Abstract
Kobellite is a Pb-Bi-Sb sulfosalt with minor amounts of (Cu, Fe) and with the crystal structure composed of two types of rods, one of which has unusual lateral extensions (‘lobes’), which depart from the usual lozenge-shaped rod cross-section in sulfosalts. Several Pb-Bi-Sb and Pb-Sb-rich sulfosalts form a small group built on similar principles. Some of them are related by homology (e.g., izoklakeite), and differ by the perpendicular dimensions of rods (length and multiplicity of atomic layers in a rod; e.g., sterryite), and especially by different combinations of archetypes and archetype portions which participate in the rods (PbS archetype and the two orientations of SnS archetype). The present article summarizes and discusses the published data on the group. Homeotypism makes the group interesting and potentially a fertile source of further structural varieties.
Similar content being viewed by others
Introduction
A key factor of the archetypal rod description of (especially) lead sulfosalt structures in the nineteen-eighties, was the choice of archetypal structures. This happened by the introduction of PbS and SnS archetypes, as the two archetypal structures with very different lone-pair activity of their cations (Makovicky 1981, 1985). A part of this problem was how to describe the relation akin to that which separates bismuthinite from bournonite. This was achieved in the simplest way as 2 Å shear along the 4 (or 8) Å direction (Makovicky 1985). This concept was then cleverly used for the sterryite – parasterryite pair when they were discovered (Moëlo et al. 2011).
The unusual and highly interesting combination of the two archetype concepts in one structure produces the homeotypic cluster of mutually related sulfosalt crystal structures, which is described here. As always, the structural differences and variations described have obvious background in the differences in chemistry and in the behavior of component cations, especially in their lone electron pair activity and preferred bond schemes.
All these compounds have already been included in various structural classifications more than once, e.g., as members of the kobellite homologous series (Makovicky and Mumme 1986), of the rod based sulfosalt structures (Makovicky 1993), of the sterryite plesiotypic series (Biagioni et al. 2016), of structures based on concatenation of arms of triangular ‘paddle-wheels’ (Moëlo et al. 2011), or (partly) as members of the group of lead-antimony oxysalts (Biagioni et al. 2016). The work submitted here does not suggest a revision of these groups. Instead, it describes one more, yet largely unnoted kinship category which exists between, and among, several very complex sulfosalts.
The type structures: kobellite and izoklakeite
The crystal structure of a sulfosalt mineral of special kind, kobellite, (Cu1.12Fe0.88Pb12Bi7.89Sb6.11S35), was published by Miehe (1971) and Mumme et al. (2013). On the one hand, kobellite is a mineral with important contents of both Bi and Sb, which are two elements with profoundly different lone electron pair activity, and on the other hand, it was the first known structure, in which one of the rod-like Pb-(Bi,Sb) configurations differed in cross-section from the usual lozenge or truncated-lozenge shape, by attachment of lateral lobes with additional cations (cation rows) at the opposing ends of the largest lozenge (Fig. 1).
The crystal structure of kobellite (Miehe 1971) projected along [001] (the direction of the rods). Yellow spheres: S; red and pink spheres: Bi and Sb (Sb preferably in N = 2 rods); blue: Pb; small orange: Cu. Two z levels, 2 Å apart, are indicated for all atom species by light and dark shading. The A-type rod is outlined in brown shades, the B-type rod in green. Long bonds across the interior of LEP micelles are indicated by simple lines (even in overlap) and the LEP-filled volumes by lighter color
The crystal structure of kobellite is composed of two rod types in a quasi-chessboard arrangement, which more exactly are arranged in agreement with a space group Pnnm. The smaller rod type (designated as A below) has rod cross-sections two coordination pyramids long along the a direction, and is four atomic planes thick in the b direction. The A rods are in antiparallel arrangement to one another. They are of the SnS type, with a mutual 2 Å shift of cation levels in their two halves, when compared with a simple PbS-like arrangement (Fig. 1). They are dominated by coordination polyhedra of Sb.
The larger rods (type B in what follows), dominated by coordination polyhedra centered by bismuth atoms, are parallel to (110), respectively (\(1\bar {1}0\)). The bulk length of their double-layers is 6 cation polyhedra, although their truncated outer atom planes are only 5 polyhedra wide. The B rod is only three atomic planes thick (Fig. 1) and is built according to the PbS archetype. The remarkable property of kobellite and of the related phases described here are the lateral attachments of these rods. In kobellite, they consist of another atomic level, oriented in parallel with the main rod body and forming, when taken together with the rest of the rod, a ‘doubly-bulging’ rod (Fig. 1). The formation of this ‘dumb-bell’ rod avoids formation of a large vacancy which would otherwise separate PbS-like rods, which are arranged according to the glide planes of the space group Pnnm.
The cation sites in outer atomic planes of the two types of rods complete their coordinations by linking with S atoms from the outer planes of the neighboring rods. Notably, they are represented by Pb atoms in monocapped trigonal prismatic coordinations in the B rods, and by bicapped trigonal prismatic coordinations in both the A and B rods. A conspicuous feature are the pseudotrigonal channels formed on junctions of two B rods and one A rod, as a combination of three bicapped prisms of Pb.
Makovicky and Mumme (1986) called kobellite the N = 2 homologue of a potential homologous series, which, remarkably, was soon enlarged by the member N = 4, izoklakeite (Cu2.6Fe1.4Pb55.4Bi23.1Sb13.6S114) (Makovicky and Mumme 1986). In this structure, the lozenge-shaped SnS-archetype rods (rods A), with large cations (Pb) on pseudotetragonal N = 4 surfaces, and with S atoms, exposed from the Sb-based coordination polyhedra and situated on the diagonally oriented cutting surfaces, alternate with sizable PbS-like (B) rods which are 5 atomic levels thick. These rods are augmented by addition of two more rudimentary levels (i.e., by one polyhedron height) on blunt corners. Bulk polyhedron sequences in these ‘dumb-bell’ rods are 6 polyhedra, as in kobellite, except for corner truncation. Again, B rods are dominated by coordination polyhedra of bismuth, whereas the A rods by those of Sb. A difference to kobellite is in the occupancy of the central four cationic sites, which in izoklakeite house important to dominant Bi amounts.
Both kobellite and izoklakeite have a tetrahedral cation site situated between two PbS-like B rods and one SnS-like A rod. This site, in agreement with charge requirements, is occupied by mixed Cu and Fe. The N = 2 member is also known with the (ideally) pure antimonian composition, as tintinaite Cu2Pb10Sb16S35 (Moëlo et al. 1984), and the Bi-rich monoclinic N = 4 member has been described as giessenite (Cu,Fe)2Pb26.4(Bi,Sb)19.6S57 (Graeser 1963).
Makovicky and Mumme (1986) spent considerable time investigating possible N = 3 and similar odd variants. Their conclusion was that in all variations attempted, substantial local changes were needed to make such a model viable, although no problems were encountered for N = 4, for which a complete model was derived even before izoklakeite (N = 4) was found. In one of the N = 3 models, the only difference from sterryite structure was that Makovicky and Mumme (1986) assumed the ‘lobes’ being one cation polyhedron thick but in sterryite entire fragments of an atomic double layer are attached. Therefore, the way in which the Nature proceeded to get N = 3 homeotypes makes a fascinating story.
Sterryite and parasterryite
Unlike the N = 2 kobellite, sterryite (Ag1.86Cu1.73Hg0.24Tl0.24Pb19.13Sb16.47As7.06Bi0.1S56) (Moëlo et al. 2012) has the N = 3 A-rods of the PbS type, with only slight sideways shift of double-layers against one another. Their PbS-like archetypal internal arrangement holds well also in the side-views. The elongation of cross-sections of the N = 3 rod is parallel to the b axis (Fig. 2). The large dumb-bell B rod is composed of four atomic layers, which are six polyhedra broad, with the lobes two atomic layers thick (they were only one atomic level thick in kobellite). These lobes are only two Pb polyhedra broad. An additional, split position of lead, which is situated next to them, is to be considered just as a fill of the niche in the recess of the dumb-bell rod, similar to what is found in the closely related parasterryite.
The crystal structure of sterryite (Moëlo et al. 2012) in projection along [100]. Pink spheres: Sb; blue spheres: Pb; green spheres: predominant As; small red and grey spheres: Cu and Ag; yellow: S. Long interactions across LEP micelles do not overlap. Asterisks indicate insertion of an Ag atom and a Sb atom with distorted coordinations as correction of the rod fitting scheme. For further conventions see caption to Fig. 1
The central LEP micelle of the B rod has long bonds arranged in an SnS-like pattern, with mutual shift of the walls by ½ of the pseudotetragonal subcell parameter in the (100) plane, i.e., perpendicular to the rod elongation. Looking from the side, these bonds are not quite perpendicular to the rod axis but the same holds for the short bonds inside the tightly-bonded layer. There are steric problems which the crystal structure might experience on the sulfur-covered diagonal surfaces of this rod, and caused by the just described sideways shift. They are largely corrected, however, by insertion of a silver atom and an antimony atom, both with distorted octahedral environment (indicated in Fig. 2 by asterisks; see also Moëlo et al. 2012), into the inner atomic layers, closest to their marginal portions. They alter locally the pronounced SnS-like shift into a distorted PbS-like shift, and avoid in this way any excessive warping of the sulfur-covered surface. The two lateral LEP micelles, which form the lobes, are attached to the central portions of the B rod via long bonds. These are again arranged in a SnS manner but with mutual shift of the walls along the rod elongation. Thus, the B rod combines both orientations of the SnS archetype.
The well-ordered bonding scheme of sterryite (Fig. 2) accommodates a tetrahedrally coordinated Cu atom in the niche created by closing the above mentioned lobe. Further three copper and silver atoms are adjacent to this site as well, as also are the inflated-configured coordination polyhedra of two arsenic sites. Thus, the interesting covalently bonded pair As14-As15 is a part of this ‘receding step’ configuration. The observed covalent As-As bond length appears to lie at the higher range of known As-As distances (Kasatkin et al. 2022), and the two short As-S bonds of both As atoms are oriented (i.e., pointing) the same way, not like in gungerite (Kasatkin et al. 2022) in which they point the opposite ways.
In all these crystal structures we can additionally discern twinned-octahedral layers running through and crossing the dumb-bell rod scheme in the direction which is parallel to the b axis of sterryite. In sterryite, there are two amalgamated, parallel and uninterrupted twinned-octahedral layers of this kind, although the octahedra and layers are always a bit distorted. This simple inconspicuous scheme determines the spacing of A rods in these structures, and is disturbed only in the structure of meerschautite, by insertion of fragments of the third layer of this kind.
In parasterryite (Ag4Pb20.21Sb13.56As10.23S58; Moëlo et al. 2012) the dumb-bell rod is again composed of tightly-bonded double layers, which are six cation polyhedra (or 6 pseudotetragonal subcells) wide. The side-lobes, again, are only two coordination polyhedra of lead long, with one adjacent polyhedron which has a very heterogeneous composite occupation by cations, attached to them. Therefore, it will not be described as a portion of the lobe. With the lobes included, the dumb-bell rod is 8 atomic planes broad, as in sterryite.
The central LEP interspace of the dumb-bell rod, situated between identical (but anti-oriented) double-layers can be interpreted as indicating SnS archetype, with the layer shift perpendicular to the rod and long Me-S distances visible in the projection plane [i.e., (100)]. However, it is less expressed and regular than in sterryite. The LEP interspaces between the main double-layers and the side-lobes keep their PbS-like character, although they show a degree of counter-inclination against the large LEP space. These deviations differ slightly for the two wings of the dumb-bell.
The N = 3 A-rod has a well-defined PbS-like configuration. However, the marginal arsenic atom flips its short (i.e., vertex-oriented) bond into the LEP space of the surrounding Sb. Columns which contained the split lead site in sterryite, and one of those containing antimony in sterryite, are occupied by a number of combined cations with differing coordinations. The N = 3 A-rods deviate slightly from the cell axis in their orientation and are re-arranged en echelon. Tetrahedrally coordinated Ag atom is situated in the void at the descending portion of the lobe, close to where lead atoms are concentrated. The effects of the main SnS-like shift are partly moderated in a way similar to sterryite, i.e., by insertion of the Sb-Ag combination in one column at both ends of the double-layers. Again, both these cations have a strongly asymmetric bond scheme.
Meerschautite
The SnS-like A-rods of meerschautite [(Ag,Cu)5.5Pb42.4(Sb,As)45.1S112O0.8 (Biagioni et al. 2016)] retain their SnS-like character, with overall shifts parallel to the rod axis, and a width of three cation polyhedra, as in a typical N = 3 homologue (Fig. 3). The ‘dumb-bell’ B-rod has 4 individual atomic (cation and anion) layers, unlike to kobellite, which has 3 such layers per rod, and izoklakeite, with 5 layers per rod. It corresponds to the situation in sterryite. The fundamental length of the dumb-bell rod is 6 pseudotetragonal subcells.
Formally, the SnS-like N = 3 A-rods can be considered truncated at the sharp edges, because one of the last, terminal polyhedra of an untruncated type is occupied by a mixture of elements, which have close contacts to a cation in the adjacent polyhedron. The corresponding polyhedron at the opposite corner of the rod also is shared by a B rod (Fig. 3).
The latter sites are sandwiched between one SnS-like and two dumb-bell rods. This represents a difference against the simpler arrangement in kobellite. The principal reason is that in meerschautite the ‘bulge’ of the dumb-bell is two atomic planes thick, and not a single atomic plane, as it was in kobellite.
Meerschautite is structurally close to the N = 3 homologue of the series, sterryite. However, when compared to sterryite, meerschautite structure is locally enlarged by addition of fragments of one more ‘twinned’ octahedral layer (010)kob. As a consequence, the (010) spacing is larger than in kobellite and also in sterryite, and the distortion of the structure causes slight rotations of the SnS-like rods. The en echelon orientation of the N = 3 rods results in rod rotations by about one short [010]-pointing cation-anion bond length for each gained [100] segment.
As mentioned above, the N = 3 A-rods are of the SnS type, with the mutual shift of their two halves parallel to the rod axis, although the full structure projection shows variations in Sb-S short-bond orientations along the rod. The central LEP micelle of the dumb-bell B rod has SnS character, with the shifts perpendicular to the rod axis (similar to sterryite). On one side, the bulge created by additional double-layer represents the SnS-like connection (mutual shifts parallel to the rod axis) to the central double-layers. The opposite bulge, however, is attached to the central double-layers by a somewhat distorted set of longer distances, and is intermediate between the PbS-like and the SnS-like principles (with the shift perpendicular to the rod axis). The resulting asymmetry of B-rod architecture is astounding and might be connected with the geometric distribution of all three archetype configurations dispersed all over the structure (Fig. 3).
In general, the ubiquitous presence/development of LEP micelles in meerschautitte conforms clearly with the pure Pb – Sb(As) character of meerschautite, which is a structure without Bi. Smaller but significant amounts of Ag and Cu, and presence of some oxygen are inevitable components of the structure (Biagioni et al. 2016).
Ciriottiite
Ciriottiite [Cu(Cu,Ag)3Pb19(Sb,As)22(As2)S56 (Bindi et al. 2016)] is according to the authors a Cu-analogue of sterryite and even of meerschautite. Again, it has been described in terms of ‘broken three-fold paddle-wheels’ joined into complex interlocking chains parallel to [010] (Bindi et al. 2016). As meerschautite, this structure is full of lone electron pair micelles, always placed between tightly-bonded double-layers of cations and anions.
The N = 3 rods remain of the PbS type in ciriottiite, and we note the absence of the split long cation-anion distances oriented ‘up’ and ‘down’. Different orientation, however, is valid for the two ‘side-lobes’ of the dumb-bell rod; both are attached as in the SnS archetype, with mutual shifts of the two double-layers along the rod axis. Contrary to these, the central, long and narrow LEP micelle has shift perpendicular to those just described, in the (100) plane of the structure, reaching a perfect ½ subcell shift of one surface against another.
As observed in previous cases, at the ends of the 6 subcells (6 polyhedra) wide cross-section of the dumb-bell rod, the effects of the pronounced mutual SnS-like displacement are partly eliminated by insertion of highly asymmetric coordination polyhedra of Sb (critical bonds 2.40/2.51 Å versus 3.21 Å), combined with those of Cu, into marginal [100] rows. Copper plays a role corresponding to Ag in the previous structures. In this way, the array of marginal sulfur atoms achieves the structurally required near-planarity. The waning of the interatomic distance distribution seen in the lobe portions, which takes place in the center of the long dumb-bell rod, takes place via insertion of the symmetric (octahedral) polyhedra of bismuth (bonds of 2.47–2.66 Å) combined with the Cu positions which alternate with them. They are connected to As rows, which also contribute to the width adjustments of the lobe arrays. This array is completed by copper in non-planar threefold coordination, combined with an additional, longer (2.57 Å) distance. This position is closest to the Cu position in kobellite.
Intergrowths with other structure types: eclarite and marrucciite
The interesting, well characterized crystal structures of the kobellite and sterryite type form intergrowths with at least two other structure types. In the present paper we deal with a bismuth-rich case, the mineral eclarite, and with a case in which coordination polyhedra of mercury caused a pronounced reorganization of the crystal structure, the mineral marrucciite.
Eclarite, (Cu,Fe)Pb9Bi12S28 (Kupčík 1984; Topa and Makovicky 2012), has the dumb-bell rods cut in approximate halves which become removed widely from one another. Such truncated rods form kobellite-type (001) slices, which occur in regular intergrowths with slices similar to those of rod-based layer structures, namely cosalite (Topa and Makovicky 2010). In the cosalite-like pairs of rods, the PbS-like N = 2 rods are interconnected by single-octahedral strings (Fig. 4). In these structure portions, every second contact of the kobellite-like slices of N = 2 elements has been reduced, so that along this contact, the N = 2 rods form a tight sequence of rods on the sides of the (001) plane (Fig. 4). The broader slices between such contacts are kobellite-like, with three atomic planes per B-like rod. However, along the reduced contacts, halves of these B-like rods are missing, having only one bulge left (Fig. 4).
The crystal structure of marrucciite (Hg3Pb16Sb18S46; Orlandi et al. 2007, Sejkora et al. 2011) consists of slabs, in which perpendicular-oriented N = 3 rods are arranged in alternation with diagonally oriented, truncated N = 3 rods, all slanting in one direction (unlike to kobellite). Both rod types are truncated at the sharp corners and the diagonally oriented rods lost their additional bulging cations. The coarsely grooved surface of this structural slab is on both sides accompanied by a layer of Hg-containing N = 3 rods, which are interconnected via an additional Hg octahedron, surrounded by Pb and Sb. The large and broad coordination polyhedra of Hg are all concentrated in this layer and make it sufficiently extended to match the complex periodicity of the kobellite-related slab.
Conclusions
Phases of the kobellite homeotypic series are composed of rods of PbS-like and/or SnS-like structure; these are formed by Pb-Bi-S and/or Pb-Sb-S combinations. It is a chess-board like arrangement of smaller A rods of the above types (with lozenge-like cross-section), with or without truncation on sharp corners, and of larger B rods with more complicated boundaries. The latter display addition of an atomic-layer fragment or of an atomic double-layer fragment at two opposing corners. This addition fills up the otherwise empty channel, which would be created by the rod composition. The crystal structure of kobellite represents the simplest model of these structures.
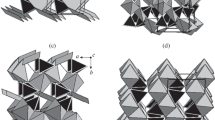
Although the crystal structures of this family look very much alike in the projection along the rod direction, the existing combinations of Pb-Bi-Sb (or only two of these elements), together with minor contents of Cu, Ag, Fe, etc., necessary for both local and overall charge compensation, cause that the PbS archetypal arrangement, and the variously complicated SnS-archetype arrangement, can and often do combine in one structure (Fig. 5a-d). In kobellite and izoklakeite, the parallel N = 2 and N = 4 A-rods are SnS-like, with the mutual 2 Å double-layer shifts in the rod interior oriented parallel to the rod axis, whereas in the Pb-Sb sulfosalts, sterryite and parasterryite, these N = 3 A-rods are of the PbS archetype (Fig. 5b,c). Ciriottiite has the A-rods of PbS type, with no shifts parallel or perpendicular to the rod axis, very similar to sterryite. Meerschautite, however, has the A rods (N = 3) of the SnS type, with shifts parallel to the rod axis (Fig. 5d). Whether this is connected with the appearance of the PbS-like arrangement in one of the B-rod lobes, is an interesting open question.
Projections of kobellite homeotypes along the direction of the structural rods. Contacts of rods (left uncolored) are fragments of non-commensurate interspaces, which are akin to contacts of pseudotetragonal and pseudohexagonal layers in cannizzarite (Matzat 1979). In the rod interior, tightly-bonded double-layers (left uncolored) are interconnected via lone-electron pair-filled interspaces (colored): either as PbS-like continuation of the structure across the interspace (green areas), or as SnS-like continuation with mutual ½-period shifts of layers in the direction parallel to the rods (light blue areas), or as SnS-like continuation with mutual shifts perpendicular to the rod direction (yellow areas). a: kobellite; b: sterryite, c: parasterryite, d: meerschautite. Note the homeotypism within the group
In the dumb-bell shaped B rods of ciriottiite, the central LEP micelle is a flat long interspace between the ‘inner’ double-layers of the rod, with shifts perpendicular to the rod axis. The same occurs in sterryite (Figs. 2 and 5b), whereas the lateral LEP micelles (forming the ‘bulges’of the B rod in sterryite), which are much shorter than the central LEP space, are SnS-archetypal in both minerals, with shifts parallel to this axis and the rods (Fig. 5b). Parasterryite has a more complicated situation (Fig. 5c), apparently due to a number of substituting cations present in its structure. Its PbS-archetype is developed in the N = 3 A-rods, whereas a transitional type of weak bonding (sidewise-distorted PbS-like arrangement) was found in the attachment of both bulges to the rest of the rod structure. The SnS-like arrangement with shifts perpendicular to the rod axis constitutes the central LEP gap of the B rod.
The complicated crystal structure of marrucciite has N = 3 rods of the PbS type as well, but the diagonally oriented rods have the SnS-like shift parallel to the rod axis. The complex situation in the kobellite homeotypes is best illustrated in Fig. 5a-c, in which coloring has been limited to the LEP interspaces in the rod interiors and the tightly-bonded double-layers interconnected by the activity in these interspaces were left uncolored.
The different arrangements of archetype fragments in the rods are reflected in the unit cell parameters and symmetry. According to the results of crystal structure determinations, unit cells are very similar, with the a parameter varied within 8.19 Å and 8.40 Å, the b parameter 27.95 Å and 28.53 Å, and c within 42.45 Å and 43.88 Å. In sterryite, ciriottiite and parasterryite, the monoclinic angle has the same orientation, and is comprised by the 8 Å and ~ 43 Å parameters. However, in sterryite and ciriottiite the β angle varies only between 94.9° and 93.6° but in parasterryite it is very different, about 90° (Moëlo et al. 2012). In agreement with its different archetype distribution in the structure, the monoclinic angle in meerschautite is comprised by the 8.2 Å and 28.4 Å parameters, and is equal to ~ 94.1°. Coordination or occupancy variations manifested by the 8.2 Å – 8.4 Å doubling of the basic 4 Å a parameter, reflect the alternation of two Sb coordinations or/and the Sb/Pb alternation along the rod elongation.
The space groups of sterryite and ciriottiite are P21/n, confirming their structural proximity, that of parasterryite is P21/c, in agreement with its different archetype content and the resulting 2 Å-shifts. Meerschautite has symmetry lowered to P21, with a quite different orientation of the b direction.
Because of extremely complicated original compositions and, let us admit, a number of occupancy assumptions made by the original authors about many mixed cation sites, we selected two reasonably simple structures – kobellite and sterryite for the analysis of observed crystal chemical features. The large, dumb-bell shaped rod in sterryite, M34S42, has the composition defined by the values from the structure refinement as Pb13.26Tl0.24Ag1.86Cu0.9Hg0.24Sb12.01As3.49S42. The smaller rod, Me10S14, has the structurally refined composition Pb3.90Bi0.1Sb4.29As1.57S14, and the three atoms in interspace were refined as Pb1.83Cu0.83Sb0.17. Total charge error is about 3.5% (undervalued cation charge) and there are 21 mixed-occupancy positions in the structure. Average cation valence in the rods is similar, 2.47 for the large rod, and 2.8 for the small rod in sterryite, plus 3 positive valences divided between the two rods, and positioned in their rather narrow contact zone.
Kobellite has the dumb-bell rods with configuration simpler than sterryite (Fig. 1). The composition of the dumb-bell rod is Pb8Bi6.22Sb3.78S23 (i.e., 10 cations have a pronounced LEP character), whereas the smaller rods are Pb4Bi1.67Sb2.33S12. The additional, inter-rod cation was defined as Cu2 + 0.56Fe2+0.44 (Miehe 1971). The average valence of a cation in the dumb-bell rod is 2.56, in the small lozenge-shaped rod it would be required to be 3.0 for charge balance, but is 2.5 instead, leaving an excess of -4 valence. However, the smaller rod is flanked on two oposite sharp corners by inter-rod cations (Cu, Fe) that compensate this excess valence.
Unlike kobellite, the dumb-bell rod of sterryite concentrates LEP elements, which results in a structure characterized by pronounced LEP micelles. Interrestingly, its smaller rods are of the PbS-like character, which is apparently determined by the best fit on rod-to-rod boundaries. In kobellite, in which the LEP active element, Sb, is concentrated into smaller rods, and the area in which three rods meet configured differently from that in sterryite (Figs. 1 and 5a and b), the opposite solution is preferred.
Although the percentage of SnS-like archetype ‘applied’ in the particular structure appears to be primarily the function of the Sb/Bi ratio, in the majority of cases both archetypes participate in the same structure and, most remarkably, they often ‘exchange places’ between the N = 2,3,4 A-rods and the dumb-bell shaped B-rods (as it was specified for individual structures above). The structure of the rod interior expresses mostly the Sb/Bi proportions of the LEP cations which participate in it.
On the level of rod surfaces, the main difference between these various structures (besides the widths of exposed surfaces of the rods) should be the presence of the pseudohexagonal sulfur-populated rod-contact surface in the case of the PbS-like rod structure versus the presence of the sheared pseudohexagonal S-populated contact surface in the case of the internal SnS-based rod structure. The sulfur-populated surfaces of both kinds usually face the pseudotetragonal surface of the opposite rod (Figs. 1 and 2). May-be, preferable configurations for the contacts of adjacent rods exist and they influence the final choice of archetype, when the two rod types combine and face one another.
References
Biagioni C, Moëlo Y, Orlandi P, Stanley CJ (2016) Lead-antimony sulfosalts from Tuscany (Italy). XVII. Meerschautite, (Ag,Cu)5.5Pb42.4(Sb,As)45.1S112O0.8, a new expanded derivative of owyheeite from the Pollone mine, Valdicastello Carducci: occurrence and crystal structure. Min Mag 80:675–690
Bindi L, Biagioni C, Martini B, Salvetti A (2016) Ciriottiite, Cu(Cu,Ag)3Pb19(Sb,As)22(As2)S56, the Cu-analogue of sterryite from the Tavagnasco Mining District, Piedmont, Italy. Minerals 6:8
Graeser S (1963) Giessenit - ein neues Pb-Bi-Sulfosalz aus dem Dolomit des Binnatales. Schweiz Min Petrog Mitt 43:471–478
Kasatkin A, Plášil J, Makovicky E, Chukanov NV, Škoda R, Agakhanov AA, Tsyganko MV (2022) Gungerite, TlAs5Sb4S13, a new thallium sulfosalt with a complex structure containing covalent As-As bonds. Am Min 107:1164–1173
Kupčík V (1984) Die Kristallstruktur des Minerals Eclarite, (Cu,Fe)Pb9Bi12S28. TMPM 32:250–269
Makovicky E (1981) The building principles and classification of bismuth-lead sulphosalts and related compounds. Fortschr Min 59:137–190
Makovicky E (1985) The building principles and classification of sulphosalts based on the SnS archetype. Fortschr Min 63:45–89
Makovicky E (1993) Rod-based sulphosalt structures derived from the SnS and PbS archetypes. Eur J Min 5:545–591
Makovicky E, Mumme WG (1986) The crystal structure of izoklakeite, Pb51.3Sb20.4Bi19.5Ag1.2Cu2.9Fe0.7S114. The kobellite homologous series and its derivatives. N Jb Min Abh 153:121–148
Matzat E (1979) Cannizzarite Acta Cryst B35:133–136
Miehe G (1971) Crystal structure of kobellite. Nat Phys Sci 231:133–134
Moëlo Y, Jambor JL, Harris DC (1984) Tintinaïte et sulfosels associés de Tintina (Yukon): la cristallochimie de la série de la kobellite. Can Min 22:219–226
Moëlo Y, Guillot-Deudon C, Evain M, Orlandi P, Biagioni C (2012) Comparative modular analysis of two complex sulfosalt stuctures: sterryite, Cu(Ag,Cu)3Pb19(Sb,As)22(As-As)S56, and parasterryite, Ag4Pb20(Sb,As)24S58. Acta Cryst B68:480–492
Mumme WG, Gable RW, Wilson N (2013) A crystal structure determination of (selenian) kobellite from the Boliden Mine, Sweden. N Jb Min Abh (J Min Geochem) 2013:109–115
Orlandi P, Moëlo Y, Campostrini I, Meerschaut A (2007) Lead-antimony sulfosalts from Tuscany (Italy). Marrucciite Hg3Pb16Sb18S46, a new sulfosalt from Buca della Vena mine, Apuan Alps: definition and crystal structure. Eur J Min 19:267–279
Sejkora J, Ozdín D, Laufek F, Plášil J, Litochleb J (2011) Marrucciite, a rare Hg-sulfosalt from the Gelnica ore deposit (Slovak Republic), and its comparison with the type occurrence at Buca della Vena mine (Italy). J Geosci 56:399–408
Topa D, Makovicky E (2010) The crystal chemistry of cosalite based on new electron-microprobe data and single-crystal determinations of the structure. Can Min 48:1081–1107
Topa D, Makovicky E (2012) Eclarite, new data and interpretation. Can Min 50:371–386
Acknowledgements
One of the tasks of mineralogy as a science is to organize the (mostly inorganic) compounds of natural origin into a combined chemical and structural system which will be helpful for the coming generations in further research and, especially for this large group of complex sulfides (‘sulfosalts’), in further investigations by methods of materials science. The submitted paper is authors’ little contribution to this task, dedicated to the memory of Professor Dr. Josef Zemann, an outstanding mineralogist and crystallographer, who was always willing to help us and many others of our generation. Our thanks to Cristian Biagioni for the data on meerschautite. Many thanks to the editor, Hertha Silvia Effenberger, as well as to Yves Moëlo and Cristian Biagioni for their valuable comments.
Funding
Open access funding provided by Royal Danish Library
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: H. Effenberger.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makovicky, E., Balić-Žunić, T. On the homeotypes of kobellite. Miner Petrol 117, 209–217 (2023). https://doi.org/10.1007/s00710-023-00828-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-023-00828-z