallo-beta-lactamase variants reveals differences in protein stability. J. Antimicrob. Chemother. 70, 463–469 (2015).
Chung, S. & Darwin, A. J. The C terminus of substrates is critical but not sufficient for their degradation by the Pseudomonas aeruginosa CtpA protease. J. Bacteriol. 202, e00174–20 (2020).
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, 2001).
Tomatis, P. E., Rasia, R. M., Segovia, L. & Vila, A. J. Mimicking natural evolution in metallo-beta-lactamases through second-shell ligand mutations. Proc. Natl Acad. Sci. USA 102, 13761–13766 (2005).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Uzzau, S., Figueroa-Bossi, N., Rubino, S. & Bossi, L. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl Acad. Sci. USA 98, 15264–15269 (2001).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Osborn, M. J. & Munson, R. Separation of the inner (cytoplasmic) and outer membranes of gram-negative bacteria. Methods Enzymol. 31, 642–653 (1974).
Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard - Ninth Edition. CLSI Document M07-A9 (CLSI, 2012).
Hinchliffe, P. et al. Cross-class metallo-beta-lactamase inhibition by bisthiazolidines reveals multiple binding modes. Proc. Natl Acad. Sci. USA 113, E3745–E3754 (2016).
Gonzalez, J. M. et al. Metallo-beta-lactamases withstand low Zn(II) conditions by tuning metal-ligand interactions. Nat. Chem. Biol. 8, 698–700 (2012).
Maciejewski, M. W. et al. NMRbox: a resource for biomolecular NMR computation. Biophys. J. 112, 1529–1534 (2017).
Vranken, W. F. et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 (2005).
Lipari, G. & Szabo, A. Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J. Am. Chem. Soc. 104, 4546–4559 (1982).
Dosset, P., Hus, J.-C., Blackledge, M. & Marion, D. Efficient analysis of macromolecular rotational diffusion from heteronuclear relaxation data. J. Biomol. NMR 16, 23–28 (2000).
Hansen, D. F., Vallurupalli, P. & Kay, L. E. An improved 15N relaxation dispersion experiment for the measurement of millisecond time-scale dynamics in proteins. J. Phys. Chem. B 112, 5898–5904 (2008).
Zaballa, M.-E., Abriata, L. A., Donaire, A. & Vila, A. J. Flexibility of the metal-binding region in apo-cupredoxins. Proc. Natl Acad. Sci. USA 109, 9254–9259 (2012).
Acknowledgements
This research was supported by grants from the National Institutes of Health (grant nos. R01AI100560 to R.A.B. and A.J.V., R01AI063517 to R.A.B. and R01AI072219 to R.A.B.) and Agencia I+d+i to A.J.V. (grant no. PICT 2020-0031) and L.J.G. (grant no. PICT 2020-1923). This study was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award no. 1I01BX001974 to R.A.B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 to R.A.B. The NMR instrumentation was provided by PLABEM at IBR. G.B. and M.M.G. were recipients of fellowships from CONICET. A.J.V. and L.J.G. are staff members from CONICET. We are grateful to Marina Avecilla for excellent technical assistance. We thank L. Giono for designing and drawing Fig. 6 and the Graphical Abstract.
Author information
Authors and Affiliations
Contributions
L.J.G. and A.J.V. crafted the main hypothesis and designed research. L.J.G. performed the microbiological, molecular biology and biochemical experiments, and NMR experiments on interactions and NDM-6 dynamics. G.B. carried out the experiments involving proteins in liposomes and part of the proteolysis experiments and M.M.G. performed the NMR experiments on NDM-1 and BcII. L.J.G., G.B., R.A.B. and A.J.V. wrote the paper, and all authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Emily Que and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 SEC and negative staining TEM demonstrate formation of complexes between Prc K477A and apo-rNDM-1.
a) SDS-PAGE analysis of SEC fractions corresponding to samples containing Prc K477A and either holo-rNDM-1 (top) or apo-rNDM-1 (bottom). b) Negative staining TEM of Prc K477A (top) or complexes of Prc K477A and apo NDM-1 (bottom). Samples for TEM analysis were obtained from SEC of the corresponding mixtures (shown in Fig. 4a), at the elution volume (VE in mL) indicated. Panels a, b show one of two independent experiments.
Extended Data Fig. 2 NlpI blocks association of apo-rNDM-1 to Prc K477A, possibly by occupying the same binding site on the protease.
a) SDS-PAGE analysis of SEC fractions from the left panel, corresponding to samples containing NlpI and apo-rNDM-1, with (bottom) or without (top) Prc K477A (two independent repetitions). b) Structural alignment of the Prc:NlpI complex (PDB 5WQL) and the proposed complex of Prc and apo-rNDM-1 (from Fig. 4b). NlpI and apo-rNDM-1 are shown as yellow and green ribbons, respectively, while Prc is shown in a magenta surface representation.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21; Tables 1–4; full, uncut gel images for Figs. 2, 8, 9 and 10; full, uncut gel images for Figs. 11, 12, 14, 15 and 17 and references.
Source data
Source Data Fig. 1
Source data Excel file for Fig. 1.
Source Data Fig. 2
Source data Excel file for Fig. 2.
Source Data Fig. 3
Source data Excel file for Fig. 3.
Source Data Fig. 4
Source data Excel file for Fig. 4.
Source Data Fig. 5
Source data Excel file for Fig. 5.
Source Data Extended Data Fig. 1
Source data Excel file for Extended Data Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
González, L.J., Bahr, G., González, M.M. et al. In-cell kinetic stability is an essential trait in metallo-β-lactamase evolution. Nat Chem Biol 19, 1116–1126 (2023). https://doi.org/10.1038/s41589-023-01319-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01319-0
This article is cited by
-
Epistasis arises from shifting the rate-limiting step during enzyme evolution of a β-lactamase
Nature Catalysis (2024)