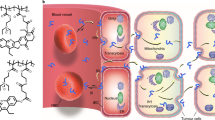
Abstract
Although cyclodextrin-based renal-clearable nanocarriers have a high potential for clinical translation in targeted cancer therapy, their designs remain to be optimized for tumour retention. Here we report on the design of a tailored structure for renal-clearable zwitterionic cyclodextrin for colorectal cancer-selective drug delivery. Twenty cyclodextrin derivatives with different charged moieties and spacers are synthesized and screened for colloidal stability. The resulting five candidates are evaluated for biodistribution and an optimized structure is identified. The optimized cyclodextrin shows a high tumour accumulation and is used for delivery of doxorubicin and ulixertinib. Higher tumour accumulation and tumour penetration facilitates tumour elimination. The improved antitumour efficacy is demonstrated in heterotopic and orthotopic colorectal cancer models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files. Source data is available for Figs. 1c,e, 2d,f,g, 3e,f, 4a,b,d,e and 5a–c,e,f, and Supplementary Figs. 8d, 9c, 11a,b, 13a,c, 14b,c, 15a,d,f, 16a,b, 17a,b, 18a,b,d, 19a–c, 20a–c, 21a,b, 22 and 25a–c in the associated source data files.
References
Blanco, E. et al. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Yu, M. X. & Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 9, 6655–6674 (2015).
Nurunnabi, M. et al. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 7, 6858–6867 (2013).
Li, B. & Lane, L. A. Probing the biological obstacles of nanomedicine with gold nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 11, e1542 (2019).
Zhang, Y. N. et al. Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. J. Control. Release 240, 332–348 (2016).
Longmire, M. et al. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 3, 703–717 (2008).
Cheng, Y. H. et al. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano 14, 3075–3095 (2020).
Lammers, T. Macro-nanomedicine: targeting the big picture. J. Control. Release 294, 372–375 (2019).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Liu, J. B. et al. Renal clearable inorganic nanoparticles: a new frontier of bionanotechnology. Mater. Today 16, 477–486 (2013).
Zhou, C. et al. Luminescent gold nanoparticles with efficient renal clearance. Angew. Chem. Int. Ed. 50, 3168–3172 (2011).
Zhou, C. et al. Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew. Chem. Int. Ed. 51, 10118–10122 (2012).
Liu, J. B. et al. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: long tumor retention and fast normal tissue clearance. J. Am. Chem. Soc. 135, 4978–4981 (2013).
Burns, A. A. et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 9, 442–448 (2009).
Ruggiero, A. et al. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl Acad. Sci. USA 107, 12369–12374 (2010).
Peng, C. Q. et al. Correlating anticancer drug delivery efficiency with vascular permeability of renal clearable versus non-renal clearable nanocarriers. Angew. Chem. Int. Ed. 58, 12076–12080 (2019).
Peng, C. Q. et al. Renal clearable nanocarriers: overcoming the physiological barriers for precise drug delivery and clearance. J. Control. Release 322, 64–80 (2020).
Peng, C. et al. Tuning the in vivo transport of anticancer drugs using renal-clearable gold nanoparticles. Angew. Chem. Int. Ed. 58, 8479–8483 (2019).
Liu, J. et al. PEGylation and zwitterionization: pros and cons in the renal clearance and tumor targeting of near-IR-emitting gold nanoparticles. Angew. Chem. Int. Ed. 52, 12572–12576 (2013).
Kang, H. et al. Renal clearable theranostic nanoplatforms for gastrointestinal stromal tumors. Adv. Mater. 32, e1905899 (2020).
Kang, H. et al. Renal clearable organic nanocarriers for bioimaging and drug delivery. Adv. Mater. 28, 8162–8168 (2016).
Wang, H. et al. Renal-clearable porphyrinic metal–organic framework nanodots for enhanced photodynamic therapy. ACS Nano 13, 9206–9217 (2019).
Choi, H. S. et al. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 5, 42–47 (2010).
Huang, H. et al. Inorganic nanoparticles in clinical trials and translations. Nano Today 35, 100972 (2020).
Missaoui, W. N. et al. Toxicological status of nanoparticles: what we know and what we don’t know. Chem. Biol. Interact. 295, 1–12 (2018).
Kang, H. et al. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 9, 1901223 (2020).
Choi, H. S. et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol. 31, 148–153 (2013).
Verbeek, F. P. R. et al. Near-infrared fluorescence imaging of both colorectal cancer and ureters using a low-dose integrin targeted probe. Ann. Surg. Oncol. 21, S528–S537 (2014).
Sofias, A. M. et al. Tumor targeting by αvβ3-integrin-specific lipid nanoparticles occurs via phagocyte hitchhiking. ACS Nano 14, 7832–7846 (2020).
McNeeley, K. M. et al. Decreased circulation time offsets increased efficacy of PEGylated nanocarriers targeting folate receptors of glioma. Nanotechnology 18, 385101 (2007).
Shuhendler, A. J. et al. A novel solid lipid nanoparticle formulation for active targeting to tumor αvβ3 integrin receptors reveals cyclic RGD as a double-edged sword. Adv. Healthc. Mater. 1, 600–608 (2012).
Cheng, W. W. & Allen, T. M. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: a comparison of whole monoclonal antibody, Fab′ fragments and single chain Fv. J. Control. Release 126, 50–58 (2008).
Zhang, Y. et al. Strategies and challenges to improve the performance of tumor-associated active targeting. J. Mater. Chem. B 8, 3959–3971 (2020).
Zhao, Z. et al. Targeting strategies for tissue-specific drug delivery. Cell 181, 151–167 (2020).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 70, 313–313 (2020).
McCleary, N. J. et al. Personalizing adjuvant therapy for stage II/III colorectal cancer. Am. Soc. Clin. Oncol. Educ. Book. 37, 232–245 (2017).
Jalaeikhoo, H. et al. Effectiveness of adjuvant chemotherapy in patients with stage II colorectal cancer: a multicenter retrospective study. J. Res. Med. Sci. 24, 39 (2019).
Taieb, J. & Gallois, C. Adjuvant chemotherapy for stage III colon cancer. Cancers 12, 2679 (2020).
Braun, M. S. & Seymour, M. T. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther. Adv. Med. Oncol. 3, 43–52 (2011).
Xie, Y. H. et al. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 5, 22 (2020).
Ooi, H. W. et al. Multivalency enables dynamic supramolecular host-guest hydrogel formation. Biomacromolecules 21, 2208–2217 (2020).
Lee, D. W. et al. Supramolecular assembly based on host–guest interaction between beta-cyclodextrin and adamantane for specifically targeted cancer imaging. J. Ind. Eng. Chem. 57, 37–44 (2018).
Galema, H. A. et al. Fluorescence-guided surgery in colorectal cancer; a review on clinical results and future perspectives. Eur. J. Surg. Oncol. 48, 810–821 (2022).
Tringale, K. R. et al. Image-guided surgery in cancer: a strategy to reduce incidence of positive surgical margins. Wiley Interdiscip. Rev. Syst. Biol. 10, e1412 (2018).
Keller, D. S. et al. Indocyanine green fluorescence imaging in colorectal surgery: overview, applications, and future directions. Lancet Gastroenterol. Hepatol. 2, 757 (2017).
Shukla, A. et al. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol. Cancer 9, 314 (2010).
Salaroglio, I. C. et al. ERK is a pivotal player of chemo-immune-resistance in cancer. Int. J. Mol. Sci. 20, 2505 (2019).
Christowitz, C. et al. Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer 19, 757 (2019).
Ortiz, R. et al. Nanomedicine to overcome multidrug resistance mechanisms in colon and pancreatic cancer: recent progress. Cancers 13, 2058 (2021).
Kim, D. H. et al. Effects of kefir on doxorubicin-induced multidrug resistance in human colorectal cancer cells. J. Funct. Food 78, 104371 (2021).
Yuan, C. et al. Inclusion complex of astaxanthin with hydroxypropyl-beta-cyclodextrin: UV, FTIR, H-1 NMR and molecular modeling studies. Carbohydr. Polym. 89, 492–496 (2012).
Hamdi, H. et al. Spectroscopic studies of inclusion complex of beta-cyclodextrin and benzidine diammonium dipicrate. Spectrochim. Acta A 75, 32–36 (2010).
Lv, S. et al. High drug loading and sub-quantitative loading efficiency of polymeric micelles driven by donor–receptor coordination interactions. J. Am. Chem. Soc. 140, 1235–1238 (2018).
Hiensch, A. E. et al. Doxorubicin-induced skeletal muscle atrophy: elucidating the underlying molecular pathways. Acta Physiol. 229, e13400 (2020).
Ou, H. C. et al. Low-level laser prevents doxorubicin-induced skeletal muscle atrophy by modulating AMPK/SIRT1/PCG-1alpha-mediated mitochondrial function, apoptosis and up-regulation of pro-inflammatory responses. Cell Biosci. 11, 200 (2021).
Henriksen, P. A. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 104, 971–977 (2018).
Tian, Z. et al. High cumulative doxorubicin dose for advanced soft tissue sarcoma. BMC Cancer 20, 1139 (2020).
Luo, R. et al. Distinct biodistribution of doxorubicin and the altered dispositions mediated by different liposomal formulations. Int. J. Pharm. 519, 1–10 (2017).
Patel, K. J. et al. Distribution of the anticancer drugs doxorubicin, mitoxantrone and topotecan in tumors and normal tissues. Cancer Chemother. Pharmacol. 72, 127–138 (2013).
Speth, P. A. et al. Clinical pharmacokinetics of doxorubicin. Clin. Pharmacokinet. 15, 15–31 (1988).
Terasaki, T. et al. Pharmacokinetic study on the mechanism of tissue distribution of doxorubicin: interorgan and interspecies variation of tissue-to-plasma partition coefficients in rats, rabbits, and guinea pigs. J. Pharm. Sci. 73, 1359–1363 (1984).
Tredan, O. et al. Drug resistance and the solid tumor microenvironment. J. Natl Cancer Inst. 99, 1441–1454 (2007).
Torok, S. et al. Limited tumor tissue drug penetration contributes to primary resistance against angiogenesis inhibitors. Theranostics 7, 400–412 (2017).
Ziemys, A. et al. Progression-dependent transport heterogeneity of breast cancer liver metastases as a factor in therapeutic resistance. J. Control. Release 291, 99–105 (2018).
Cabral, H. et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6, 815–823 (2011).
Zhou, Q. et al. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 14, 799–809 (2019).
Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
Waite, C. L. & Roth, C. M. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities. Crit. Rev. Biomed. Eng. 40, 21–41 (2012).
Sun, D. X. et al. What went wrong with anticancer nanomedicine design and how to make it right. ACS Nano 14, 12281–12290 (2020).
Feng, H. Y. et al. Targeted micellar phthalocyanine for lymph node metastasis homing and photothermal therapy in an orthotopic colorectal tumor model. Nanomicro Lett. 13, 145 (2021).
Hackl, C. et al. Metronomic oral topotecan prolongs survival and reduces liver metastasis in improved preclinical orthotopic and adjuvant therapy colon cancer models. Gut 62, 259–271 (2013).
Song, W. et al. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 9, 2237 (2018).
Zhu, C. et al. Suppress orthotopic colon cancer and its metastasis through exact targeting and highly selective drug release by a smart nanomicelle. Biomaterials 161, 144–153 (2018).
Blackman, L. D. et al. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 48, 757–770 (2019).
Okamatsu, A. et al. Design and evaluation of folate-appended alpha-, beta-, and gamma-cyclodextrins having a caproic acid as a tumor selective antitumor drug carrier in vitro and in vivo. Biomacromolecules 14, 4420–4428 (2013).
Okamatsu, A. et al. Folate-appended beta-cyclodextrin as a promising tumor targeting carrier for antitumor drugs in vitro and in vivo. Bioconjugate Chem. 24, 724–733 (2013).
Hyun, H. et al. 700-nm zwitterionic near-infrared fluorophores for dual-channel image-guided surgery. Mol. Imaging Biol. 18, 52–61 (2016).
Shao, Q. & Jiang, S. Influence of charged groups on the properties of zwitterionic moieties: a molecular simulation study. J. Phys. Chem. B 118, 7630–7637 (2014).
Dwivedi, R. et al. Design of therapeutically improved analogue of the antimicrobial peptide, indolicidin, using a glycosylation strategy. Amino Acids 51, 1443–1460 (2019).
Xu, X. D. et al. In situ recognition of cell-surface glycans and targeted imaging of cancer cells. Sci. Rep. 3, 2679 (2013).
Kasashima, H. et al. Mouse model of colorectal cancer: orthotopic co-implantation of tumor and stroma cells in cecum and rectum. STAR Protoc. 2, 100297 (2021).
Gontijo, S. M. L. et al. Erlotinib/hydroxypropyl-beta-cyclodextrin inclusion complex: characterization and in vitro and in vivo evaluation. J. Incl. Phenom. Macrocycl. Chem. 83, 267–279 (2015).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Ministry of Science and ICT (grant nos. NRF-2021R1C1C1009320 (J.-Y.L.); NRF-2018R1A5A2024425, NRF-2018M3A7B4071203 and NRF-2020R1A2C2099983 (D.-D.K.); and NRF-2018H1A2A1062046, (M.-J.B.)).
Author information
Authors and Affiliations
Contributions
M.-J.B., J.-Y.L. and D.-D.K. conceived and designed the experiments. M.-J.B., D.-T.N., D.K., S.-Y.Y. and S.M.L. performed the experiments. M.-J.B., J.-Y.L. and D.-D.K. interpreted the data and developed the discussion. M.-J.B., J.-Y.L. and D.-D.K. composed the paper. J.-Y.L. and D.-D.K. supervised the entire project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Jean-Luc Coll and Roger Gomis for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Magnified version of Fig. 1a, Supplementary Figs. 1–25 and References.
Supplementary Data 1
Statistical source data for supplementary figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baek, MJ., Nguyen, DT., Kim, D. et al. Tailoring renal-clearable zwitterionic cyclodextrin for colorectal cancer-selective drug delivery. Nat. Nanotechnol. 18, 945–956 (2023). https://doi.org/10.1038/s41565-023-01381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01381-8