Abstract
In this study, a flower-like SnIn4S8/SmVO4 heterostructure was synthesized by applying a hydrothermal approach and used as a visible-light-driven photocatalyst to remove hexavalent chromium (Cr6+) and Rhodamine B (RhB). The process design and optimization were carried out by response surface methodology (RSM). An artificial neural network (ANN) was also used to determine the relative importance of operational factors. Under RSM-based optimal conditions, the Cr6+ and RhB removal efficiency reached 90.93 and 97.57%, respectively. Process modeling by ANN revealed pH as the most influential factor in Cr6+ and dye removal. The predominant photocatalytic activity of SnIn4S8/SmVO4 was attributed to its Z-scheme structure, leading to the significant separation of charge carriers and conserving the redox capacity of the photogenerated electron-holes. Kinetic studies revealed that the SnIn4S8/SmVO4 could achieve considerable rate constants of 0.036 and 0.080 min−1 to remove Cr6+ and RhB, respectively. Furthermore, the catalyst’s reusability was confirmed under optimal conditions.
Similar content being viewed by others
Introduction
Contamination of aquatic environments by organic and inorganic pollutants has always been a concern from an environmental perspective. Dyes and heavy metals are among the main organic and inorganic compounds existing in most industries’ wastewater. Rhodamine B (RhB) and hexavalent chromium (Cr(VI) or Cr+6), selected as the organic dye and heavy metal ion models in this research, are among the water pollutants whose harms have been widely reported. RhB is a synthetic and fluorescein dye commonly used in textile and dyeing with proven carcinogenic and teratogenic effects on public health. In 2019, Cr6+ compounds were introduced among the first category of hypertoxic water contaminants. When released directly into the water, such chemicals will cause irreparable damage to flora and fauna life by disrupting photosynthetic processes and cytotoxicity. In addition, they lead to various diseases, such as ulcers, respiratory problems, kidney and liver damage, and lung cancer in humans1,2,3,4,5,6,7,8,9. Therefore, urgent action is necessary to prevent them from entering aquatic environments.
Accordingly, tremendous efforts have been devoted to addressing water pollutants. However, advanced oxidation processes (AOPs) seem to have aroused plentiful interest due to their unique features in converting pollutants into harmless compounds10,11,12,13,14. Photocatalysis mediated by semiconductors is one of the AOPs capable of forming a reduction/oxidation (redox) process to efficiently reduce heavy metals and organic dyes without secondary pollution efficiently15,16,17,18. Regarding energy crisis issues, exploiting responsive semiconductors for cost-effective irradiation sources like visible/solar light is indispensable. Stannum indium sulfide (SnIn4S8) is a ternary chalcogenide semiconductor with potential uses in photocatalysis regarding its relatively narrow band gap, high stability, and strong visible-light absorption capacity. Generally, the short lifetime of photoinduced electron-hole (e--h+) pairs dramatically restricts the performance of the simplex catalyst, where SnIn4S8 is no exception19,20. Among the extensive efforts to overcome this dilemma and improve efficiency, constructing SnIn4S8-based heterostructures enhance its performance21,22,23,24,25. In this respect, photocatalysts with Z-scheme heterostructure have a privileged position because of separating charge carriers and conserving the redox capacity of the photogenerated e--h+ pairs26,27,28. Researchers have reported various SnIn4S8-based Z-scheme photocatalysts. For instance, Shi et al. prepared Z-scheme flower-like Bi2S3/SnIn4S8 heterostructure and reported its higher performance than pure SnIn4S8 and Bi2S3 in photocatalytic degradation of RhB29. Zhang et al. successfully synthesized the Z-scheme heterojunction of SnIn4S8-CdS with enhanced photocatalytic redox capabilities30. Shen et al. showed that Z-scheme SnIn4S8/CeO2 heterojunction could be used in the photocatalytic reduction of Cr6+ under visible light31. Tang et al. reported the g-C3N4/SnIn4S8 composite as a direct Z-scheme photocatalyst to enhance photoactivity and stability in eliminating dyes and heavy metals32. Despite the tremendous advances in this field, choosing an efficient semiconductor with suitable band edges is still essential for fabricating more SnIn4S8-based Z-scheme heterostructures with high photocatalytic efficiency and practical applications. Recently, rare-earth elements and their compounds have been considered in photocatalytic studies regarding their unique electronic and optical features33. In this respect, samarium vanadate (SmVO4) can be considered a promising semiconductor candidate owning to its relatively narrow band gap, visible-light harvesting capability, and suitable band structure for photoredox reactions34. The capability of SmVO4 to form heterojunctions with other semiconductors has been investigated in various studies35,36,37,38,39,40.
Considering the energy band alignment with SnIn4S8, SmVO4 is expected as a good alternative for fabricating a Z-scheme heterojunction with high photocatalytic activity. However, to our knowledge, no related study has yet been conducted on SnIn4S8/SmVO4 heterojunction.
In the present study, SnIn4S8/SmVO4 photocatalyst was synthesized with a proposed Z-scheme heterojunction to reduce Cr6+ and degrade RhB in a visible-light-driven redox process. Since cost and time saving and accurate estimation of results are research priorities, this work aims to design experiments, optimize, and rank operational factors using response surface methodology (RSM) and artificial neural network (ANN), as two multivariate statistics procedures. Furthermore, the kinetics and mechanism of the involved process were studied.
Results and Discussion
Crystallographic, morphology, and surface area studies
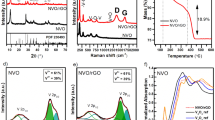
The crystalline structure of the synthesized samples was explored by X-ray diffraction (XRD) patterns depicted in Fig. 1. For bare SnIn4S8, the peaks at 2θ angels of 9.65°, 19.10°, 28.20°, 32.80°, 47.82°, and 49.65° correspond to the (200), (202), (600), (602), (174), and (001) planes of tetragonal phase SnIn4S8, respectively (JCPDS Card No. 42–1305)1,31. Similarly, for pure SmVO4, seven peaks appeared at 18.5°, 24.64°, 33.06°, 48.92°, 61.64°, 63.63°, and 69.4°, which are indexed with the (101), (200), (112), (312), (332), (204), and (224) planes of tetragonal phase SmVO4, respectively (JCPDS Card No. 17–0876)41,42. In the case of SnIn4S8/SmVO4 samples, predominant diffraction peaks of SnIn4S8 and SmVO4 were observed, confirming the successful formation of SnIn4S8/SmVO4.
The field emission-scanning electron microscope (FE-SEM) images of SmVO4, SnIn4S8, and SnIn4S8/SmVO4 with two magnifications are shown in Fig. 2. According to Fig. 2a, aʹ, the SmVO4 sample formed of uniform spherical nanoparticles. Fig. 2b, c represent the formation of flower-like SnIn4S8 and SnIn4S8/SmVO4 microspheres. The high-magnification FE-SEM images of SnIn4S8 and SnIn4S8/SmVO4 (Fig. 2bʹ, cʹ) reveal that these microspheres were loosely assembled by numerous nanosheets.
The mechanism behind the formation of flower-like morphology was followed through the synthesis of SnIn4S8 at different hydrothermal reaction times. Supplementary Fig. 1 illustrates the FE-SEM images of synthesized samples at different hydrothermal times. As shown in Supplementary Fig. 1a, irregular wrinkled nanosheets with a clinging tendency were formed at 4 h. By increasing hydrothermal time to 8 h, the SnIn4S8 hierarchical microspheres begin to form by self-assembling a large number of nanosheets (Supplementary Fig. 1b). When the hydrothermal time prolongs to 12 h, the microspheres obtained from the nanosheets found a more regular arrangement and formed a perfect flower-shaped sphere (Supplementary Fig. 1c).
The constituent elements of the SnIn4S8/SmVO4 composite was unraveled by energy dispersive X-ray (EDX) spectrum (Supplementary Fig. 2). The symmetrical distribution of relevant elements on the surface of the SnIn4S8/SmVO4 composite was depicted well by the EDX elemental mapping (Supplementary Fig. 3).
The microstructure of the binary SnIn4S8/SmVO4 composite was further scrutinized by transmission electron microscope (TEM) images (Supplementary Fig. 4), by which SnIn4S8 nanosheets decorated with SmVO4 nanoparticles are seen. Close contact between composite components aids heterojunction formation, transfer of photoexcited charge carriers, and their separation in photocatalytic processes8,30,31.
The nitrogen adsorption-desorption curves and Brunauer-Emmett-Teller (BET) analysis results were surveyed to evaluate the surface characteristics of the SnIn4S8, SmVO4, and binary SnIn4S8/SmVO4 composite. As per Fig. 3a, the N2 adsorption-desorption curves of all samples showed a type-IV isotherm with an H3 hysteresis loop (IUPAC classification)43, indicating the presence of mesopores in the structure of the samples. The obvious hysteresis (0.3–1.0 p/p0) between the adsorption and desorption branches of SnIn4S8/SmVO4 indicates the presence of considerable mesopores in its texture. This hysteresis can be attributed to the voids in the SnIn4S8 hierarchical microsphere. This result was confirmed by calculated pore size distribution ranging 1 to 10 nm from the desorption branch of the Barrett-Joyner-Halenda (BJH) plot (Fig. 3b). Besides, the values of the specific surface area of SnIn4S8, SmVO4, and SnIn4S8/SmVO4 were estimated from BET analysis as 17.18, 32.93, and 28.56 m2 g−1, respectively. Obviously, introducing SmVO4 spherical nanoparticles led to a significant increase in SnIn4S8/SmVO4 specific surface area compared with SnIn4S8 nanosheets. Notably, the obtained specific surface area for SnIn4S8/SmVO4 has an acceptable value compared with its similar binary composites, such as SnIn4S8/Bi2MoO6 (14.3 m2 g−1), AgInS2/SnIn4S8 (21.7 m2 g−1), CdS/SnIn4S8 (32.1 m2 g−1), and g-C3N4/SnIn4S8 (18.7 m2 g−1)8,23,24,32.
Band gap energy, band edge potentials, and e--h+ separation studies
The band gap energies (Eg) of synthesized samples were calculated by the UV–Vis diffuse reflectance spectra (Fig. 4a) and Kubelka-Munk function (F(R) = (1-R)2/2 R, where R denotes the reflectance value). The Eg can be obtained by drawing a plot of [F(R)hυ]1/2 versus the photon energy (hυ) and extrapolating the plots to [F(R)hυ]1/2 = 0 (Fig. 4b)44. The Eg values were determined to be about 2.11, 1.87, and 2.03 eV for SnIn4S8, SmVO4, and SnIn4S8/SmVO4, respectively, thereby guaranteeing their utilization under visible light. In this regard, the introduced SmVO4 reduces the Eg value of SnIn4S8 from 2.11 to 2.03 eV.
The band edge potentials of studied semiconductors were estimated using the Mott-Schottky plots (Fig. 4c) and Eg values. According to Fig. 4c, the slope of the plots is positive, indicating the n-type nature of semiconductors. The flat-band potential (EFB) values of SnIn4S8 and SmVO4 were determined as −0.85 and 0.14 V vs. Ag/AgCl, respectively, by extrapolating the plots to C−2 = 0. It is of note that the potential of the conduction band (ECB) of an n-type semiconductor is approximately equal to EFB vs. NHE (Normal Hydrogen Electrode), which can be computed from the following empirical formula:
where E°(Ag/AgCl)= 0.21 V (vs. NHE)45. Accordingly, the ECB values of SnIn4S8 and SmVO4 were calculated as −0.69 and 0.29 V vs. NHE, respectively. Meanwhile, the valence band potentials (EVB) of SnIn4S8 and SmVO4 were computed as 1.42 and 2.16 V vs. NHE, respectively, using the EVB = ECB + Eg equation.
In the next step, the e--h+ separation ability of individual and binary samples was explored using the photoluminescence (PL) spectra. A low-intensity PL emission spectrum normally indicates a lower recombination rate of e--h+, and vice versa46. As shown in Fig. 4d, the weakest PL intensity belongs to SnIn4S8/SmVO4, indicating suppression of charge recombination by constructing SnIn4S8/SmVO4 heterojunction. Such a desirable outcome can be attributed to the band structure modulation in heterojunction, which enhances the photocatalytic activity. For detailed explanations, see the mechanism section.
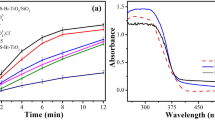
Comparison of the performance of the synthesized powders toward Cr6+ and RhB removal
The adsorption and photocatalytic performance of simplex and binary powders were investigated by monitoring the removal of Cr6+ and RhB. As shown in Fig. 5, the contribution of Cr6+ and RhB adsorption by the synthesized powders was little compared to their photocatalytic removal. Figure 5 also illustrates that all binary SnIn4S8/SmVO4 composites had higher photocatalytic activity than the single-phase component samples under visible light irradiation. As mentioned above, constructing SnIn4S8/SmVO4 heterostructure suppresses e--h+ recombination and enhances photocatalytic activity. However, the SnIn4S8/SmVO4 composite with an equal molar ratio of components exhibited top-flight photocatalytic performance as compared to other ratios. According to the literature, flower-like structures such as SnIn4S8 have potential photocatalytic applications because of their particular configuration, especially the presence of hierarchical pores. According to Yan et al., the pores inside hierarchical architectures can act as light transmission paths to penetrate photon energy to the depths of the photocatalyst structure, increasing its light-harvesting significantly. In addition, the catalyst photoabsorption improves by increasing the light absorption, reflection, and scattering within such porous architectures. Furthermore, abundant pores in this structure provide numerous active sites for adsorbing more target species19. It seems that increasing SmVO4 content in the SnIn4S8/SmVO4 texture from an optimal ratio could shield the SnIn4S8 surface, thereby impeding the light absorption by SnIn4S8 and also blocking the active sites. Therefore, a proper molar ratio of composite components could substantially enhance the photoactivity of the composite. In continuation of our investigation, the role of SnIn4S8/SmVO4 (1:1) toward Cr6+ reduction and RhB degradation at visible light exposure was evaluated.
Assessing the role of operational factors and process optimization by RSM
As mentioned in the experimental section, the effect of four influential factors was studied during 30 RSM-designed experiments to find the optimal condition for photocatalytic Cr6+ reduction or RhB degradation (Supplementary Tables 1, 2).
After importing the laboratory results to the DX7 software and implementing stepwise command (to remove the insignificant linear, quadratic, and interaction effects), theoretical results were predicted by the following reduced quadratic polynomial equations:
The accuracy and precision of the proposed models were assessed by analysis of variance (ANOVA) statistical testing. Statistically, a high F-value and a low p-value (<0.05) indicate the significance of a model47. According to Supplementary Tables 3, 4, the F and p-values of both models at a 95% confidence interval indicate their statistical significance. The lack of fit F-values of models also suggests the lack of fits is not significant relative to the pure error. In addition, the predicted and adjusted R-Squared values close to 1 confirmed the satisfactory correlation between experimental and theoretical removal efficiency values. Furthermore, according to diagnostic plots depicted in Supplementary Figs. 5, 6, the normality of residuals’ dispersion (Supplementary Fig. 5), and random errors in runs (Supplementary Fig. 6) indicate the precision of the proposed models. Based on the mentioned points, Eqs. (2) and (3) were satisfactory models for predicting the efficiency of Cr6+ and RhB removal.
As mentioned above, running the stepwise command in the DX7 software removes the negligible effects and represents the major linear, quadratic, and mutual effects. As can be seen in Supplementary Table 3 and Eq. (2), the binary interaction of solution pH with the initial Cr6+ concentration, catalyst dosage, and irradiation time have pivotal roles in the photocatalytic reduction of Cr6+. These outcomes are graphically illustrated by 3D response surfaces in Fig. 6a–c to interpret these interactions. Similarly, Supplementary Table 4 and Eq. (3) show that interactions of [RhB]0*Time, [Catalyst]0*pH, [Catalyst]0*Time, and pH*Time are major mutual effects in photocatalytic degradation of RhB. The response surfaces of these effects are depicted in Fig. 7a–d.
As shown in Fig. 6a–c, acidic pHs were favorable to the photocatalytic reduction of Cr6+. In this respect, Cr6+ exists in forms of \({{{\mathrm{HCrO}}}}_4^ -\) and \({{{\mathrm{Cr}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}_7^{2 - }\) in acidic solutions, and its photocatalytic reduction occurs according to Eqs. (4) and (5)48,49. It is noteworthy that the abundance of H+ is suitable for reducing Cr6+. In alkaline environments, \({{{\mathrm{CrO}}}}_4^{2 - }\) ion is the dominant species of Cr6+, whose photocatalytic reduction produces \({{{\mathrm{Cr(OH)}}}}_{{{\mathrm{3}}}}\) precipitation (Eq. (6)). These precipitations cover the catalyst surface, blocking many active sites and hindering photon penetration3.
Furthermore, the point of zero charge (pHpzc) of the SnIn4S8/SmVO4 composite is 7.2 (Supplementary Fig. 7), meaning that the composite surface has positively and negatively charged at pHs below and beyond 7.2, respectively. Thus, the electrostatic attraction between \({{{\mathrm{HCrO}}}}_4^ -\) and \({{{\mathrm{Cr}}}}_{{{\mathrm{2}}}}{{{\mathrm{O}}}}_7^{2 - }\) with positively charged catalyst surfaces at acidic pHs caused the remarkable adsorption of these anions on the catalyst surface and, thus, substantial photocatalytic reduction of Cr6+. Consequently, the cleanup pathway of Cr6+ is more favorable under strong acidic conditions. In Fig. 6a, b, the pH interactions with catalyst dosage and irradiation time show that increasing the catalyst amount and irradiation duration in relatively strong acidic environments increases the efficiency. This enhancement might be due to the increased available active sites and the possibility of creating more active species. Besides, according to Fig. 6c, the removal efficiency was decreased at higher Cr6+ concentrations because of huge amounts of Cr6+ ions and the non-responsiveness of involved active species30,31.
According to Fig. 7a–d, increasing the initial dye concentration, catalyst dosage, and duration of irradiation increases the RhB removal efficiency. In fact, all three mentioned factors raise the chance of the RhB molecules’ collision with the catalyst surface, thereby increasing the photocatalytic eradication of RhB. Although an increment in dye concentration may prevent the penetration of photons deep inside the solution, it can be compensated by the prolonged illumination time (Fig. 7a). The obtained results are consistent with the results observed in previous studies44,50,51.
According to Fig. 7b, d, alkaline pHs were favorable to photocatalytic degradation of RhB. Indeed, the electrostatic attraction between negatively charged catalyst surfaces and cationic molecules of RhB caused the remarkable pulling of RhB toward the catalyst surface at basic pHs, leading to substantial photocatalytic degradation of RhB dye. This conclusion is entirely consistent with the results obtained by other studies52.
Typically, determining the optimal conditions is the final step in experimental design. Table 1 summarizes the RSM-based optimum conditions for the removal efficiency of Cr6+ and RhB.
Figure 8 presents the UV-Vis spectra and photographs of Cr6+ and RhB solutions before and after the photocatalytic process. The fading of the solutions’ color and a significant decrease in absorbance at the corresponding maximum wavelengths indicate the remarkable photocatalytic removal of Cr6+ and RhB under the optimal RSM-based conditions.
The extent of RhB mineralization in the photocatalytic process was estimated using a total organic carbon (TOC) analyzer (Shimadzu TOC-L-CPN). The obtained results demonstrate that TOC was removed up to 73.98% at optimal RSM-based conditions, suggesting effective mineralization of RhB dye. In addition, the produced by-products during the RhB degradation were identified by gas chromatography-mass spectrometry (GC-MS) according to the instructions reported in the Supplementary Methods. The GC chromatogram of degraded RhB and corresponding Mass spectra are presented in Supplementary Figs. 8, 9, respectively. Figure 9 illustrates the chemical structure of 7 by-products produced during RhB photocatalytic degradation. These compounds’ appearance confirmed the remarkable degradation of RhB dye through the SnIn4S8/SmVO4 photocatalyzed process.
Process re-optimization and ranking of operational factors by ANN
The operational factors were ranked, and the process was re-optimized using the ANN approach. As mentioned in the experimental section, 30 experiments were designed by RSM and laboratory results were normalized and imported to the MATLAB software as input and output data, respectively. The process was modeled using a two-layer feed-forward/back-propagation network. Here, the Sigmoid and Purlin functions were used as activation functions and the Levenberg-Marquardt as the training algorithm. Networks with various numbers of hidden neurons were tried to find the optimum network. Generally, a network with an appropriate number of hidden neurons has the least mean square error (MSE)53. In Supplementary Fig. 10, networks with 8 and 10 hidden neurons had the least MSE in ANN modeling of Cr6+ reduction and RhB degradation, respectively. The accuracy and precision of the selected networks were assessed by correlation coefficient (R) values of graphs of training, validation, test, and total data (Supplementary Figs. 11, 12). All plots exhibit R values close to unity, indicating the networks would cover the data well enough. This result is supported by congruence between experimental and ANN-predicted removal efficiency values listed in Supplementary Tables 1, 2.
After validating the prepared ANNs, the operational factors were ranked using the connection weights of the networks listed in Supplementary Tables 5, 6 and Garson’s equation (Supplementary Equation 4). The relative importance of operational factors is illustrated by pie charts in Supplementary Fig. 13. As shown in Supplementary Fig. 13a, b, all factors are effective in photocatalytic Cr6+ reduction and RhB degradation, but pH is more important factor.
Finally, the performance of RSM and ANN multivariate statistics procedures was compared in modeling the photocatalytic process. According to Supplementary Fig. 14, both approaches have a good capability to predict the removal efficiency; however, a closer look reveals that ANN’s model can explain the process behavior more accurately and reliably than RSM because of its broader capabilities.
Mechanism of the photocatalytic process
Light-harvesting ability, a sufficient lifetime of photoinduced e--h+ pairs, and sufficient redox ability are three main determinants of photocatalytic performance. A narrow bandgap photocatalyst is desirable for excitation with cost-effective irradiation sources like visible light. Although a photocatalyst with a high CB and low VB (i.e., a wide bandgap photocatalyst) plays a critical role in achieving a high redox ability, these two conditions are contradictory.
The design of heterojunction photocatalysts is among the promising methods to overcome the mentioned problems. Based on the position of the CB and VB of the involved semiconductors or depending on their types (n or p-type), the architecture of heterojunction photocatalysts is divided into five categories, which is abundantly reported in the literature: Type-I heterojunction, Type-II heterojunction, p-n junction, Schottky junction, and Z-scheme heterojunction. Notably, there are two main types of Z-scheme heterojunctions, namely direct Z-scheme (without any electron mediator) and indirect Z-scheme (with an electron mediator)54,55,56.
Typically, two important steps to provide the architecture of a heterojunction photocatalyst are: determining the band alignment of involved semiconductors and figuring out the involved active species. Based on the above Mott-Schottky and UV-Vis DRS analysis, the CB and VB potentials were determined as −0.69 and 1.42 V for SnIn4S8 and 0.29 and 2.16 V for SmVO4, respectively. Supplementary Fig. 15a depicts the relative position of the band edge potential of mentioned semiconductors. Besides, clarifying the active species was followed by trapping tests under RSM-based optimal conditions. Ascorbic acid (AA), tert-butanol (TB), potassium bromate (PB), and ethylene diamine tetra-acetic acid (EDTA) were used as scavenging agents of superoxide radicals (\({}^ \bullet {{{\mathrm{O}}}}_2^ -\)), hydroxyl radicals (\({}^ \bullet {{{\mathrm{OH}}}}\)), photoinduced electrons (e-), and holes (h+), respectively. As displayed in Supplementary Fig. 15b, Cr6+ reduction was significantly declined by suppressing \({}^ \bullet {{{\mathrm{O}}}}_2^ -\) and e- in the presence of AA and PB; meanwhile, RhB removal efficiency was reduced by quenching \({}^ \bullet {{{\mathrm{O}}}}_2^ -\) and h+ in the presence of AA and EDTA, respectively. In short, superoxide radicals and photoinduced electrons and holes played vital roles in the photocatalytic removal of Cr6+ and RhB under visible light irradiation.
To realize the architecture of the studied heterojunction photocatalyst, two configurations in Fig. 10 were regarded as defaults.
The Eg values of SnIn4S8 and SmVO4 indicate that their exposure to visible light leads to the transfer of photoinduced electrons from the VB to the CB of each of them, maintaining the holes on their VB. The trapping tests also demonstrated that \({}^ \bullet {{{\mathrm{O}}}}_2^ -\), e-, and h+ are dominant species in the photocatalytic redox reactions.
Since the CB potential of SmVO4 was more positive than the standard potential of \({}^ \bullet {{{\mathrm{O}}}}_2^ - /{{{\mathrm{O}}}}_2\) (−0.33 V vs. NHE)57, the hypothesis of photogenerated electrons transmission from SnIn4S8-CB to SmVO4-CB and, \({}^ \bullet {{{\mathrm{O}}}}_2^ -\) production by accumulated electrons in SmVO4-CB is rejected. Hence, the formation of type-II heterojunction between SnIn4S8 and SmVO4 through the electrons migration from SnIn4S8-CB to SmVO4-CB simultaneous with the transfer of holes from SmVO4-VB to SnIn4S8-VB is far from the expected. Alternatively, the CB potential of SnIn4S8 was negative enough compared with the standard potential of \({}^ \bullet {{{\mathrm{O}}}}_2^ - /{{{\mathrm{O}}}}_2\); thus, the adsorbed O2 molecules on the catalyst surface could be reduced to \({}^ \bullet {{{\mathrm{O}}}}_2^ -\) by electron injection from the SnIn4S8-CB. Such an approach can be possible through e--h+ recombination between the CB of SmVO4 and VB of SnIn4S8. Accordingly, the electrons and holes remain on the CB of SnIn4S8 and VB of SmVO4, respectively. As mentioned, gathered electrons on SnIn4S8-CB could reduce O2 to yield \({}^ \bullet {{{\mathrm{O}}}}_2^ -\) to participate in the photocatalytic removal of pollutants. It is of note that the VB potential of SmVO4 was not positive enough compared with the standard potential of \({{{\mathrm{H}}}}_{{{\mathrm{2}}}}{{{\mathrm{O/}}}}{}^ \bullet {{{\mathrm{OH}}}}\) (+2.40 V vs. NHE)58. Therefore, the adsorbed H2O molecules on the catalyst surface could not produce hydroxyl radicals. Hence, collected holes on SmVO4-VB would directly participate in the photocatalytic removal of pollutants. This finding is consistent with the trapping test results.
Based on the mentioned points, photocatalytic Cr6+ reduction and RhB degradation were taken by superoxide radicals and electron-holes by establishing a direct Z-scheme heterojunction between SnIn4S8 and SmVO4 under visible light irradiation.
Accordingly, the plausible reactions during the photocatalytic Cr6+ reduction and RhB degradation through the Z-scheme heterojunction of SnIn4S8/SmVO4 under RSM-based optimal conditions were assessed. Since the CB potential of SnIn4S8 was more negative than the standard reduction potential of Cr6+/Cr3+ (1.33 V vs. NHE)59, Cr6+ could be reduced to Cr3+ by photoinduced electrons. In addition, superoxide anion radicals could reduce Cr6+ to Cr3+. Besides, RhB could be oxidized and reduced by photoinduced holes and superoxide anion radicals, respectively. According to the mentioned points and based on the related literature, the following reactions are expected in the involved process:
Reusability test and kinetics study
Catalyst recovery and reuse are vital in its practical applications. Hence, the catalyst reusability was tested under RSM-based optimal conditions, according to the method described in the Supplementary Methods. As it is clear from Supplementary Fig. 16a, the ability to use the recovered catalyst can be justified up to four times. However, more reuse is not reasonable, probably due to blocking active sites or unavoidable loss of SnIn4S8/SmVO4 powders during the repetitive tests60. Supplementary Fig. 16b shows the XRD patterns of fresh and recycled photocatalysts. As can be seen, the XRD diffraction peaks of SnIn4S8/SmVO4 photocatalysts remain the same as that of fresh SnIn4S8/SmVO4 after four runs. In addition, comparing the FE-SEM images of fresh and recycled photocatalysts (Supplementary Fig. 17) indicates that no noticeable change occurred in SnIn4S8/SmVO4 composite morphology after four consecutive uses. These results suggest the photostability and reusability of the SnIn4S8/SmVO4 as a photocatalyst from the practical aspect.
Studying the kinetics of photocatalytic processes is another important aspect in proving their applicability. The literature review indicates that Langmuir-Hinshelwood’s pseudo-first-order model is the most likely kinetics model for describing photodegradation processes (see the detailed explanations of this model in the Supplementary Methods). Hence, this model was studied in the present research under RSM-based optimal conditions. As can be inferred from Supplementary Fig. 18, the experimental data for both Cr6+and RhB dye were consistent with the pseudo-first-order kinetics model (R2 values close to one) with the apparent rate constants (kapp) of 0.036 and 0.080 min−1, respectively.
The advantages of the prepared SnIn4S8/SmVO4 heterojunction were studied by comparing its photocatalytic performance for Cr6+ reduction and RhB degradation with recently published other photocatalysts. According to Tables 2 and 3, the obtained removal efficiency values in this study are quite comparable with other photocatalysts. These results indicate that the synthesized SnIn4S8/SmVO4 heterojunction could be considered a prominent visible-light-driven photocatalyst to control organic and inorganic pollutants.
Methods
The chemical materials, characterization methods, and the methods of the point of zero charge (pHpzc) determination and Mott-Schottky tests are provided in the Supplementary Methods.
Preparation of SnIn4S8/SmVO4
The SnIn4S8/SmVO4 composite was prepared as follows:
Synthesis of SnIn4S8
The SnIn4S8 was synthesized via a solvothermal procedure proposed in the literature1. Firstly, 0.438 g (1.25 mmol) of SnCl4.5H2O and 1.466 g (5 mmol) of InCl3.4H2O were dissolved in 150 mL of C2H5OH under magnetic stirring at room temperature. Then, 0.939 g (12.5 mmol) of CH3CSNH2 was added to the above solution, and the mixture was further stirred until a uniformly dispersed solution was formed. The obtained blend was transferred into a 250 mL Teflon-lined autoclave and hold on 160 °C for 12 h. The final product was achieved by centrifuging, rinsing thrice with ethanol and deionized water, and drying at 60 °C for 8 h.
Synthesis of SmVO4
The SmVO4 was synthesized by precipitation method with an equal molar ratio of Sm:V36. Firstly, 0.5 g of Sm(NO3)2.6H2O (A) and 0.132 g of NH4VO3 (B) were separately dissolved in 50 mL of deionized water and stirred for 30 min at room temperature. Then, solution B was dropwise added into a round-bottom flask containing solution A under vigorously stirring, whiles the pH of the mixture was kept at 7–8 using NH3. The mixture was stirred for another 2 h. Afterwards, the precipitates were collected centrifugally and rinsed with deionized water to neutrality. The obtained product was oven-dried at 100 °C for 12 h, and calcined in the furnace for 2 h at 500 °C.
Preparation of SnIn4S8/SmVO4
The SnIn4S8/SmVO4 binary composite was synthesized hydrothermally at variable molar ratios of SnIn4S8:SmVO4 (4:1, 2:1, 1:1, 1:2, and 1:4)37. Firstly, certain amounts of SnIn4S8 and SmVO4 were separately dispersed in 100 mL of deionized water under ultrasonication at room temperature. Then, both suspensions were transferred into a 250 mL Teflon-lined autoclave to be heated at 120 °C for 8 h. The final product was obtained by centrifuging, rinsing with deionized water, and drying at 80 °C for 8 h.
Designing the photocatalytic experiments, RSM, and ANN modeling of the process
The photocatalytic experiments were conducted under a 300 W halogen lamp irradiation based on the method described in the Supplementary Methods. Supplementary Fig. 19 illustrates the photocatalytic experimental setup.
The experiments were designed using the response surface methodology (RSM) to reduce costs, save time, and obtain optimal conditions. The design and optimization processes were performed using the RSM-based central composite design (CCD) toolbox of the Design-Expert® Version 7 (DX7) package. After preliminary trials (Supplementary Figs. 20, 21), factors contributed were selected at five levels, and the software suggested 30 experiments (Supplementary Tables 1, 2). After conducting the laboratory tests and importing the experimental results to the DX7 software, theoretical results were predicted by a quadratic polynomial equation, Supplementary Equation 2.
Operational factors were ranked and re-optimized by modeling in MATLAB R2020 b software based on the artificial neural network (ANN) toolbox. The mentioned 30 experiments were designed by RSM, and laboratory results were normalized by Supplementary Equation 3. In the next step, they were imported into the software as input and output data, respectively. The photocatalytic process was modeled using a two-layer feed-forward/back-propagation network. After selecting a network with a suitable topology, the operational factors were ranked using the connection weights and Garson’s equation, Supplementary Equation 461. For more information, see the detailed explanations in the Supplementary Methods.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Wang, L. et al. Efficient photocatalytic reduction of aqueous Cr (VI) over flower-like SnIn4S8 microspheres under visible light illumination. J. Hazard. Mater. 244, 681–688 (2013).
Chen, D. et al. Fabrication of Bi modified Bi2S3 pillared g-C3N4 photocatalyst and its efficient photocatalytic reduction and oxidation performances. Appl. Surf. Sci. 426, 427–436 (2017).
Lu, D. et al. Solvothermal-assisted synthesis of self-assembling TiO2 nanorods on large graphitic carbon nitride sheets with their anti-recombination in the photocatalytic removal of Cr (VI) and rhodamine B under visible light irradiation. Nanoscale 9, 3231–3245 (2017).
Zhang, L. et al. One-step in situ synthesis of CdS/SnO2 heterostructure with excellent photocatalytic performance for Cr (VI) reduction and tetracycline degradation. Chem. Eng. J. 352, 863–875 (2018).
Ou, B., Wang, J., Wu, Y., Zhao, S. & Wang, Z. Efficient removal of Cr (VI) by magnetic and recyclable calcined CoFe-LDH/g-C3N4 via the synergy of adsorption and photocatalysis under visible light. Chem. Eng. J. 380, 122600 (2020).
Cui, H., Dong, S., Wang, K., Luan, M. & Huang, T. Synthesis of a novel Type-II In2S3/Bi2MoO6 heterojunction photocatalyst: Excellent photocatalytic performance and degradation mechanism for Rhodamine B. Sep. Purif. Technol. 255, 117758 (2021).
Weng, R. et al. Efficient mineralization of TBBPA via an integrated photocatalytic reduction/oxidation process mediated by MoS2/SnIn4S8 photocatalyst. Chemosphere 285, 131542 (2021).
Wang, Z. et al. Novel core-shell SnIn4S8@ Bi2MoO6 heterojunction with highly-enhanced photocatalytic activity for visible light-driven Cr (VI) reduction. Appl. Surf. Sci. 589, 152888 (2022).
Mehrizad, A. & Gharbani, P. Novel ZnS/Carbon Nanofiber Photocatalyst for Degradation of Rhodamine 6G: Kinetics Tracking of Operational Parameters and Development of a Kinetics Model. Photochem. Photobiol. 93, 1178–1186 (2017).
Qu, X., Alvarez, P. J. J. & Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res 47, 3931–3946 (2013).
Bolisetty, S., Peydayesh, M. & Mezzenga, R. Sustainable technologies for water purification from heavy metals: review and analysis. Chem. Soc. Rev. 48, 463–487 (2019).
Ghernaout, D. & Elboughdiri, N. Advanced oxidation processes for wastewater treatment: facts and future trends. Open Access Lib 7, 1–15 (2020).
Tabatabaei, S. M., Dastmalchi, S., Mehrizad, A. & Gharbani, P. Enhancement of 4-nitrophenol ozonation in water by nano ZnO catalyst. Iran. J. Environ. Health Sci. Eng. 8, 363–372 (2011).
Mehrizad, A. & Gharbani, P. Decontamination of 4-chloro-2-nitrophenol from aqueous solution by graphene adsorption: Equilibrium, kinetic, and thermodynamic studies. Pol. J. Environ. Stud. 23, 2111–2116 (2014).
Belver, C., Bedia, J., Gómez-Avilés, A., Peñas-Garzón, M. & Rodriguez, J. J. Semiconductor photocatalysis for water purification. in Nanoscale materials in water purification 581–651 (Elsevier, 2019).
Tahir, M. B. et al. Advances in photo-catalysis approach for the removal of toxic personal care product in aqueous environment. Environ. Dev. Sustain. 22, 6029–6052 (2020).
Wang, H. et al. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chinese J. Catal. 43, 178–214 (2022).
Ataei, A., Mehrizad, A. & Zare, K. Photocatalytic degradation of cefazoline antibiotic using zeolite-supported CdS/CaFe2O4 Z-scheme photocatalyst: Optimization and modeling of process by RSM and ANN. J. Mol. Liq. 328, 115476 (2021).
Yan, T., Li, L. & Li, G. Solvothermal synthesis of hierarchical SnIn4S8 microspheres and their application in photocatalysis. Res. Chem. Intermed. 37, 297–307 (2011).
Yan, T. et al. Porous SnIn4S8 microspheres in a new polymorph that promotes dyes degradation under visible light irradiation. J. Hazard. Mater. 186, 272–279 (2011).
Wang, T., Zhang, Y. & Ding, T. One-step solvothermal synthesis of SnIn4S8/TiO2 nanocomposite with enhanced visible-light-activated photocatalytic activity. Mater. Lett. 123, 153–155 (2014).
Deng, F. et al. Facile low-temperature co-precipitation method to synthesize hierarchical network-like g-C3N4/SnIn4S8 with superior photocatalytic performance. J. Mater. Sci. 51, 6998–7007 (2016).
Deng, F. et al. One-step hydrothermal fabrication of visible-light-responsive AgInS2/SnIn4S8 heterojunction for highly-efficient photocatalytic treatment of organic pollutants and real pharmaceutical industry wastewater. Appl. Catal. B 219, 163–172 (2017).
Deng, F. et al. One-step in situ hydrothermal fabrication of octahedral CdS/SnIn4S8 nano-heterojunction for highly efficient photocatalytic treatment of nitrophenol and real pharmaceutical wastewater. J. Hazard. Mater. 340, 85–95 (2017).
Lu, M. et al. Construction of cobalt phthalocyanine sensitized SnIn4S8/g-C3N4 composites with enhanced photocatalytic degradation and hydrogen production performance. Synth. Met. 268, 116480 (2020).
Guo, H. et al. Construction of direct Z-scheme AgI/Bi2Sn2O7 nanojunction system with enhanced photocatalytic activity: accelerated interfacial charge transfer induced efficient Cr (VI) reduction, tetracycline degradation and Escherichia coli inactivation. ACS Sustain. Chem. Eng. 6, 8003–8018 (2018).
Zhu, Z. et al. Fabrication of magnetically recoverable photocatalysts using g-C3N4 for effective separation of charge carriers through like-Z-scheme mechanism with Fe3O4 mediator. Chem. Eng. J. 331, 615–625 (2018).
Wang, T. et al. A Z-scheme TiO2 quantum dots fragment-Bi12TiO20 composites for enhancing photocatalytic activity. Renew. Energy 147, 856–863 (2020).
Shi, H. et al. Highly efficient visible light driven photocatalytic inactivation of E. coli with Ag QDs decorated Z-scheme Bi2S3/SnIn4S8 composite. Appl. Catal. B 254, 403–413 (2019).
Zhang, S. et al. In-situ constructing of one-dimensional SnIn4S8-CdS core-shell heterostructure as a direct Z-scheme photocatalyst with enhanced photocatalytic oxidation and reduction capabilities. Appl. Surf. Sci. 542, 148618 (2021).
Shen, C.-H. et al. Efficient photocatalytic H2 evolution and Cr (VI) reduction under visible light using a novel Z-scheme SnIn4S8/CeO2 heterojunction photocatalysts. J. Hazard. Mater. 416, 126217 (2021).
Tang, C. et al. Spatially distributed Z-scheme heterojunction of g-C3N4/SnIn4S8 for enhanced photocatalytic hydrogen production and pollutant degradation.Appl. Surf. Sci. 598, 153870 (2022).
Yi, M., Park, S.-K., Seong, C.-Y., Piao, Y. & Yu, T. The general synthesis and characterization of rare earth orthovanadate nanocrystals and their electrochemical applications. J. Alloys Compd. 693, 825–831 (2017).
Ge, X., Zhang, Y., Wu, H., Zhou, M. & Lin, T. SmVO4 nanocrystals with dodecahedral shape: Controlled synthesis, growth mechanism and photoluminescent properties. Mater. Res. Bull. 97, 81–88 (2018).
He, Y. et al. Photodegradation of organics over a new composite catalyst V2O5/SmVO4. Catal. Commun. 10, 1354–1357 (2009).
Li, T. et al. Synthesis, characterization and photocatalytic activity of visible-light plasmonic photocatalyst AgBr-SmVO4. Appl. Catal. B 138, 95–103 (2013).
Li, T. et al. Synthesis of g-C3N4/SmVO4 composite photocatalyst with improved visible light photocatalytic activities in RhB degradation. Appl. Catal. B 129, 255–263 (2013).
Eghbali-Arani, M., Sobhani-Nasab, A., Rahimi-Nasrabadi, M. & Pourmasoud, S. Green synthesis and characterization of SmVO4 nanoparticles in the presence of carbohydrates as capping agents with investigation of visible-light photocatalytic properties. J. Electron. Mater. 47, 3757–3769 (2018).
Shandilya, P. et al. Fabrication of fluorine doped graphene and SmVO4 based dispersed and adsorptive photocatalyst for abatement of phenolic compounds from water and bacterial disinfection. J. Clean. Prod. 203, 386–399 (2018).
Chen, J. et al. One-step MOF assisted synthesis of SmVO4 nanorods for photocatalytic degradation of tetracycline under visible light. Mater. Lett. 276, 128213 (2020).
Leeladevi, K., Kumar, J. V., Arunpandian, M., Thiruppathi, M. & Nagarajan, E. R. Investigation on photocatalytic degradation of hazardous chloramphenicol drug and amaranth dye by SmVO4 decorated g-C3N4 nanocomposites. Mater. Sci. Semicond. Process. 123, 105563 (2021).
Quach, T.-A. et al. Direct Z-scheme mediated SmVO4/UiO-66-NH2 heterojunction nanocomposite for the degradation of antibiotic tetracycline hydrochloride molecules under sunlight. Chemosphere 303, 134861 (2022).
Rouquerol, J., Rouquerol, F., Llewellyn, P., Maurin, G. & Sing, K. S. W. Adsorption by powders and porous solids: principles, methodology and applications. (Academic press, 2013).
Hemmatpour, P. & Nezamzadeh-Ejhieh, A. A Z-scheme CdS/BiVO4 photocatalysis towards Eriochrome black T: An experimental design and mechanism study. Chemosphere 307, 135925 (2022).
Zuo, G., Wang, Y., Teo, W. L., Xian, Q. & Zhao, Y. Direct Z-scheme TiO2–ZnIn2S4 nanoflowers for cocatalyst-free photocatalytic water splitting. Appl. Catal. B 291, 120126 (2021).
Liu, H. et al. Preparation and photocatalytic activity of Gd3+-doped trititanate nanotubes. Micropor. Mesopor. Mat. 142, 439–443 (2011).
Bezerra, M. A., Santelli, R. E., Oliveira, E. P., Villar, L. S. & Escaleira, L. A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76, 965–977 (2008).
Wang, D. et al. In situ construction efficient visible-light-driven three-dimensional Polypyrrole/Zn3In2S6 nanoflower to systematically explore the photoreduction of Cr (VI): Performance, factors and mechanism. J. H. Mater. 384, 121480 (2020).
Li, Y.-X., Han, Y.-C. & Wang, C.-C. Fabrication strategies and Cr (VI) elimination activities of the MOF-derivatives and their composites. Chem. Eng. J. 405, 126648 (2021).
Khairnar, S. D., Patil, M. R. & Shrivastava, V. S. Hydrothermally synthesized nanocrystalline Nb2O5 and its visible-light photocatalytic activity for the degradation of congo red and methylene blue. Iran. J. Catal. 8, 143–150 (2018).
Chaker, H., Ferouani, G., Chikhi, I., Djennas, M. & Fourmentin, S. A novel statistical approach for the synthesis of Chalcones viaClaisen-Schmidt condensation catalyzed by Pd nanoparticles modified mesoporous TiO2 as an efficient heterogeneous catalyst. Colloid Interface Sci. Commun 43, 100461 (2021).
Abdi, J., Yahyanezhad, M., Sakhaie, S., Vossoughi, M. & Alemzadeh, I. Synthesis of porous TiO2/ZrO2 photocatalyst derived from zirconium metal organic framework for degradation of organic pollutants under visible light irradiation. J. Environ. Chem. Eng. 7, 103096 (2019).
Behnajady, M. A., Eskandarloo, H. & Eskandarloo, F. Artificial neural network modeling of the influence of sol–gel synthesis variables on the photocatalytic activity of TiO2 nanoparticles in the removal of Acid Red 27. Res. Chem. Intermed. 41, 6463–6476 (2015).
Teranishi, T. & Sakamoto, M. Charge separation in type-II semiconductor heterodimers. J. Phys. Chem. Lett. 4, 2867–2873 (2013).
Xu, Q. et al. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 21, 1042–1063 (2018).
Zhang, G. et al. A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application. Green Energy Environ 7, 176–204 (2020).
Chen, S., Hu, Y., Meng, S. & Fu, X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3. Appl. Catal. B 150, 564–573 (2014).
Zhao, W. et al. Fabrication of a novel p–n heterojunction photocatalyst n-BiVO4@ p-MoS2 with core–shell structure and its excellent visible-light photocatalytic reduction and oxidation activities. Appl. Catal. B 185, 242–252 (2016).
Yuan, G. et al. Research progress on photocatalytic reduction of Cr (VI) in polluted water. Bull. Chem. Soc. Jpn. 94, 1142–1155 (2021).
Wang, J. et al. Visible-light-driven double-shell SnIn4S8/TiO2 heterostructure with enhanced photocatalytic activity for MO removal and Cr (VI) cleanup. Appl. Surf. Sci. 587, 152867 (2022).
Mehrizad, A. & Gharbani, P. Application of central composite design and artificial neural network in modeling of reactive blue 21 dye removal by photo-ozonation process. Water Sci. Technol 74, 184–193 (2016).
Xu, P. et al. Surfactant-assisted hydrothermal synthesis of rGO/SnIn4S8 nanosheets and their application in complete removal of Cr (vi). RSC Adv 8, 5749–5759 (2018).
Ai, L. et al. Controlled growth of single-crystalline Bi. 333 (Bi6S9) Br nanorods under hydrothermal conditions for enhanced photocatalytic reduction of Cr (VI). J. Alloys Compd. 842, 155879 (2020).
Yao, L., Chen, Z., Li, J. & Shi, C. Creation of oxygen vacancies to activate lanthanum-doped bismuth titanate nanosheets for efficient synchronous photocatalytic removal of Cr (VI) and methyl orange. J. Mol. Liq. 314, 113613 (2020).
Sun, M. et al. Fabrication of MOF-derived tubular In2O3@ SnIn4S8 hybrid: Heterojunction formation and promoted photocatalytic reduction of Cr (VI) under visible light. J. Colloid Interface Sci 596, 278–287 (2021).
Nguyen, V. H. et al. Composite photocatalysts containing MIL-53 (Fe) as a heterogeneous photo-Fenton catalyst for the decolorization of rhodamine B under visible light irradiation. J. Environ. Chem. Eng. 6, 7434–7441 (2018).
Xu, S., Liu, Z., Zhang, M. & Guo, L. Piezotronics enhanced photocatalytic activities of Ag-BaTiO3 plasmonic photocatalysts. J. Alloys Compd. 801, 483–488 (2019).
Xu, P. et al. Z-Schemed WO3/rGO/SnIn4S8 sandwich nanohybrids for efficient visible light photocatalytic water purification. Catalysts 9, 187 (2019).
Spilarewicz-Stanek, K., Jakimińska, A., Kisielewska, A., Batory, D. & Piwoński, I. Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation. Materials 13, 4653 (2020).
Acknowledgements
The authors would like to thank Tabriz Branch, Islamic Azad University for providing facilities and technical support.
Author information
Authors and Affiliations
Contributions
S.A.: Investigation, Original draft preparation; A.M.: Supervision, Software, Formal analysis, Reviewing and Editing; M.A.B.: Project administration, Supervision; M.E.R.: Visualization, Reviewing and Editing; P.G.: Data Curation, Validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asoubar, S., Mehrizad, A., Behnajady, M.A. et al. Hexavalent chromium reduction and Rhodamine B degradation by visible-light-driven photocatalyst of stannum indium sulfide-samarium vanadate. npj Clean Water 6, 27 (2023). https://doi.org/10.1038/s41545-023-00246-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-023-00246-w