Abstract
Cells respond to perturbations such as inflammation by sensing changes in metabolite levels. Especially prominent is arginine, which has known connections to the inflammatory response. Aminoacyl-tRNA synthetases, enzymes that catalyse the first step of protein synthesis, can also mediate cell signalling. Here we show that depletion of arginine during inflammation decreased levels of nuclear-localized arginyl-tRNA synthetase (ArgRS). Surprisingly, we found that nuclear ArgRS interacts and co-localizes with serine/arginine repetitive matrix protein 2 (SRRM2), a spliceosomal and nuclear speckle protein, and that decreased levels of nuclear ArgRS correlated with changes in condensate-like nuclear trafficking of SRRM2 and splice-site usage in certain genes. These splice-site usage changes cumulated in the synthesis of different protein isoforms that altered cellular metabolism and peptide presentation to immune cells. Our findings uncover a mechanism whereby an aminoacyl-tRNA synthetase cognate to a key amino acid that is metabolically controlled during inflammation modulates the splicing machinery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
RNA-seq and TGIRT-seq data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under accession code GSE165513 and on NIH Bioproject with accession number PRJNA561913. Mass spectrometry data have been deposited in ProteomeXchange with the primary accession codes PXD015692, PXD024091 and PXD036232. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Custom code for the analysis of TGIRT-seq data can be found at https://github.com/wckdouglas/tgirt_map.
Change history
26 May 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41556-023-01172-2
References
Netea, M. G. et al. A guiding map for inflammation. Nat. Immunol. 18, 826–831 (2017).
Bronte, V. & Zanovello, P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5, 641–654 (2005).
Murray, P. J. Amino acid auxotrophy as a system of immunological control nodes. Nat. Immunol. 17, 132–139 (2016).
Bar-Peled, L. & Sabatini, D. M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 24, 400–406 (2014).
Weichhart, T., Hengstschläger, M. & Linke, M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 15, 599–614 (2015).
Schimmel, P. R. & Söll, D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu. Rev. Biochem. 48, 601–648 (1979).
He, X.-D. et al. Sensing and transmitting intracellular amino acid signals through reversible lysine aminoacylations. Cell Metab. 27, 151–166.e6 (2018).
Vo, M.-N. et al. ANKRD16 prevents neuron loss caused by an editing-defective tRNA synthetase. Nature 557, 510–515 (2018).
Cui, H. et al. Regulation of ex-translational activities is the primary function of the multi-tRNA synthetase complex. Nucleic Acids Res. 49, 3603–3616 (2021).
Lee, S. W., Cho, B. H., Park, S. G. & Kim, S. Aminoacyl-tRNA synthetase complexes: beyond translation. J. Cell Sci. 117, 3725–3734 (2004).
Ray, P. S., Arif, A. & Fox, P. L. Macromolecular complexes as depots for releasable regulatory proteins. Trends Biochem. Sci. 32, 158–164 (2007).
Lund, E. & Dahlberg, J. E. Proofreading and aminoacylation of tRNAs before export from the nucleus. Science 282, 2082–2085 (1998).
Guo, M. & Schimmel, P. Essential nontranslational functions of tRNA synthetases. Nat. Chem. Biol. 9, 145–153 (2013).
Sajish, M. & Schimmel, P. A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature 519, 370–373 (2015).
Yannay-Cohen, N. et al. LysRS serves as a key signaling molecule in the immune response by regulating gene expression. Mol. Cell 34, 603–611 (2009).
Shi, Y. et al. tRNA synthetase counteracts c-Myc to develop functional vasculature. eLife 3, e02349 (2014).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. A phase separation model for transcriptional control. Cell 169, 13–23 (2017).
Zhu, L. & Brangwynne, C. P. Nuclear bodies: the emerging biophysics of nucleoplasmic phases. Curr. Opin. Cell Biol. 34, 23–30 (2015).
Spector, D. L. & Lamond, A. I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 3, a000646 (2011).
Galganski, L., Urbanek, M. O. & Krzyzosiak, W. J. Nuclear speckles: molecular organization, biological function and role in disease. Nucleic Acids Res. 45, 10350–10368 (2017).
Guo, Y. E. et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 572, 543–548 (2019).
Galarza-Muñoz, G. et al. Human epistatic interaction controls IL7R splicing and increases multiple sclerosis risk. Cell 169, 72–84.e13 (2017).
Braunschweig, U. et al. Widespread intron retention in mammals functionally tunes transcriptomes. Genome Res. 24, 1774–1786 (2014).
Pan, Q. et al. Revealing global regulatory features of mammalian alternative splicing using a quantitative microarray platform. Mol. Cell 16, 929–941 (2004).
Chen, Y. & Belmont, A. S. Genome organization around nuclear speckles. Curr. Opin. Genet. Dev. 55, 91–99 (2019).
Smith, K. P., Hall, L. L. & Lawrence, J. B. Nuclear hubs built on RNAs and clustered organization of the genome. Curr. Opin. Cell Biol. 64, 67–76 (2020).
Blencowe, B. J., Issner, R., Nickerson, J. A. & Sharp, P. A. A coactivator of pre-mRNA splicing. Genes Dev. 12, 996–1009 (1998).
Blencowe, B. J. et al. The SRm160/300 splicing coactivator subunits. RNA 6, 111–120 (2000).
Ilik, İ. A. et al. SON and SRRM2 are essential for nuclear speckle formation. eLife 9, e60579 (2020).
Zhang, X. et al. An atomic structure of the human spliceosome. Cell 169, 918–929.e14 (2017).
Gautam, A., Grainger, R. J., Vilardell, J., Barrass, J. D. & Beggs, J. D. Cwc21p promotes the second step conformation of the spliceosome and modulates 3′ splice site selection. Nucleic Acids Res. 43, 3309–3317 (2015).
Kaplanis, J. et al. Evidence for 28 genetic disorders discovered by combining healthcare and research data. Nature 586, 757–762 (2020).
Tomsic, J. et al. A germline mutation in SRRM2, a splicing factor gene, is implicated in papillary thyroid carcinoma predisposition. Sci. Rep. 5, 10566 (2015).
Shehadeh, L. A. et al. SRRM2, a potential blood biomarker revealing high alternative splicing in Parkinson’s disease. PLoS ONE 5, e9104 (2010).
Nathanson, L. & Deutscher, M. P. Active aminoacyl-tRNA synthetases are present in nuclei as a high molecular weight multienzyme complex. J. Biol. Chem. 275, 31559–31562 (2000).
Horiguchi, N. et al. Dissociation between liver inflammation and hepatocellular damage induced by carbon tetrachloride in myeloid cell-specific signal transducer and activator of transcription 3 gene knockout mice. Hepatology 51, 1724–1734 (2010).
Keilhauer, E. C., Hein, M. Y. & Mann, M. Accurate protein complex retrieval by affinity enrichment mass spectrometry (AE–MS) rather than affinity purification mass spectrometry (AP–MS). Mol. Cell. Proteom. 14, 120–135 (2015).
Kim, M. H. & Kim, S. Structures and functions of multi-tRNA synthetase complexes. Enzymes 48, 149–173 (2020).
Costes, S. V. et al. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86, 3993–4003 (2004).
Ofir-Birin, Y. et al. Structural switch of lysyl-tRNA synthetase between translation and transcription. Mol. Cell 49, 30–42 (2013).
Hayano, M., Yang, W. S., Corn, C. K., Pagano, N. C. & Stockwell, B. R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 23, 270–278 (2016).
Seburn, K. L., Nangle, L. A., Cox, G. A., Schimmel, P. & Burgess, R. W. An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a charcot-marie-tooth 2D mouse model. Neuron 51, 715–726 (2006).
Schmidt, E. K., Clavarino, G., Ceppi, M. & Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6, 275–277 (2009).
Boivin, V. et al. Simultaneous sequencing of coding and noncoding RNA reveals a human transcriptome dominated by a small number of highly expressed noncoding genes. RNA 24, 950–965 (2018).
Watkins, C. P., Zhang, W., Wylder, A. C., Katanski, C. D. & Pan, T. A multiplex platform for small RNA sequencing elucidates multifaceted tRNA stress response and translational regulation. Nat. Commun. 13, 2491 (2022).
Tapial, J. et al. An atlas of alternative splicing profiles and functional associations reveals new regulatory programs and genes that simultaneously express multiple major isoforms. Genome Res. 27, 1759–1768 (2017).
Han, H. et al. Multilayered control of alternative splicing regulatory networks by transcription factors. Mol. Cell 65, 539–553.e7 (2017).
Anders, S., Reyes, A. & Huber, W. Detecting differential usage of exons from RNA-seq data. Genome Res. 22, 2008–2017 (2012).
Blencowe, B. J. The relationship between alternative splicing and proteomic complexity. Trends Biochem. Sci. 42, 407–408 (2017).
Kleifeld, O. et al. Identifying and quantifying proteolytic events and the natural N terminome by terminal amine isotopic labeling of substrates. Nat. Protoc. 6, 1578–1611 (2011).
Aass, K. R., Kastnes, M. H. & Standal, T. Molecular interactions and functions of IL-32. J. Leukoc. Biol. 109, 143–159 (2021).
Itsumi, M. et al. Idh1 protects murine hepatocytes from endotoxin-induced oxidative stress by regulating the intracellular NADP+/NADPH ratio. Cell Death Differ. 22, 1837–1845 (2015).
Rock, K. L., Reits, E. & Neefjes, J. Present yourself! By MHC Class I and MHC Class II molecules. Trends Immunol. 37, 724–737 (2016).
Bassani-Sternberg, M., Pletscher-Frankild, S., Jensen, L. J. & Mann, M. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol. Cell. Proteom. 14, 658–673 (2015).
Calis, J. J. A. et al. Properties of MHC class I presented peptides that enhance immunogenicity. PLoS Comput. Biol. 9, e1003266 (2013).
Geiger, R. et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016).
Bertram, K. et al. Structural insights into the roles of metazoan-specific splicing factors in the human Step 1 spliceosome. Mol. Cell 80, 127–139.e6 (2020).
Hu, S., Lv, P., Yan, Z. & Wen, B. Disruption of nuclear speckles reduces chromatin interactions in active compartments. Epigenetics Chromatin 12, 43 (2019).
Kincaid, E. Z., Murata, S., Tanaka, K. & Rock, K. L. Specialized proteasome subunits have an essential role in the thymic selection of CD8+ T cells. Nat. Immunol. 17, 938–945 (2016).
van den Eshof, B. L., Medfai, L., Nolfi, E., Wawrzyniuk, M. & Sijts, A. J. A. M. The function of immunoproteasomes—an immunologists’ perspective. Cells 10, 3360 (2021).
Kincaid, E. Z. et al. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat. Immunol. 13, 129–135 (2011).
Lu, S. X. et al. Pharmacologic modulation of RNA splicing enhances anti-tumor immunity. Cell 184, 4032–4047.e31 (2021).
Preger, C. et al. Generation and validation of recombinant antibodies to study human aminoacyl-tRNA synthetases. J. Biol. Chem. 295, 13981–13993 (2020).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 (2019).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Koushik, S. V., Chen, H., Thaler, C., Puhl, H. L. & Vogel, S. S. Cerulean, Venus, and VenusY67C FRET reference standards. Biophys. J. 91, L99–L101 (2006).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Saharia, A. et al. Flap endonuclease 1 contributes to telomere stability. Curr. Biol. 18, 496–500 (2008).
Dull, T. et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72, 8463–8471 (1998).
Kanaji, T. et al. Tyrosyl-tRNA synthetase stimulates thrombopoietin-independent hematopoiesis accelerating recovery from thrombocytopenia. Proc. Natl Acad. Sci. USA 115, E8228–E8235 (2018).
Beebe, K., Waas, W., Druzina, Z., Guo, M. & Schimmel, P. A universal plate format for increased throughput of assays that monitor multiple aminoacyl transfer RNA synthetase activities. Anal. Biochem. 368, 111–121 (2007).
Phair, R. D. & Misteli, T. High mobility of proteins in the mammalian cell nucleus. Nature 404, 604–609 (2000).
Adamczak, R., Porollo, A. & Meller, J. Accurate prediction of solvent accessibility using neural networks-based regression. Proteins 56, 753–767 (2004).
Qin, Y. et al. High-throughput sequencing of human plasma RNA by using thermostable group II intron reverse transcriptases. RNA 22, 111–128 (2016).
Xu, H., Yao, J., Wu, D. C. & Lambowitz, A. M. Improved TGIRT-seq methods for comprehensive transcriptome profiling with decreased adapter dimer formation and bias correction. Sci. Rep. 9, 7953 (2019).
Wu, D. C., Yao, J., Ho, K. S., Lambowitz, A. M. & Wilke, C. O. Limitations of alignment-free tools in total RNA-seq quantification. BMC Genomics 19, 510 (2018).
Didion, J. P., Martin, M. & Collins, F. S. Atropos: specific, sensitive, and speedy trimming of sequencing reads. PeerJ 5, e3720 (2017).
Chan, P. P. & Lowe, T. M. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 44, D184–D189 (2016).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Mi, H., Muruganujan, A., Ebert, D., Huang, X. & Thomas, P. D. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 47, D419–D426 (2019).
Ge, S. X., Jung, D. & Yao, R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36, 2628–2629 (2020).
Irimia, M. et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell 159, 1511–1523 (2014).
Purcell, A. W., Ramarathinam, S. H. & Ternette, N. Mass spectrometry-based identification of MHC-bound peptides for immunopeptidomics. Nat. Protoc. 14, 1687–1707 (2019).
Xu, T. et al. ProLuCID: an improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J. Proteom. 129, 16–24 (2015).
Reynisson, B., Alvarez, B., Paul, S., Peters, B. & Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 48, W449–W454 (2020).
Acknowledgements
We thank the Center for Metabolomics, the Microscopy core, the High Performance Computing team and Flow Cytometry Core, all at Scripps Research, for training and instrument maintenance, and the staff at the Genomic Sequencing and Analysis Facility at the University of Texas at Austin for poly(A) library preparation and sequencing. Access to a plate reader and flow cytometry software was provided by J. Paulson and access to a ChemiDoc Imager by J. Kelly, both at Scripps Research. We thank A. Dutta for preliminary analysis of ArgRS- and MetRS-dependent signalling pathways. We also thank U. Braunschweig, R. Barutcu and M. Wu (all University of Toronto) for feedback on the experimental design (U.B.) and earlier versions of the manuscript (R.B. and M.W.). This work was supported by the National Foundation for Cancer Research to P.S., the DFG (327097878) and the HFSP (LT000207) to H.C., NIH grant P41 GM103533 to J.R.Y., NIH grant R35 GM136216 and Welch Foundation grant F-1607 to A.M.L. and a CIHR Foundation Grant to B.J.B. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
H.C. planned and conducted experiments and analysed data. J.K.D., J.J.M. and J.R.Y. performed, analysed or supervised mass spectrometry experiments. D.C.W., R.M.N. and A.M.L. performed, analysed or supervised TGIRT-seq and RNA-seq library preparation and sequencing. J.J.L. and B.B. analysed RNA-seq results. P.S., A.M.L. and H.C. designed the research and wrote the manuscript with input and contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
Thermostable Group II Intron Reverse Transcriptase (TGIRT) enzymes and methods for their use are the subject of patents and patent applications that have been licensed by the University of Texas to InGex, LLC. A.M.L. and the University of Texas are minority equity holders in InGex, and A.M.L., some members of A.M.L.’s laboratory, and the University of Texas receive royalty payments from the sale of TGIRT enzymes. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ArgRS localized to the nucleus in murine cells and tissues and verification of decreased plasma arginine levels.
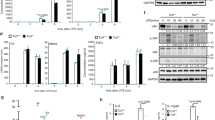
(a) ArgRS in different cellular compartments of murine embryonic fibroblasts (MEF, left), mouse spleen (middle), and mouse liver (right) probed by western blot following cell fractionation. Far right: Cell fractionation of HepG2 cells shown with alternative markers for the cytoplasmic and nuclear fraction. GAPDH: cytoplasmic loading control, Histone H3: nuclear loading control. Lamin A/C: nuclear loading control, Tubulin: cytoplasmic loading control. lys: lysate, cyt: cytoplasm, nuc: nucleus. Experiments were repeated at least 3 times. (b) Active site and nuclear localization signal mutations in ArgRS do not abrogate nuclear import. Left panel: Schematic of the ArgRS active site with amino acids coordinating arginine highlighted and aminoacylation assays with purified recombinant wt and D197E mutant ArgRS proteins. Middle left panel: A mutation in the ArgRS active site reduced the increase of ArgRS in response to arginine but did not abrogate it completely. Western blot of the nuclear fraction of wt-V5 and D137E mutated ArgRS-V5. A C-terminal 14-amino acid V5-tag that can be recognized with an anti-V5 antibody was used to differentiate between endogenous and mutant ArgRS. Representative image of 4 replicates. Middle right panel: Mutations in the ArgRS nuclear localization sequence reduced but did not abrogate the increase of nuclear ArgRS in response to arginine. ΔNLS: K287N, K288N, K309N. Representative image of 3 replicates. Right panel: Densitometric quantification of ArgRS relative to the nuclear loading control Histone H3 and normalized to 0x arginine. n = 4, 4, 4, 4, 3, 3 biologically independent transfections, p = *0.048. One-way Anova, Tukey’s multiple comparisons test. (c) LC/MS measurement of plasma arginine after (left panel) treatment with recombinant Arginase-1, an arginine-degrading enzyme, or (right panel) by induction of systemic inflammation with the liver toxin CCl4. n = 4, 3, 4, 4 independent mice. p = **0.002, p = ***0.0009. Unpaired, two-tailed t-test.
Extended Data Fig. 2 Verification of the interaction between ArgRS and SRRM2 and domain mapping.
(a) Venn diagrams comparing 3 different ArgRS and the MetRS interactomes. Statistical significance of overrepresentation by hypergeometric test: [nuc] vs [HepG2]: p = 7.6e-10, [HepG2] vs [293T]: p = 1.9e-10, [ArgRS 293T] vs [MetRS 293T]: p = 2.0e-7. [nuc]: HepG2 cells, nuclear fraction, IP: ArgRS, [HepG2]: HepG2 cells, whole cell lysate, IP: ArgRS, [293T]: 293T cells, whole cell lysate, IP: ArgRS, [MetRS]: 293T cells, whole cell lysate, IP: MetRS. Significance was calculated based on triplicate experiments (different cell passages). (b-f) Verification of ArgRS and SRRM2 interaction by immunoprecipitation (IP). Different antibodies are indicated by different colors of the antibody symbol. Input is indicated in the Figure. (g-j) Mapping of the interaction site between ArgRS and SRRM2. (g) Domain mapping of ArgRS to identify which domains are necessary for the interaction with SRRM2 using purified, biotinylated ArgRS fragments. Purified proteins were added to cell lysate and retrieved with streptavidin beads. N- and C-terminally truncated variants were used to retrieve SRRM2. Schematic of ArgRS domain architecture. (h) Immunoprecipitation of SRRM2 retrieved catalytically inactive ArgRS mutants as shown by western blot. A C-terminal V5-tag was used to differentiate between endogenous and mutant ArgRS. D197E and Y384F are mutations in the arginine binding site of ArgRS. See Extended Data Figs. 1b and 7e for confirmation of the loss of activity. (b-h, j, k) Experiments were repeated at least 3 times. (i) Volcano plot of the interactome of ArgRS with a deleted leucine zipper (remaining amino acids: 72–660). Axes denote enrichment (Welch’s t-test enrichment, x-axis) and reproducibility (Welch’s t-test significance, y-axis). Color: number of unique peptides detected (see scale at bottom right). Significance was calculated based on triplicate experiments (different cell passages). (j) Domain mapping of SRRM2 to identify which domains are necessary for interaction with ArgRS using purified, biotinylated fragments. The N-terminal coiled-coil domain (1–276) and an arginine-rich stretch of SRRM2 (445–800) were used to retrieve ArgRS. (k) SRRM2 immunoprecipitation did not enrich for MetRS but ArgRS. Detection of ArgRS, MetRS, and SRRM2 by western blot. (b-g, k) SRRM2 western blotting led to a smear-like appearance due to heavy post-translational modifications and inefficient transfer due to the high molecular weight of SRRM2.
Extended Data Fig. 3 Fluorescent microscopy of ArgRS and SRRM2.
(a) Colocalization (yellow) of SRRM2 (green) and nuclear speckle protein SON (red) by immunofluorescent staining and imaging by confocal microscopy. 84% of SON colocalized with SRRM2 and 34% of SRRM2 colocalized with SON. n = 16, 16. Bar: 2 μm. (b) Maximum intensity projections of ArgRS immunofluorescent staining in HepG2 shSCR control and shRARS_1 cells to validate antibody specificity. Comparable experiments were repeated at least 3 times. Bar: 10 μm. (c) Manders coefficients of SRRM2, ArgRS, and MetRS. Hoechst staining was used to identify the cell nucleus. Manders coefficients were calculated for individual cell nuclei (n = 11, 12) or for an average for 2–3 cells (C, n = 8). (d) Manders coefficient of ArgRS and SRRM2 colocalization when Costes thresholding was used. n = 14, 14. (e) Colocalization (yellow) of ArgRS (green) and SRRM2 (red) with different antibodies than used in Fig. 3a. Bar: 2 μm. (f) Immunofluorescent staining of ArgRS and SRRM2 in representative nuclei. Bar: 2 μm. (g) SRRM2 staining without signal intensity threshold shows diffuse, connected areas where punctae accumulate. SRRM2 signal is stronger in DNA-low areas. (h) Verification of antibodies by ArgRS (shRARS) and SRRM2 (shSRRM2) knock down. Bar: wt and shRARS, 20 μm. wt and shSRRM2: 5 μm. Antibody validations for immunofluorescence were performed once.
Extended Data Fig. 4 Fluorescent microscopy to determine the nuclear ArgRS localization.
(a) Colocalization of ArgRS (green) and paraspeckle protein SFPQ (red). Manders coefficient of colocalization after Costes thresholding. n = 15, 15. Bar: 2 μm. (b) Colocalization of ArgRS (green) and Cajal body protein Coilin (red). Manders coefficient of colocalization. n = 11, 11. Bar: 2 μm. (c) Pearson correlation coefficient of SRRM2 and ArgRS calculated from immunofluorescence staining during arginine starvation (0x arginine) and high arginine (5x arginine). Representative immunofluorescent staining of ArgRS and SRRM2. p = *0.01, n = 14, 9 independent cells. Unpaired, two-tailed t-test. Arginine concentrations are relative to DMEM. Bar: 2 μm. (d) Confocal microscopy following immunofluorescent staining of MetRS/SRRM2. A single slice of a series of z-stacks is shown. Colocalized areas in yellow. Bar: 2 µm. (e) Localization of MetRS and ArgRS in whole cells and in the nucleus. Bar: 5 µm. (d, e) Experiments were repeated at least 3 times. Quantification in Extended Data Fig. 3c. (f) Scheme of nuclear ArgRS localization relative to other MSC components and nuclear proteins.
Extended Data Fig. 5 Fluorescent microscopy to assess SRRM2 labeling and dynamics.
(a) Labeling of endogenous SRRM2 by fusion with the fluorescent protein mVenus. mVenus was inserted four amino acids from the C-terminus of SRRM2. Verification of genomic mVenus insertion by PCR. Predicted amplification length of wildtype amplicon: 238 bp; predicted length of SRRM2-mVenus amplicon: 2071 bp. (b) Labeling with mVenus did not alter SRRM2 localization in 293T cells. Endogenous, unlabeled SRRM2 was visualized by immunofluorescence (upper panel). SRRM2-mVenus fluorescence displayed a similar pattern with fluorescence restricted to the cell nucleus and defined foci (middle panel). In comparison, stable expression of mVenus alone did not display a distinct nuclear localization (lower panel). Bar: 10 μm. (c) Fluorescence Recovery After Photobleaching (FRAP) measured over 90 s. Recovery plateaued after 60 s. (d) Absolute immobile SRRM2-mVenus fraction calculated from one-exponential curve fits of FRAP measurements and relative immobile fraction calculated by subtraction of residual fluorescence directly after bleaching. n = 6, 7, 6 with each derived from an average of > 16 regions of interest. Means with SEM are shown. (e) Recovery of fluorescent signal is faster in the absence of arginine. Fluorescent signal was corrected for background and normalized. Each measurement included 14 regions of interest and the geometric mean of 8 measurements is shown. Means with SEM are shown. (f) Cell viability upon ArgRS knock down in SRRM2-mVenus cells, measured with Alamar Blue. Technical quadruplets and means with SEM are shown. (g) Knock down of ArgRS did not affect SRRM2-mVenus protein levels measured by flow cytometry.
Extended Data Fig. 6 Characterization of ArgRS knock down.
(a, b) ArgRS knock down was confirmed on protein level by western blot (A) and (B) on mRNA level by RT-qPCR (RQ and RQ max are shown) and RNA-seq. A representative of at least 3 repeats is shown for western blots and RT-qPCR. Replicates are different cell passages. (c) Protein synthesis was not inhibited by ArgRS knock down in HepG2 cells. Western blot verification of ArgRS knock down with 4 different shRNAs (shRARS_1–4). Western blot of puromycin incorporation. Puromycin is incorporated into newly synthesized proteins and detected with an anti-puromycin antibody to infer protein synthesis. A representatives of 6 (shRARS_1, 2) or 3 repeats (shRARS_3, 4) with different shRNAs are shown. Quantification of puromycin incorporation: shSCR: 1, shRARS_1: 1.5 ± 0.4, shRARS_2: 0.9 ± 0.3, shRARS_3: 2.6 ± 1.5, shRARS_4: 1.9 ± 1.1. (d) Alamar blue based cell viability assay, quadruplets of technical replicates with 4 different shRNAs are shown. Means with SEM are shown. (e) Volcano plot of differentially expressed exons upon ArgRS knock down as found by TGIRT-seq. Dashed vertical lines: 2-fold change, dashed horizontal line: p-value 1e-6. (a-d) ctrl: shSCR control, shRARS: ArgRS knock down. Significance was calculated with DEXSeq2.
Extended Data Fig. 7 ArgRS knock down affected mRNA processing and protein isoforms.
(a, b) RT-PCR of splice isoforms of (A) FGFR3 and (B) PLEKHG5. Genes were detected as alternatively spliced in HepG2 cells by RNA-seq and confirmed for HEK 293T cells. A representative image from 3 or more replicates is shown as well as the densitometric quantification for 293T. PSI: percent spliced in. Ctrl: shSCR control. p = *0.04, n = 4, 4. p = *0.03, n = 4, 4 cell passages. Unpaired, two-tailed t-test. (c) Rescue of ArgRS knock down by overexpression of ArgRS-GFP by transient transfection in 293T cells, shown by western blot. (d) Rescue of FGFR3 splicing by overexpression of ArgRS-GFP. Densitometric analysis of 3 repeats is shown. p = *0.003, n = 3, 3, 3, 3 independent transfections. One-way Anova, Tukey’s multiple comparisons test. (e) FGFR3 splicing change upon ArgRS knock down is not rescued by expression of catalytically inactive ArgRS mutants. Top: Aminoacylation assay with purified wild-type ArgRS and ArgRS mutants. Lower left: Western blot demonstrating restoration of ArgRS expression levels by overexpression of ArgRS-V5 mutants. Lower right: Densitometric analysis of RT-PCR of FGFR3 splice isoforms without or with overexpression of catalytically inactive ArgRS-V5 mutants. p = **0.003 (shSCR vs shRARS), **0.001 (shSCR vs shRARS + RARS_D197E), ***0.0006 (shSCR vs shRARS + RARS_Y384F), n = 7, 7, 7, 7, independent transfections. One-way Anova, Tukey’s multiple comparisons test. (f) Genes and their corresponding proteins shared between differentially expressed exons or splice junction changes (RNA-seq) and N-terminal peptides (proteomics) in ArgRS knock down vs control cells. (g) N-terminal peptides found for PTBP1 in mass spectrometry. (h) Western blot verification of preference for a higher molecular weight form of PTBP1 after ArgRS knock down. p = *0.003, n = 4, 4. Paired, two-tailed t-test.
Extended Data Fig. 8 Verification of stable and transient SRRM2 knock down and its effects on mRNA splicing.
(a) Left to right: Knock down of SRRM2 in 293T_SRRM2_mVenus cells to verify shRNAs later used in HepG2 cells, assessed by flow cytometry. < 50% medium fluorescence intensity reduction upon SRRM2 knock down. Knock down of SRRM2 in HepG2 verified by qRT-PCR, three repeats, each measured in triplicates. Means with SEM are shown. Normalized SRRM2 counts in RNAseq. (b) Left: Verification of transient SRRM2 knock down by a heterogenous pool of siRNAs (esiRNA) in 293T_mVenus cells using flow cytometry. esi_SRRM2 1 and 2 are different batches of the same pool. ctrl: non-targeting pool of siRNAs. Right: RT-PCR of CDK5RAP3 and FGFR3 upon transient knock down of SRRM2 with a pool of esiRNA in 293T cells. CDK5RAP3: p = *0.03 paired, two-tailed t-test. n = 4, 4. FGFR3: p = **0.01, *0.04 paired, two-tailed t-test, n = 3, 3, 3. Replicates are independent transfections.
Extended Data Fig. 9 Characterization of MetRS knock down and verification of splicing events regulated by ArgRS and SRRM2 in opposite directions.
(a) Verification of MetRS knock down (shMARS) by western blot and RNAseq. (b) Splicing events with |ΔPSI| > 0.1, MV > 0 and differential exon usage (padj < 0.05 DEXSeq). Dashed vertical lines: 2-fold change, dashed horizontal line: 1e-6. Significance was calculated with DEXSeq. (c) Venn diagram of shared splicing event changes upon knock down of ArgRS, SRRM2, or MetRS. All splicing event changes relative to shSCR control. MV > 0, no criterion for |ΔPSI|. (d) Inversely correlated alternative splicing events shared between ArgRS and SRRM2 or MetRS and SRRM2. Hypergeometric test for overrepresentation was calculated in R. (e) Knock down of ArgRS and SRRM2 had opposite effects on CDK5RAP3, RAD52, and STX16 processing as shown by RT-PCR. Representatives of 4 or 5 replicates shown with quantification by densitometry. CDK5RAP3: p = *0.02, n = 5, 4, 5. IL32: p = *0.03, n = 4, 4, 3. Unpaired, two-tailed t-test. RAD52: p = *0.02, *0.04, n = 5, 5, 5. STX16: p = *0.04, *0.04, n = 4, 4, 4. Paired, two-tailed t-test. Replicates are different cell passages.
Extended Data Fig. 10 SRRM2 and ArgRS induced changes on gene expression, exon and splice junction usage, and MHCI peptide presentation.
(a) Comparison of genes with differential gene expression, differential exons, and splice junctions for shSRRM2 and shRARS. (b) Genes with differentially expressed exons enriched in MSigDB hallmark categories upon SRRM2 and ArgRS knock down (padj < 0.05) in HepG2. (c) Gene Ontology (GO) enrichment of genes with differentially expressed exons upon SRRM2 and ArgRS knock down (padj < 0.05) in HepG2. (d) GO enrichment of genes with changed splice junctions upon SRRM2 and ArgRS knock down (MV > 0) in HepG2. (c, d) All expressed genes were used as reference. (e) MSigDB hallmark categories and GO enrichment of genes with differential gene expression shared between SRRM2 and ArgRS knock down. (b-e) Circle size and Venn diagram visualize the proportion of shared exons, junctions, or genes. (f) Quantification of differentially expressed exons by qRT-PCR. PSMB5: p = *0.03, *0.04. UBE2D3: p = **0.006, **0.008. Unpaired, two-tailed t-test. n = 9, 9, 9, 9, 9, 8 different passages. PSMB5: Splicing in ENST00000493471 led to a frameshift, resulting in PSMB5 isoform 3. UBE2D3: Skipping of ENSE00003638329 leads to an alternative start site, resulting in UBE2D3 isoform 4. (g) (top left) Length distribution histogram of MHCI peptides confirmed that isolated peptides consisted predominantly of 9 amino acid peptides. (top right) Flow cytometry of MHCI on HepG2 shSCR ctrl, ArgRS knock down, and SRRM2 knock down cells after antibody staining. (bottom) Comparable overrepresentation of amino acids were found with WebLogo in 9 amino acid-long peptides. (h) Venn diagrams of MHCI peptides identified by mass spectrometry following the isolation of MHCI complex. Comparison between biological replicates (top panel), proteins corresponding to the detected peptides found in all three replicates (middle panel), and peptides found in at least one of three replicates (lower panel). All replicates are different cell passages.
Supplementary information
Supplementary Tables
Supplementary Table 1. ArgRS nuclear interactome in HepG2. Two-sample t-test, n = 3 replicates are different cell passages. Supplementary Table 2. ArgRS interactome in HepG2 whole cell lysate. Two-sample t-test, n = 3 replicates are different cell passages. Supplementary Table 3. ArgRS interactome in 293T whole cell lysate. Two-sample t-test, n = 3 replicates are different cell passages. Supplementary Table 4. MetRS interactome in 293T whole cell lysate. Two-sample t-test, n = 3 replicates are different cell passages. Supplementary Table 5. Proteins shared between interactomes. Supplementary Table 6. tRNA expression upon ArgRS knockdown as calculated from TGIRT-seq. Significance was calculated with DESeq2. Supplementary Table 7. Splice junctions with MV >0 and |ΔPSI| >0.1 upon ArgRS knockdown. Supplementary Table 8. Differentially expressed exons upon ArgRS knockdown (padj >0.05). Significance was calculated with DEXSeq2. Supplementary Table 9. Peptides identified after enrichment of N-terminal peptides with |pDiff| >1 upon ArgRS knockdown. Two-sample t-test, n = 3 replicates are different cell passages. Supplementary Table 10. Splice junctions with MV >0 and |ΔPSI| >0.1 upon SRRM2 knockdown. Supplementary Table 11. Differentially expressed exons upon SRRM2 knockdown (padj >0.05). Significance was calculated with DEXSeq2. Supplementary Table 12. Splice junctions with MV >0 and |ΔPSI| >0.1 upon MetRS knockdown. Supplementary Table 13. Genes associated with Hallmark and GO terms that are inversely modulated by ArgRS and SRRM2. Supplementary Table 14. Mapping statistics for TGIRT-seq. Numbers on the top of each cell represent numbers of read pairs. Numbers within parentheses represent percentages. Raw pairs indicate the number of read pairs for the sample after Illumina sample de-multiplexing. Trimmed pairs indicate the number of read pairs that passed adaptor and quality trimmings, and length cut-offs, number in parentheses indicates percentage of trimmed pairs respect to raw pairs. tRNA/rRNA pairs indicates number of read pairs that can be concordantly aligned to ribosomal RNA repeat units (GenBank accession numbers: X12811.1 and U13369.1) or tRNA (GtRNAdb; 75, percentage is computed respect to trimmed pairs). Non-tRNA/rRNA pairs indicate the number of read pairs that are not mappable to rRNA or tRNA, percentage is computed respect to trimmed pairs. HISAT2 mapped pairs indicates number of read pairs that can be aligned concordantly to human genome (hg19) by HISAT2 77, percentage is computed with respect to non-tRNA/rRNA pairs. BOWTIE2 mapped pairs indicates number of read pairs that aligned to human genome by BOWTIE2 76, percentage is computed with respect to non-tRNA/rRNA pairs. Mapping rate is computed by the sum of percentage of HISAT mapper pairs and BOWTIE2 mapped pairs. Uniquely mapped indicates number of read pairs that are aligned to the human genome at a single location without ambiguity by HISAT2 (with alignment tag NH:1) or BOWTIE2 (MAPQ, 255). Unique map rate is computed respective to the sum of HISAT mapped pairs and BOWTIE2 mapped pairs. Splice read rate is computed by dividing the number of read pairs with either read being split by HISAT2 aligner (with an N cigar string) to the sum of HISAT mapped pairs and BOWTIE2 mapped pairs. Supplementary Table 15. Mapping statistics for RNA-seq. Supplementary Table 16. MHCI peptides. Supplementary Table 17. Additional information on reagents used.
Supplementary Video 1
SON/SRRM2 distribution in the nucleus. Visualization of SON and SRRM2 signal in a HepG2 hepatocellular carcinoma cell by immunofluorescent staining. Blue: Hoechst staining of DNA; green: SRRM2; red: SON, yellow: co-localization. Sequence in video: SON only – SRRM2 only – SON and SRRM2 – SON and SRRM2, co-localized areas highlighted in yellow.
Supplementary Video 2
ArgRS/SRRM2 co-localization in the nucleus. Visualization of ArgRS and SRRM2 nuclear signal in a HepG2 hepatocellular carcinoma cell by immunofluorescent staining. The cell nucleus was identified by Hoechst staining. Blue: Hoechst staining of DNA; red: ArgRS; green: SRRM2; yellow: co-localization between ArgRS and SRRM2. Sequence in video: ArgRS only – SRRM2 only – ArgRS and SRRM2 – ArgRS and SRRM2, co-localized areas highlighted in yellow.
Supplementary Video 3
MetRS/SRRM2 co-localization in the nucleus. Visualization of MetRS and SRRM2 nuclear signal in a HepG2 hepatocellular carcinoma cell by immunofluorescent staining. The cell nucleus was identified by Hoechst staining. Blue: Hoechst staining of DNA; red: MetRS; green: SRRM2; yellow: co-localization between MetRS and SRRM2. Sequence in video: MetRS only – SRRM2 only – MetRS and SRRM2 – MetRS and SRRM2, co-localized areas highlighted in yellow.
Supplementary Video 4
MetRS/ArgRS co-localization in the cell. Visualization of ArgRS and MetRS in a HepG2 hepatocellular carcinoma cell by immunofluorescent staining. The cell nucleus was identified by Hoechst staining. Blue: Hoechst staining of DNA; red: MetRS; green: ArgRS; yellow: co-localization between ArgRS and SRRM2. Sequence in video: MetRS only – ArgRS only – ArgRS and MetRS – ArgRS and MetRS, co-localized areas highlighted in yellow.
Supplementary Video 5
Time lapse of fluorescence recovery after photobleaching (FRAP) of SRRM2-mVenus fluorescence in 293T cells expressing a non-targeting shRNA (ctrl). Bleaching occurs between frames 4 and 5 (1 s into the video).
Supplementary Video 6
Time lapse of fluorescence recovery after photobleaching (FRAP) of SRRM2-mVenus fluorescence in 293T cells expressing a shRNA against the 3′UTR of ArgRS (shRARS_1). Bleaching occurs between frames 4 and 5 (1 s into the video).
Supplementary Video 7
Time lapse of fluorescence recovery after photobleaching (FRAP) of SRRM2-mVenus fluorescence in 293T cells expressing a shRNA against the coding region of ArgRS (shRARS_2). Bleaching occurs between frames 4 and 5 (1 s into the video).
Source data
Source Data Fig. 1
Uncropped blots and gels.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Uncropped blots and gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Uncropped blots and gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Uncropped blots and gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Uncropped blots and gels, and flow cytometry gating strategy.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Uncropped blots and gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Uncropped blots and gels.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Uncropped blots and gels.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Uncropped blots and gels.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Extended Data Fig. 10
Flow cytometry gating strategy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, H., Diedrich, J.K., Wu, D.C. et al. Arg-tRNA synthetase links inflammatory metabolism to RNA splicing and nuclear trafficking via SRRM2. Nat Cell Biol 25, 592–603 (2023). https://doi.org/10.1038/s41556-023-01118-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01118-8
This article is cited by
-
A viral pan-end RNA element and host complex define a SARS-CoV-2 regulon
Nature Communications (2023)