Abstract
Upon stimulation by extrinsic stimuli, stem cells initiate a programme that enables differentiation or self-renewal. Disruption of the stem state exit has catastrophic consequences for embryogenesis and can lead to cancer. While some elements of this stem state switch are known, major regulatory mechanisms remain unclear. Here we show that this switch involves a global increase in splicing efficiency coordinated by DNA methyltransferase 3α (DNMT3A), an enzyme typically involved in DNA methylation. Proper activation of murine and human embryonic and haematopoietic stem cells depends on messenger RNA processing, influenced by DNMT3A in response to stimuli. DNMT3A coordinates splicing through recruitment of the core spliceosome protein SF3B1 to RNA polymerase and mRNA. Importantly, the DNA methylation function of DNMT3A is not required and loss of DNMT3A leads to impaired splicing during stem cell turnover. Finally, we identify the spliceosome as a potential therapeutic target in DNMT3A-mutated leukaemias. Together, our results reveal a modality through which DNMT3A and the spliceosome govern exit from the stem state towards differentiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under accession code GSE222906. Source data are provided with this study. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Change history
15 May 2023
In the version of this article initially published, there was a typo in the Figure 5g lower left y-axis label, now reading “α-SRSF2”, which appeared originally as “α-sc-SRSF2”; the label is now corrected in the HTML and PDF versions of the article.
References
Baldridge, M. T. et al. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature 465, 793–797 (2010).
Essers, M. A. G. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Scheller, M. et al. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nat. Immunol. 7, 1037–1047 (2006).
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Tazikawa, H. et al. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J. Exp. Med. 208, 273–284 (2011).
Yu, V. W. C. et al. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell 167, 1310–1322 (2016).
Bick, A. G. et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586, 763–768 (2020).
Watson, C. J. et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367, 1449–1454 (2020).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Buscarlet, M. et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 130, 753–762 (2017).
Xie, M. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 20, 1472–1478 (2014).
Pich, O. et al. Discovering the drivers of clonal hematopoiesis. Nat. Commun. 13, 4267 (2022).
McKerrell, T. et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 10, 1239–1245 (2015).
Zink, F. et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130, 742–752 (2017).
Chen, T. et al. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol. 23, 5594–5605 (2003).
Challen, G. A. et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 44, 23–31 (2011).
Yang, L. et al. DNMT3A in haematological malignancies. Nat. Rev. Cancer 15, 152–165 (2015).
Challen, G. A. et al. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell 15, 350–364 (2014).
Jeong, M. et al. Loss of Dnmt3a immortalizes hematopoietic stem cells in vivo. Cell Rep. 23, 1–10 (2018).
Mayle, A. et al. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood 125, 629–638 (2015).
Wu, H. et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science 329, 444–448 (2010).
Banaszak, L. G. et al. Abnormal RNA splicing and genomic instability after induction of DNMT3A mutations by CRISPR/Cas9 gene editing. Blood Cells Mol. Dis. 69, 10–22 (2018).
Ladle, B. H. et al. De novo DNA methylation by DNA methyltransferase 3a controls early effector CD8+ T-cell fate decisions following activation. Proc. Natl Acad. Sci. USA 113, 10631–10636 (2016).
Ghoneim, H. E. et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170, 142–157 (2017).
Barwick, B. G. et al. B cell activation and plasma cell differentiation are inhibited by de novo DNA methylation. Nat. Commun. 15, 1900 (2018).
Prinzing, B. et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity.Sci. Transl. Med. 13, eabh272 (2021).
Yoshihara, H. et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1, 685–697 (2007).
Walter, D. et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 520, 549–552 (2015).
Dong, S. et al. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature 591, 117–123 (2021).
Semrau, S. et al. Dynamics of lineage commitment revealed by single-cell transcriptomics of differentiating embryonic stem cells. Nat. Commun. 8, 1096 (2017).
Maunakea, A. K. et al. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 23, 1256–1269 (2013).
Shukla, S. et al. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479, 74–79 (2011).
Maor, G. L. et al. The alternative role of DNA methylation in splicing regulation. Trends Genet. 31, 274–280 (2015).
Liao, J. et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat. Genet. 47, 469–478 (2015).
Erb, M. A. et al. Transcription control by the ENL YEATS domain in acute leukaemia. Nature 543, 270–274 (2017).
Nabet, B. et al. The dTAG system for immediate and target-specific protein degradation. Nat. Chem. Biol. 14, 431–441 (2018).
Gowher, H. et al. Mutational analysis of the catalytic domain of the murine Dnmt3a DNA-(cytosine C5)-methyltransferase. J. Mol. Biol. 357, 928–941 (2006).
Holz-Schietinger, C. et al. Mutations in DNA methyltransferase (DNMT3A) observed in acute myeloid leukemia patients disrupt processive methylation. J. Biol. Chem. 287, 30941–30951 (2012).
Guo, Y. E. et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 572, 543–548 (2019).
Caizzi, L. et al. Efficient RNA polymerase II pause release requires U2 snRNP function. Mol. Cell 81, 1920–1934 (2021).
Lee, S. C. et al. Synthetic lethal and convergent biological effects of cancer-associated spliceosomal gene mutations. Cancer Cell 34, 225–241 (2018).
Liu, Z. et al. Mutations in the RNA Splicing Factor SF3B1 promote tumorigenesis through MYC stabilization. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-19-1330 (2020).
Seiler, M. et al. H3B-8800, an orally available small-molecule splicing modulator, induces lethality in spliceosome-mutant cancers. Nat. Med. 24, 497–504 (2018).
Lee, S. C. et al. Modulation of splicing catalysis for therapeutic targeting of leukemia with mutations in genes encoding spliceosomal proteins. Nat. Med. 22, 672–678 (2016).
Bowling, E. A. et al. Spliceosome-targeted therapies trigger an antiviral immune response in triple-negative breast cancer. Cell 184, 384–403 (2021).
Huang, Y. et al. Systematic profiling of DNMT3A variants reveals protein instability mediated by the DCAF8 E3 ubiquitin ligase adaptor. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-21-0560 (2021).
Stroud, H. et al. Early-life gene expression in neurons modulates lasting epigenetic states. Cell 171, 1151–1164 (2017).
LaPlant, Q. et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat. Neurosci. 13, 1137–1143 (2010).
Lavery, L. A. et al. Losing Dnmt3a dependent methylation in inhibitory neurons impairs neural function by a mechanism impacting Rett syndrome. eLife 9, 1–27 (2020).
Rinaldi, L. et al. Dnmt3a and Dnmt3b associate with enhancers to regulate human epidermal stem cell homeostasis. Cell Stem Cell 19, 491–501 (2016).
Shen, H. et al. Mouse totipotent stem cells captured and maintained through spliceosomal repression. Cell 184, 2843–2859 (2021).
Gu, T. et al. DNMT3A and TET1 cooperate to regulate promoter epigenetic landscapes in mouse embryonic stem cells. Genome Biol. 19, 1–15 (2018).
Acknowledgements
We thank A. Jain, A. Malovannaya and S. Jung at the proteomics core at Baylor College of Medicine; J. Sederstrom in the flow cytometry core; T. Chen at M.D. Anderson for providing Dnmt3a KO J1 6aa mouse ESCs and S. Kornblau at M.D. Anderson, Department of Leukemia for access to AML patient samples. This work was supported by NIH grants DK092883, CA183252, AG036695, F31DK113705, CA215226, CA186784 and CA125123. Also supporting this work were CPRIT (RP160884), the CDMPR/DOD Breast Cancer Research Program (1W81XWH1810573), the McNair Medical Institute, the Edward P. Evans Foundation and the American Federation of Aging Research. BCM Mass Spectrometry Proteomics Core is supported by the Dan L. Duncan Comprehensive Cancer Center NIH award (P30 CA125123), CPRIT Core Facility Award (RP210227) and NIH High End Instrument award (S10 OD026804). In addition, this project was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the CPRIT Core Facility Support Award (CPRIT-RP180672) and the NIH (CA125123 and RR024574).
Author information
Authors and Affiliations
Contributions
R.R. and M.A.G. designed the study with input from T.F.W. R.R., A.G.G., L.B., M.C.G., C.R., A.T., S.T., H.L., S.M.C., R.E.R. and J.J.K. performed the experiments. J.H.W. and J.M.R. performed analysis of RNA and chromatin IP–sequencing data. S.M.K. provided access to AML patient samples. R.R. and M.A.G. prepared the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Maria Carmo-Fonseca, Thomas Graf and Toshio Suda for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
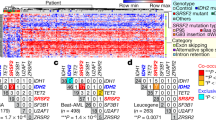
Extended Data Fig. 1 DNMT3A is frequently mutated in Clonal disorders.
(a) Number of individuals with somatic mutations in genes involved in Clonal Hematopoiesis (CH). (b) Number of individuals with somatic mutations in genes involved in Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML).
Extended Data Fig. 2 Enhanced Splicing Efficiency in Activated HSCs.
(a) Flow cytometry plot of CD150+ HSCs gated for CD34 after activation of WT and KO stem cells using 5-Flurouracil (5-FU) and Thrombopoietin (TPO) (n = 4 mice per group). (b) Schematic of Intronic Read (IR) ratio/score. Recently used in (Bowling and Wang, et al. Cell47). (c) Heatmap depicting IR ratio for each locus in PBS-treated vs. stimuli-treated mouse HSCs (n = 4 mice per replicate, 2 replicates per treatment). Red lines indicate presence of intronic reads at those loci. Raw data available in Extended Data Table 1. (d) Bar plot showing IR ratio for 437 common loci displayed greater splicing efficiency after exposure to stimuli. N = 4 animals and 2 independent experiments. Mean values + /- SD. Middle line represents mean, unpaired t-test with Welch’s correction). (e) Gene ontology enrichment analysis of commonly spliced genes displaying less intronic reads (IR) in activated stem cells after treatment with pIpC, 5-FU, and TPO. (f, g) RT-qPCR validation of relative IR (top) and changes gene expression (bottom) in WT and KO HSCs after activation with pIpC. IR ratios were normalized to β-actin (n = 4 animals and 3 independent experiments. Data shows mean values + /- SD, unpaired t-test with Welch’s correction).
Extended Data Fig. 3 Impaired Splicing in Dnmt3a KO mouse HSCs.
(a) Schematic of control and Dnmt3a KO mouse HSC transplantation. (b) Agarose gel electrophoresis image of amplified DNA from whole bone marrow of Mx1-cre and Dnmt3a KO mice after Hematopoietic Stem Cell (HSC) transplantation into CD45.1 recipient mice. (c) qPCR after ablation of Dnmt3a in mouse HSCs. (d) Flow cytometry analysis of HSCs 4 months after transplant of Dnmt3a WT and KO HSCs into recipient CD45.1 mice. Lineage- Sca1+ Ckit+ (LSK) progenitor cells were processed on flow cytometer after lineage depletion. These were then used to purify stem cells and perform mRNA sequencing. Data shown in Fig. 1j, k. (e) Gene Ontology analysis of genes displaying greater IR in KO HSCs compared to WT. (f) eCDF for 10,412 introns in WT and KO LSK progenitor cells (n = 2 biological replicates, 4 mice per group, p < 2.2 × 10−16).
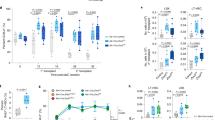
Extended Data Fig. 4 Impaired growth rate in KO mESCs after ATRA-stimulation.
(a) eCDF for 16,042 introns in WT murine ES cells (mESCs) after ATRA-stimulation for 24 hours (2 independent biological samples), p < 2.2 × 10–16). (b) eCDF for 47,880 introns in WT human ES cells (hESCs) after ATRA-stimulation for 6 hours (n = 2 biological replicates, p < 2.2 × 10–16). (c) RNA sequencing track of the Trmt1 locus in mESCs after ATRA-stimulation. (d) RNA sequencing track of the DDX17 locus in hESCs after ATRA-stimulation. (e) Gene Ontology analysis of genes displaying less intronic reads in ATRA-treated compared to DMSO-treated mESCS. (f) Gene Ontology analysis of genes displaying less intronic reads in ATRA-treated compared to DMSO-treated hESCs. (g) Changes in alternative splicing between WT and KO mouse Hematopoietic stem cells. (h) Changes in alternative splicing between WT and KO activated mouse embryonic stem cells. (i) Growth curves comparing proliferation rate of WT (black), KO (red), WT after ATRA-stimulation (ATRA-WT, blue), and KO after ATRA-stimulation (ATRA-KO, green) mESCs. N = 3 biologically independent samples and 2 independent experiments. Mean values + /- SD. Unpaired t-test with Welch’s correction, p < 0.01 = *** comparing ATRA-WT and ATRA-KO.
Extended Data Fig. 5 Increased decay rates in KO mESCs after stimulation.
(a) Decay rates of 12 mRNAs in ATRA-WT and ATRA-KO mouse embryonic stem cells (mESCs). (b) Decay rates of 12 mRNAs in WT and KO stem state mESCs. For (A) and (B), Error bars represent SD (n = 3 biologically independent samples and 2 independent experiments for each gene, unpaired t-test with Welch’s correction).
Extended Data Fig. 6 Rapid degradation of DNMT3A causes mis-splicing.
(a) Western blot of human ESC clones with the DNMT3A degron system before and after treatment with dTAG-13 for 24 hours. (b) Expression of pluripotency factors 12 hours after treatment with dTAG-13 and ATRA (c) Bar plot of genes downregulated (less than 2-fold) in dTAG-13 treated human ESCs (2 biologically independent samples, mean values + /- SD, unpaired t-test with Welch’s correction). Bars in black are genes that are associated with stem cell differentiation. (d) Bar plot of genes upregulated (greater than 2-fold) in dTAG-13 treated human ESCs (2 biologically independent samples, mean values + /- SD, unpaired t-test with Welch’s correction). Bars in black are genes that are associated with stem cell pluripotency. (e) qRT-PCR of intronic loci from mRNA and EU-labelled nascent RNA in DMSO and dTAG-13 treated human ESCs. (f) Gene expression profile of over 200 splicing-associated factors in activated Dnmt3a WT and KO stem cells. (g) Schematic for TMT mass spectrometry and analysis. (h) Volcano plot showing protein expression levels in WT ATRA and KO ATRA stem cells (n = 3 biologically independent samples, red indicates greater than 0.5 fold change and blue indicates less than 0.5 fold change in activated Dnmt3a KO mouse ESCs). Data is an integration of all samples. (i) Protein expression profile of over 200 splicing-associated factors in activated Dnmt3a WT and KO stem cells (n = 3 biologically independent samples). Data is an integration of all samples.
Extended Data Fig. 7 DNMT3A associates with components of the spliceosome.
(a) Western blot validation of biotin-tagged DNMT3A after induction with 2ug/mL doxycycline in mouse ES cells (left) and immunoprecipitation with streptavidin coated magnetic beads (right). DNMT3A (at 130 kDa) is labelled. Red box indicates fractions of cytoplasmic and nuclear cell lysates used for mass spectrometry. (b) Table showing top splicing associated factors enriched in the co-immunoprecipitation and mass spectrometry analysis of biotin-tagged DNMT3A in mouse ES cells. (c) BiFC analysis of DNMT3A-interacting spliceosome components after lentiviral expression of human DNMT3A variants (n = 4 biologically independent samples and 2 independent experiments, mean values + /- SD, unpaired student’s t-test with Welch’s correction, mean values + /- SD, p < 0.05 = **). Bar graph depicting analysis of Bimolecular Florescence complementation (BiFC) where fluorescence signal is recorded when each component of the spliceosome (prey) was introduced to (d) human DNMT3A, (e) human DNMT3A R882H, (f) human DNMT3B (bait).
Extended Data Fig. 8 Endogenous-tagging of DNMT3A and In situ interaction between DNMT3A and Splicing Factors.
(a) Western blot after introducing a flag-tag at the c-terminus of endogenous Dnmt3a in mESCs. (b) Immunofluorescence using anti-Flag antibody in Flag-DNMT3A cells versus Dnmt3a KO mESCs. (c) Expression of pluripotency factors in Flag-tagged mESCs compared to WT parental J1 cells. (d) Growth curves comparing proliferation rate of WT J1 (black) and flag-DNMT3A mESCs (blue), before and after stimulation with ATRA. (e) Proximity Ligation Assay (PLA) of DNMT3A and DNA methyltransferases. (f) PLA of DNMT3A and inactive and active RNA polymerase II in ATRA-WT and ATRA-KO mESCs. Scale bars on all immunofluorescence images represent 10 µm. (g) Co-immunoprecipitation (Co-IP) of DNMT3A before and after DNase and RNase treatment. (h) Chromatin Immunoprecipitation and sequencing (ChIP-seq) of DNMT3A before and after treatment with RNase A. (i) Box plot showing mean density of DNMT3A ChIP signal at all active genes and intronic read loci (*** = p < 0.01, n = 2 biologically independent samples). Scale bars on all immunofluorescent images represent 10 µm. 2 biologically independent experiments were performed for panels A-G.
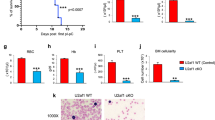
Extended Data Fig. 9 Deletion of DNMT3A in human donor stem cells.
(a) Schematic of control and sgDNMT3A edited CD34+ cord blood-derived stem cells transplanted into immunocompromised NSG mice. (b) We observed approximately 78–96% deletion efficiency across the five biological replicates as verified by amplicon sequencing. (c) Flow cytometry analysis of human progenitor cells in the bone marrow of three NSG recipient mice. Plots show expression of CD34 and CD38 in human cells (gated on hCD45) three months following injection of CD34+ cells from two experimental pairs (transfected with Cas9 only, or Cas9 and guides for DNMT3A. (d) qRT-PCR of exon 10 after electroporation of Cas9 only, or Cas9 and guides for DNMT3A in each of the 5 donor samples (error bars represent mean values + /- SD). (e) Gene Ontology (GO) enrichment analysis of mis-spliced genes in sgDNMT3A cells compared to Cas9 only CD34+ Donor HSPCs as compared to Dnmt3a WT and KO mouse HSCs (n = 2 donors, middle line, mean; top and bottom line, maxima and minima of range, respectively, two-tailed paired t-test). (f) RNA sequencing tracks of the Prpf19 locus in top WT (black) and KO (red) in mouse HSCs and bottom Cas9 (black) and sgDNMT3A (red) human HSPCs. Highlighted region in red is expanded to show increased IR in KO compared to WT HSCs. (g) RNA-sequencing tracks of the MAP3K7 locus in CD34+ cells from MDS patients with WT and Mutant SF3B1 compared with Cas9 and sgDNMT3A donor CD34+ cells. (h) qRT-PCR of NFKB and JAK-STAT pathway components in sgDNMT3A Donor HSPCs compared to Cas9 only. Error bars represent mean values + /- SD (n = 3 donors and independent experiments, unpaired t-test with Welch’s correction, ** p < 0.01, *** p < 0.001).
Extended Data Fig. 10 DNMT3A mutant leukemias are sensitive to splicing inhibition with Pladienolide B.
(a) AnnexinV apoptosis assay comparing DNMT3A WT (black) and DNMT3A mutant (red) cell lines. (b) Western blot of isogenic WT and KO K562 cells (n = 2 biologically independent KO cells and 1 biologically independent WT cell). (c) Annexin V apoptosis assay comparing DNMT3A WT (black) and KO (red) in K562 cell lines (3 biological replicates, *** p < 0.01 unpaired student’s t-test, error bars represent mean values + /- SD). (d) Kaplan−Meier survival analysis of K562 WT and KO cells transplanted recipients after injection of vehicle or Pladienolide B (5 mg/kg/day) for 5 days. Mice with less than 10% engraftment after 1.5 weeks were excluded. (n = 6 animals per group; Log-rank (Mantel-Cox) test; * p < 0.05, ** p < 0.01). (e) Bar plot of mRNA expression levels and apoptosis in WT and KO isogenic K562 cells after shRNA-mediated knockdown of SF3B3. (f) Bar plot of mRNA expression levels and apoptosis in WT and KO isogenic K562 cells after shRNA-mediated knockdown of U2AF1. (g) Upon exposure to activating stimuli, DNMT3A coordinates the upregulation of transcripts through increased splicing factor recruitment to RNA polymerase II and intronic pre-mRNA processing. Upon DNMT3A loss, stem cells are unable to effectively respond to activation signals due to impaired coordination of splicing components and efficiency of intron processing, which could negatively impact differentiation potential and alter stem cell fate.
Supplementary information
Supplementary Table 1
Extended Data Table Legends. Table 1. IR ratio from mRNA-seq of PBS-treated (control) and activated (pIpC, TPO and 5-FU) mHSCs. Data represented in Fig. 2B. Table 2. IR ratio from mRNA-seq of WT and Dnmt3a KO mHSCs. Data shown in Fig. 2F. Table 3. IR ratio from mRNA sequencing of ATRA-WT and ATRA-Dnmt3a KO mESCs. Data shown in Fig. 3B. Table 4. Alternative splicing from mRNA-seq of WT and Dnmt3a KO mHSCs. Data shown in Extended Fig. 4H. Table 5. Alternative splicing from mRNA-seq of WT and Dnmt3a KO mESCs. Data shown in Extended Fig. 4I. Table 6. IR ratio from mRNA-seq of ATRA-DMSO and ATRA-dTAG-13-treated (hESCs. Data shown in Fig. 4B,C. Table 7. IR ratio from mRNA-seq of Cas9 and sgDNMT3A donor CD34+ HSPCs. Data shown in Fig. 6C. Table 8. DNMT3A WT (n = 10) and mutant (n = 20) patient samples with AML. Data shown in Fig. 6I.Table 9. qPCR validation primers for mESCs.
Source data
Source Data Fig. 1
Source Data for main Fig. 1
Source Data Fig. 2
Source Data for main Fig. 2
Source Data Fig. 3
Source Data for main Fig. 3
Source Data Fig. 4
Source Data for main Fig. 4
Source Data Fig. 5
Source Data for main Fig. 5
Source Data Fig. 6
Source Data for main Fig. 6
Source Extended Data Fig. 1
Source Data for extended data Fig. 1
Source Extended Data Fig. 2
Source Data for extended data Fig. 2
Source Extended Data Fig. 3
Source Data for extended data Fig. 3
Source Extended Data Fig. 4
Source Data for extended data Fig. 4
Source Extended Data Fig. 5
Source Data for extended data Fig. 5
Source Extended Data Fig. 6
Source Data for extended data Fig. 6
Source Extended Data Fig. 7
Source Data for extended data Fig. 7
Source Extended Data Fig. 8
Source Data for extended data Fig. 8
Source Extended Data Fig. 9
Source Data for extended data Fig. 9
Source Extended Data Fig. 10
Source Data for extended data Fig. 10
Source Uncropped blots Fig. 4
Uncropped western blots for Fig. 4
Source Uncropped blots Fig. 5
Uncropped western blots for Fig. 5
Source Uncropped blots Extended Data Fig. 8
Uncropped western blots for Extended Data Fig. 8
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramabadran, R., Wang, J.H., Reyes, J.M. et al. DNMT3A-coordinated splicing governs the stem state switch towards differentiation in embryonic and haematopoietic stem cells. Nat Cell Biol 25, 528–539 (2023). https://doi.org/10.1038/s41556-023-01109-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01109-9
This article is cited by
-
A twist to splicing regulation in haematopoiesis
Nature Cell Biology (2023)