Abstract
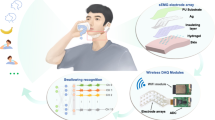
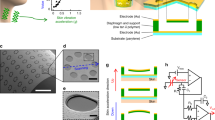
Resistive skin biosensors refer to a class of imperceptible wearable devices for health monitoring and human–machine interfacing, in which conductive materials are deposited onto or incorporated into an elastomeric polymeric sheet. A wide range of resistive skins has been developed so far to detect a wide variety of biometric signals including blood pressure, skin strain, body temperature and acoustic vibrations; however, they are typically non-specific, with one resistive signal corresponding to a single type of biometric data (one-mode sensors). Here we show a hierarchically resistive skin sensor made of a laminated cracked platinum film, vertically aligned gold nanowires and a percolated gold nanowire film, all integrated into a single sensor. As a result, hierarchically resistive skin displays a staircase-shaped resistive response to tensile strain, with distinct sensing regimes associated to a specific active material. We show that we can, through one resistive signal, identify up to five physical or physiological activities associated with the human throat speech: heartbeats, breathing, touch and neck movement (that is, a multimodal sensor). We develop a frequency/amplitude-based neural network, Deep Hybrid-Spectro, that can automatically disentangle multiple biometrics from a single resistive signal. This system can classify 11 activities—with different combinations of speech, neck movement and touch—with an accuracy of 92.73 ± 0.82% while simultaneously measuring respiration and heart rates. We validated the classification accuracy of several biometrics with an overall accuracy of >82%, demonstrating the generality of our concept.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data are available from the authors on reasonable request, and/or are included within the manuscript and its Supplementary Information. The machine learning datasets used in this study are available at https://github.com/XinZ0419/Deep-Hybrid-Spectro. Source Data are provided with this paper.
Code availability
The code that supports the plots within this paper and other findings of this study are available at https://github.com/XinZ0419/Deep-Hybrid-Spectro.
References
Kim, D.-H. et al. Epidermal electronics. Science 333, 838–843 (2011).
Miyamoto, A. et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 12, 907–913 (2017).
Wang, S. et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 555, 83–88 (2018).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Shih, B. et al. Electronic skins and machine learning for intelligent soft robots. Sci. Robot. 5, eaaz9239 (2020).
Zhou, Z. et al. Sign-to-speech translation using machine-learning-assisted stretchable sensor arrays. Nat. Electron. 3, 571–578 (2020).
Hammock, M. L., Chortos, A., Tee, B. C. K., Tok, J. B. H. & Bao, Z. 25th Anniversary article: the evolution of electronic skin (e‐skin): a brief history, design considerations, and recent progress. Adv. Mater. 25, 5997–6038 (2013).
Dagdeviren, C. et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 5, 4496 (2014).
Lee, S. et al. An ultrathin conformable vibration-responsive electronic skin for quantitative vocal recognition. Nat. Commun. 10, 2468 (2019).
Yang, J. et al. Eardrum‐inspired active sensors for self‐powered cardiovascular system characterization and throat‐attached anti‐interference voice recognition. Adv. Mater. 27, 1316–1326 (2015).
Kang, S. et al. Transparent and conductive nanomembranes with orthogonal silver nanowire arrays for skin-attachable loudspeakers and microphones. Sci. Adv. 4, eaas8772 (2018).
Kang, D. et al. Ultrasensitive mechanical crack-based sensor inspired by the spider sensory system. Nature 516, 222–226 (2014).
Jason, N. N., Ho, M. D. & Cheng, W. Resistive electronic skin. J. Mater. Chem. C. 5, 5845–5866 (2017).
Kabiri Ameri, S. et al. Graphene electronic tattoo sensors. ACS Nano 11, 7634–7641 (2017).
Gong, S. et al. Local crack‐programmed gold nanowire electronic skin tattoos for In‐plane multisensor integration. Adv. Mater. 31, 1903789 (2019).
Gong, S. et al. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 5, 3132 (2014).
Yamada, T. et al. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 6, 296–301 (2011).
Gong, S. et al. A soft resistive acoustic sensor based on suspended standing nanowire membranes with point crack design. Adv. Funct. Mater. 30, 1910717 (2020).
Amjadi, M., Kyung, K. U., Park, I. & Sitti, M. Stretchable, skin‐mountable, and wearable strain sensors and their potential applications: a review. Adv. Funct. Mater. 26, 1678–1698 (2016).
Gong, S. et al. Highly stretchy black gold e‐skin nanopatches as highly sensitive wearable biomedical sensors. Adv. Electron. Mater. 1, 1400063 (2015).
Amjadi, M., Pichitpajongkit, A., Lee, S., Ryu, S. & Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. ACS Nano 8, 5154 (2014).
Xu, X. et al. High‐strain sensors based on ZnO nanowire/polystyrene hybridized flexible films. Adv. Mater. 23, 5440 (2011).
Han, F. et al. J. Mater. Chem. C. 5, 10167 (2017).
Cao, Z., Wang, R., He, T., Xu, F. & Sun, J. Interface-controlled conductive fibers for wearable strain sensors and stretchable conducting wires. ACS Appl. Mater. Interfaces 10, 14087 (2018).
Ma, J. et al. Highly sensitive and large-range strain sensor with a self-compensated two-order structure for human motion detection. ACS Appl. Mater. Interfaces 11, 8527 (2019).
Liao, X. et al. Flexible and highly sensitive strain sensors fabricated by pencil drawn for wearable monitor. Adv. Funct. Mater. 25, 2395 (2015).
Tang, Z. et al. Highly stretchable core–sheath fibers via wet-spinning for wearable strain sensors. ACS Appl. Mater. Interfaces 10, 6624 (2018).
Wang, S. et al. Controllable synthesis of nickel nanowires and its application in high sensitivity, stretchable strain sensor for body motion sensing. J. Mater. Chem. C. 6, 4737 (2018).
Tao, L.-Q. et al. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 8, 14579 (2017).
Wei, Y. et al. A wearable skinlike ultra-sensitive artificial graphene throat. ACS Nano 13, 8639–8647 (2019).
Nayeem, M. O. G. et al. All-nanofiber–based, ultrasensitive, gas-permeable mechanoacoustic sensors for continuous long-term heart monitoring. Proc. Natl Acad. Sci. USA 117, 7063–7070 (2020).
Zhang, Q., Zeng, X., Hu, W. & Zhou, D. A machine learning-empowered system for long-term motion-tolerant wearable monitoring of blood pressure and heart rate with ear-ECG/PPG. IEEE Access 5, 10547–10561 (2017).
Zhang, Q., Zhou, D., & Zeng, X. PulsePrint: single-arm-ECG biometric human identification using deep learning. IEEE 8th Annual Ubiquitous Computing, Electronics and Mobile Communication Conference (UEMCON) 452–456 (IEEE, 2017).
Abdeldayem, S. S. & Bourlai, T. A novel approach for ECG-based human identification using spectral correlation and deep learning. IEEE Trans. Biom. Behav. Identity Sci. 2, 1–14 (2019).
Bao, X., Abdala, A. K. & Kamavuako, E. N. Estimation of the respiratory rate from localised ECG at different auscultation sites. Sensors 21, 78 (2020).
Santurkar, S., Tsipras, D., Ilyas, A. & Mądry, A. How does batch normalization help optimization? in Proc. 32nd International Conference on Neural Information Processing Systems 2488–2498 (NeurIPS, 2018).
He, K., Zhang, X., Ren, S. & Sun, J. Delving deep into rectifiers: surpassing human-level performance on ImageNet classification. In Proc. IEEE International Conference on Computer Vision 1026–1034 (IEEE, 2015).
McInnes, L. et al. UMAP: uniform manifold approximation and projection. J. Open Source Softw. https://doi.org/10.21105/joss.00861 (2018).
Gong, S. et al. Fabrication of highly transparent and flexible nanomesh electrode via self‐assembly of ultrathin gold nanowires. Adv. Electron. Mater. 2, 1600121 (2016).
Chen, Y., Ouyang, Z., Gu, M. & Cheng, W. Mechanically strong, optically transparent, giant metal superlattice nanomembranes from ultrathin gold nanowires. Adv. Mater. 25, 80–85 (2013).
Acknowledgements
This research was financially supported under Discovery Projects funding scheme (grant nos. DP180101715, DP200100624 and DP210101045) and Jack Brockhoff foundation (JBF grant no. 4659-2019). This work was performed in part at the Melbourne Centre for Nanofabrication (MCN) in the Victorian Node of the Australian National Fabrication Facility (ANFF). The authors also acknowledge the use of facilities at the Monash Centre for Electron Microscopy.
Author information
Authors and Affiliations
Contributions
S.G. and W.C. conceived the idea, designed the experiment, and composed the manuscript. W.C. and Z.G. supervised the whole project. S.G., X.Z., X.A.N., Q.S. and F.L. performed all of the experiments. All authors made technical comments on the manuscript. W.C. submitted the manuscript and was the lead contact.
Corresponding authors
Ethics declarations
Competing interests
W.C. declares that the v-AuNW growth on PDMS is related to the patented technology (patent nos. US11387012B2 and AU2018263276B2) that has been licensed to Soft Sense Pty Ltd in which W.C. is the founder. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Bin Ding and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary information.
Supplementary Video 1
Visualization demonstration of a human–robot interaction in the ‘apple-picking’ environment.
Supplementary Video 2
Visualization demonstration of a human–robot interaction in the surgical environment.
Source data
Source Data Fig. 2
SEM/TEM raw file images. Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, S., Zhang, X., Nguyen, X.A. et al. Hierarchically resistive skins as specific and multimetric on-throat wearable biosensors. Nat. Nanotechnol. 18, 889–897 (2023). https://doi.org/10.1038/s41565-023-01383-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01383-6
This article is cited by
-
Speaking without vocal folds using a machine-learning-assisted wearable sensing-actuation system
Nature Communications (2024)
-
Phase-separated stretchable conductive nanocomposite to reduce contact resistance of skin electronics
Scientific Reports (2024)
-
A fully integrated, standalone stretchable device platform with in-sensor adaptive machine learning for rehabilitation
Nature Communications (2023)
-
Artificial intelligence-powered electronic skin
Nature Machine Intelligence (2023)
-
Scalable and eco-friendly flexible loudspeakers for distributed human-machine interactions
npj Flexible Electronics (2023)