Abstract
Crimean–Congo haemorrhagic fever (CCHF) is a severe tick-borne illness with a wide geographical distribution and case fatality rates of 30% or higher. Caused by infection with the CCHF virus (CCHFV), cases are reported throughout Africa, the Middle East, Asia and southern and eastern Europe. The expanding range of the Hyalomma tick vector is placing new populations at risk for CCHF, and no licensed vaccines or specific antivirals exist to treat CCHF. Furthermore, despite cases of CCHF being reported annually, the host and viral determinants of CCHFV pathogenesis are poorly understood. CCHFV can productively infect a multitude of animal species, yet only humans develop a severe illness. Within human populations, subclinical infections are underappreciated and may represent a substantial proportion of clinical outcomes. Compared with other members of the Bunyavirales order, CCHFV has a more complex genomic organization, with many viral proteins having unclear functions in viral pathogenesis. In recent years, improved animal models have led to increased insights into CCHFV pathogenesis, and several antivirals and vaccines for CCHFV have shown robust efficacy in preclinical models. Translation of these insights and candidate therapeutics to the clinic will hopefully reduce the morbidity and mortality caused by CCHFV.
Similar content being viewed by others
Introduction
Crimean–Congo haemorrhagic fever virus (CCHFV) was first reported in the 1960s as a cause of febrile illnesses in the Congo1. Investigation of febrile illnesses in Crimea showed that the virus responsible for these cases was antigenically identical to the cause of illnesses in the Congo2. Since then, serological studies and reported human cases demonstrate that CCHFV is a widely distributed haemorrhagic fever virus endemic throughout Africa, the Middle East, Southeast Asia and southern and eastern Europe (reviewed in ref. 3), closely following the range of its Hyalomma tick reservoir host. Studies have identified ticks of the Hyalomma genus to be the principal vector and reservoir of CCHFV, although other tick species may have a role in maintaining CCHFV in endemic regions (reviewed in ref. 4). Long-range transport of CCHFV-infected ticks on birds5, global trade leading to the introduction of tick vectors to new continents6 and climate change leading to an expanding range of the Hyalomma tick as far north as Sweden7 suggest that the geographical range of CCHFV will continue to expand. Serological studies have shown that CCHFV can productively infect diverse wild animal species such as hares, small rodents, ostriches, buffalo and even rhinoceroses8 and, importantly for human exposure, livestock without apparent disease (reviewed in ref. 8). These animal species serve as important amplifying hosts for CCHFV, enabling CCHFV to spread from infected ticks to uninfected ticks through either co-feeding or feeding on a viraemic animal9. Humans are most often infected with CCHFV through tick bites or handling and butchering of infected livestock. On infection, CCHF begins as a nonspecific febrile illness that can then progress to the severe haemorrhagic manifestations. Case fatality rates vary but can be higher than 30% in some regions. There are currently no approved vaccines or therapeutics for CCHF. In recent years, we have gained an improved understanding of the function of CCHFV proteins in viral replication, and improved animal models have provided important insight into CCHFV pathogenesis and enabled preclinical testing of multiple vaccine platforms and therapeutic strategies for CCHF. In this Review, we focus on recent insights gained into the function of viral proteins in CCHFV pathogenesis along with our current understanding of CCHF and the state of treatments and vaccines for CCHFV.
Molecular biology of CCHFV
CCHFV is an enveloped negative-sense RNA virus belonging to the Orthonairovirus genus in the Nairoviridae family of the Bunyavirales order10 (Fig. 1a). In addition to CCHFV, the Nairoviridae family consists of arthropod-borne viruses such as Nairobi sheep disease virus, Dugbe virus and Hazara virus (HAZV), although these viruses seem to cause little-to-no disease in humans11. As in other bunyaviruses, the tri-segmented viral genome is coated with the viral nucleoprotein (NP) and bound by the L protein12 (Fig. 1a). The viral proteins are encoded by three genomic segments (Fig. 1b), and relative to other members of the order that can cause human disease, CCHFV has a more complex genomic organization (discussed below). On entry, these proteins produce positive-sense viral RNA using the genomic negative-sense viral RNA as a template to initiate viral protein production and replication12 (Fig. 1c). The viral glycoproteins Gn and Gc are found on the virion surface and are responsible for receptor binding and viral entry (Fig. 1a).
a, Crimean–Congo haemorrhagic fever virus (CCHFV) is an enveloped, tri-segmented, negative-sense RNA virus. The virion is studded with the glycoproteins Gn and Gc, which mediate receptor binding and entry. Some evidence suggests that the GP38 accessory protein is also found on the virion but if so, with unclear localization or function. b, The three genomic segments of CCHFV are the small (S), medium (M) and large (L) segments. The S segment encodes the viral nucleoprotein (NP) in one reading frame and the small non-structural protein (NSs) in an opposite-sense open reading frame. The M segment is complex, encoding a glycoprotein precursor (GPC) that is processed by host proteases to produce a GP160/85 domain that is further processed to a mucin-like domain (MLD) and GP38, the Gn and Gc glycoproteins and the medium non-structural protein (NSm). The L segment of CCHFV is unusually large for bunyaviruses, and the encoded protein contains the viral RNA-dependent RNA polymerase (RdRP) and an ovarian tumour-like protease (OTU) at the N terminus. c, On attachment (stage 1), CCHFV undergoes clathrin-dependent and pH-dependent entry into the host cytoplasm (stages 2 and 3). After entry into the cytoplasm, viral genomes are converted to positive-sense mRNA by the RdRP and initiate translation of viral proteins. These proteins also coordinate to produce new negative-sense viral genomes that are coated with NP and a bound L protein to initiate replication on infection of the next cell (stage 4). The GPC is translated into the endoplasmic reticulum (ER) and during trafficking through the ER and Golgi apparatus is proteolytically processed to produce mature glycoproteins along with the accessory proteins MLD, NSm and GP38. Newly produced genomes are packaged into enveloped particles and the virus buds into the Golgi apparatus for release via the secretory pathway (stage 5). New virions are then released to infect additional cells, whereas GP160/85, MLD and GP38 are also released extracellularly but with unclear consequence (stage 6). In addition to facilitating viral replication, CCHFV proteins also block host apoptosis and innate immune pathways. The CCHFV NP can block the intrinsic pathway of apoptosis at a yet-to-be-defined step, whereas the CCHFV NSs promotes apoptosis through disruption of the mitochondrial membrane or extrinsic apoptotic pathways. CCHFV may also promote apoptosis through production of tumour necrosis factor (TNF) and the TNF death receptor pathway. The CCHFV NP is also cleaved by host caspase 3 but oligomeric conformations may block this cleavage. The OTU domain of the CCHFV L protein blocks RIG-I-dependent initiation of the type I interferon response via its deubiquitylating function. MAVS, mitochondrial antiviral signalling protein; vRNA, viral RNA.
S segment
The small genomic segment (S) encodes the viral NP along with the small non-structural protein (NSs) in an opposite-sense reading frame (Fig. 1b). In addition to interactions with the viral RNA to form ribonucleoprotein complexes, NP possesses endonuclease activity, interacts with host heat shock proteins during intracellular viral replication and in infectious particles, and promotes translation of viral mRNAs13,14,15,16, demonstrating a multifactorial role for NP in the CCHFV life cycle. NP and NSs may also modulate host cell apoptosis13,17,18,19, suggesting that regulation of host cell apoptosis is important for the CCHFV life cycle. CCHFV infection induces host cell apoptosis in vitro and in vivo18,20,21, and biomarkers of apoptosis were found to be upregulated in patients infected with CCHFV22,23. However, it is unclear whether host apoptosis is proviral or antiviral. Inhibition of host apoptosis in vitro resulted in increased viral titres suggestive of an antiviral effect17. The CCHFV NP can suppress activation of caspase 3 and caspase 9, and induction of apoptosis triggered by BAX and the release of cytochrome c from mitochondria, although it is unclear where in the intrinsic apoptosis pathway NP blocks activation18 (Fig. 1c). Together, these data indicate that host apoptosis may exert antiviral activity against CCHFV and that CCHFV utilizes its NP protein to suppress this host response. Yet, the CCHFV NSs can disrupt mitochondrial membrane potential, thus triggering apoptosis19, and later during infection, CCHFV induces activation of BID (a pro-apoptotic protein), probably through extrinsic apoptotic signals18, suggesting that CCHFV may also promote apoptosis (Fig. 1c). Furthermore, the CCHFV NP and related HAZV NP contain a highly conserved DEVD or DQVD cleavage motif, respectively, that is cleaved by host caspase 3 (refs. 13,17,24) (Fig. 1c). Although it has been suggested that cleavage of NP by caspase 3 may be a host defence against CCHFV17, structural studies have shown that oligomeric conformations of NP result in shielding of this motif from host caspase 3 (ref. 25). Thus, NP may be cleaved by host caspase 3 only when present in specific conformations25. Mutation of viral NP to eliminate caspase 3 cleavage resulted in enhanced viral RNA polymerase activity25, suggesting that cleavage of NP may regulate viral RNA synthesis. However, infectious HAZV and CCHFV could be rescued when the cleavable motifs were replaced with an uncleavable DQVE or AEVA motif, respectively24,26, and a CCHFV mini-replicon system showed equivalent reporter activity between wild type NP and NP with an altered DEVD motif13, demonstrating that host caspase cleavage of this motif in orthonairoviruses is not essential to viral replication. Interestingly, infectious HAZV possessing a similarly uncleavable AQVA motif could not be rescued, suggesting that this motif may have important functions distinct from caspase cleavage24. The high conservation of the DEVD motif in CCHFV further suggests that any host antiviral activity exerted through caspase 3 cleavage of NP is offset by yet-unclear proviral functions. The role of host cell apoptosis and caspases in CCHFV infection may be distinct in mammalian and tick hosts. HAZV growth in tick cells did not induce apoptosis nor was HAZV NP cleaved by tick caspases27, yet the CCHFV possessing the uncleavable AEVA motif failed to grow in tick cell culture26.
M segment
The CCHFV medium (M) segment encodes the viral glycoprotein precursor (GPC). Compared with the M segment of other Bunyavirales, the CCHFV M segment is complex. The GPC is proteolytically processed to produce the individual viral glycoproteins Gn and Gc28, a GP160/85 protein that is further proteolytically processed29 to a heavily glycosylated mucin-like domain (MLD)28 and a GP38 protein29, and a medium non-structural protein (NSm) that promotes glycoprotein processing and virion assembly30 (Fig. 1b). Proteolytic processing of the GPC occurs through host furin-like and SKI-1 proteases as the proteins traffic through the endoplasmic reticulum and Golgi apparatus29. Among the Bunyavirales order, Gn and Gc participate in receptor binding and entry (reviewed in ref. 31), and CCHFV undergoes clathrin-dependent and pH-dependent fusion and entry32. However, the roles of the other GPC-encoded proteins are less clear. Localization signals to direct nascent proteins to the Golgi apparatus seem to be localized to Gn, although MLD and GP38 may also participate in directing proper processing of the GPC33,34. The GP160/85 protein is released from the pre-Gn protein in the Golgi apparatus by host proteases35 and is found in supernatants of infected cells29, but whether it has a biological function in this context is unknown. The MLD protein is heavily glycosylated and has little sequence conservation across diverse CCHFV isolates36,37, suggesting it is under diversifying selective pressure; yet, its role in the CCHFV life cycle is unclear. Deletion of the gene encoding the MLD protein did not impair infectivity of virus-like particles (VLPs) but did lead to a reduction in incorporation of the CCHFV glycoproteins into particles30. Ebola virus (EBOV) possesses an MLD that contributes to EBOV-induced endothelial membrane disruption and vascular permeability38 along with downregulation of major histocompatibility complex (MHC)-I on the surface of infected cells to block T cell activation39. Whether the CCHFV MLD performs similar functions is unknown. GP38 promotes proper virion assembly30 and may be secreted alone or linked to the MLD as part of the GP160/85 protein28,29. Limited evidence from the study of VLPs suggests that GP38 may be found on the viral envelope and plasma membrane40; however, other studies have failed to detect GP38 in authentic CCHFV virions29 (Fig. 1a). Nevertheless, its function in these contexts is unknown.
NSm seems non-essential for CCHFV infection of mammalian hosts as recombinant CCHFV lacking NSm was able to replicate and cause lethal disease in Ifnar−/− mice41. However, it is possible that NSm retains functions in the tick or interferon (IFN)-competent host, as adaptation of CCHFV to either resulted in mutations in the NSm protein42,43. Nevertheless, the function of the NSm in these hosts is unknown.
L segment
Consisting of more than 12,000 nt, the large (L) segments of CCHFV and related orthonairoviruses are unusually large for members of the Bunyavirales order44 (Fig. 1b). The encoded L protein contains the viral RNA-dependent RNA polymerase44 and cap-snatching functions45. Interestingly, at the very amino terminus, the Orthonairovirus L protein contains an ovarian-tumour-like (OTU) protease that possesses both de-ISGylating and deubiquitylating functions46. RIG-I has been shown to initiate the type I interferon response to CCHFV47, and the OTU domain antagonizes RIG-I-mediated host innate immunity through its deubiquitylation function48 (Fig. 1c). The de-ISGylating function may also modulate host immunity as ISG15 modifications of viral proteins and the ISG15 protein itself can be antiviral49. The OTU domain may regulate viral replication beyond antagonizing innate immunity48,50. Although some data suggest that the OTU domain may regulate the viral RNA polymerase through host ISG15 (ref. 51), other data suggest that the defect in OTU-inactive viruses is due to stable occupancy of the OTU catalytic domain by ubiquitin, resulting in impaired RNA polymerase activity48. Species-specific preferences in the OTU domains of CCHFV and related viruses for either ubiquitin or ISG15 have been hypothesized to mediate host susceptibility to disease52,53. Curiously, across the Bunyavirales order, human pathogenic viruses typically possess antagonists of innate immunity in their NSs and NSm proteins (reviewed in ref. 54). Yet, similar functions have not been shown for the CCHFV NSs or NSm proteins54, and the OTU domain is the only identified direct antagonist of the type I interferon response to CCHFV.
Genetic diversity
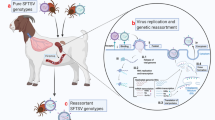
Corresponding with its wide geographical distribution, CCHFV is a genetically diverse virus. Although the NP and L proteins of CCHFV strains are conserved with approximately 95% or more amino acids conserved between strains, the CCHFV GPC is much less conserved, with divergent strains exhibiting less than 75% amino acid conservation36,37. The genetic diversity of CCHFV correlates strongly with geography, and clades of CCHFV segregate based on geographical location37,55. It is unknown what selective pressures drive the sequence diversity of CCHFV across its geographical range. Given that humans are incidental hosts for CCHFV, the selective pressures acting on CCHFV probably arise in the tick reservoir or mammalian amplifying hosts. Interestingly, CCHFV strains isolated from similar regions decades apart show strong sequence conservation56,57, suggesting that temporal evolution of CCHFV within geographical regions is limited. Instead, genetic diversity may arise from long-range migration. Strains of CCHFV circulating in southwestern Europe cluster with African rather than eastern European isolates58, indicating the long-range introduction of CCHFV to Europe from Africa, potentially through migratory birds carrying CCHFV-infected ticks5. In addition, the segmented genome of CCHFV can undergo reassortment, and CCHFV isolates possessing genomic segments with distinct geographical lineages have been used to identify historical migration and co-circulation of CCHFV over long geographical distances55,59.
Transmission to humans and risk factors
The endemicity of CCHFV is closely associated with the geographical distribution of the main arthropod vector and reservoir, Hyalomma ticks4. As an arthropod-borne virus, CCHFV is transmitted to humans primarily through the bite or handling of an infected tick, and in endemic regions, tick bites may be common and not recognized as a risk factor for CCHFV infection. CCHFV, however, can also be transmitted to humans by direct contact with blood or tissues of viraemic animals, mainly livestock. Thus, high-risk exposure exists for people with outdoor activities (for example, soldiers, farmers, forest workers and hikers) and those with close contact to livestock (for example, shepherds, farmers, butchers, slaughterhouse workers and veterinarians)56,60,61,62,63,64. In addition, surges in CCHFV may be seen during religious festivals such as Eid-ul-Adha in which potentially CCHFV-infected livestock are transported from rural to urban areas for slaughter by potentially untrained individuals65,66. Nosocomial and intrafamily transmission have been reported through needlesticks or contact with blood and secretions from patients, putting health-care workers and close family members involved inpatient care at risk for exposure64,67,68. Transmission of CCHFV during aerosol-generating medical procedures69 or sexual contact70 may be possible. However, CCHFV transmission from human to human seems to be inefficient, and widespread outbreaks sustained via human-to-human transmission (Box 1), such as those that have occurred multiple times with EBOV (reviewed in ref. 71), have not been reported for CCHFV.
Crimean–Congo haemorrhagic fever
Serological evidence demonstrates that CCHFV can productively infect a wide variety of domestic and wild animal species from rabbits to cattle to ostriches to tortoises8,72, yet only humans develop symptomatic disease. In humans, CCHF can display various outcomes ranging from asymptomatic and mild infections to severe and sometimes lethal disease64,73. Clinical diagnosis of CCHF is difficult as initial symptoms are similar to those of other febrile illnesses (Box 1). Therefore, laboratory testing has a pivotal role in case management and outbreak control (Box 2).
CCHF presents in four distinct stages: incubation, pre-haemorrhagic, haemorrhagic and convalescence64,74,75,76 (Fig. 2). The incubation period is usually less than a week (range 1–9 days) and depends on the route of exposure and virus dose. It seems shortest following a tick bite (usually 1–3 days) and slightly longer following exposure to blood, tissue and secretions of infected livestock and humans (5–6 days). The pre-haemorrhagic stage lasts about 2–4 days on average (range 1–7 days) and begins abruptly with rather nonspecific symptoms including fever (39–41 °C), headache, myalgia, dizziness, neck pain and stiffness, backache, headache, sore eyes and photophobia74. This may be accompanied by sore throat, abdominal pain, nausea, vomiting and diarrhoea63,74. Hyperaemia of the face, neck and chest, congested sclera and conjunctivitis, and jaundice may also be noticed64. In severe cases, changes in mood and sensory perception have been reported. Somnolence may replace agitation77,78. Hepatomegaly and splenomegaly may also be present63.
The clinical progression of Crimean–Congo haemorrhagic fever (CCHF) presents with four distinct stages. Initial CCHF virus (CCHFV) infection is often unrecognized following exposure via tick bites or animal husbandry (part a), but transmission may also occur via nosocomial or intrafamily transmission during the care of sick patients. After infection, the incubation period (part a) may be as short as 1–3 days depending on the route of exposure. After the incubation period, infected humans may progress to a pre-haemorrhagic stage characterized by nonspecific symptoms such as fever, malaise, myalgia and nausea (part b). This pre-haemorrhagic phase of disease can then rapidly progress to haemorrhagic disease in the first week after infection (part c). This phase of disease is characterized by uncontrolled bleeding, liver damage, inflammatory immune responses and, in severe cases, disseminated intravascular coagulation, shock and death. In patients who survive, recovery begins 10–14 days after infection and is associated with return to normal of blood chemistry and haematology and development of anti-CCHFV immunity (part d). Long-term sequelae following CCHFV infection are poorly studied.
The haemorrhagic stage is usually short (approximately 2–3 days) but can be prolonged up to 2 weeks74. Haemorrhagic manifestations range from petechia to extended ecchymoses on mucous membranes and skin, a finding particularly pronounced with CCHF compared with other viral haemorrhagic fevers (VHFs). Epistaxis, melena, haematemesis, haematuria and haemoptysis are common as is bleeding from injection sites74,77. Bleeding has occasionally been reported from other sites such as the vagina, uterus and brain79,80,81. Haematology and blood chemistry commonly show thrombocytopenia, leukopenia and elevated levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase and creatine phosphokinase74,78,82,83,84 along with elevated levels of inflammatory cytokines85,86. Coagulation may be affected, with prolonged prothrombin and activated partial thromboplastin times accompanied by a decrease in fibrinogen levels and an increase in the levels of fibrinogen degradation products74,78,82,84. The haemorrhagic stage is pronounced in severe cases, with rapid progression to disseminated intravascular coagulation, overt bleeding, kidney, liver or pulmonary failure, and shock74,87,88,89. If lethal, death usually occurs in the second week of illness.
In survivors, convalescence generally begins around 9–10 days post-onset of illness (range 9–20 days) and is associated with a return to normal for laboratory parameters74,75,90. This stage can be prolonged and may be associated with hypotension, tachycardia or bradycardia, polyneuritis, breathing issues, xerostomia, vision and hearing deficiencies, hair loss and memory loss among others73. There is no reliable evidence for relapse or a biphasic course of the disease; however, sequelae have not been studied well enough to determine long-term complications. Survivors typically develop humoral and cellular immunity against CCHFV91,92.
Correlates of disease outcome
Viraemia has prognostic significance for the outcome of a CCHFV infection. Patients with titres exceeding 109 genome copies per millilitre of plasma are more likely to have lethal disease, and mean values for fatal cases are >1,000-fold higher than those of patients who survive86,93,94. Along with high viraemia, early clinical laboratory criteria (up to 5 days after onset) that may predict fatal outcome are thrombocytopenia (≤150,000 platelets per microlitre), elevated AST and ALT levels and elevated levels of pro-inflammatory cytokines74,77,83,84,85,87,95. In addition, overt disseminated intravascular coagulation, haematemesis, melena and somnolence were associated with fatal outcomes77,84. Scoring systems that comprehensively evaluate patients for multiple risk factors have been developed and are accurate predictors of death77,84. Early host antibody responses may also be a predictor of disease outcome. Antibody responses to CCHFV may be rapid, with CCHFV-specific IgM detectable as soon as 2–3 days after symptom onset and CCHFV-specific IgG within 5–6 days96,97,98. Clearance of viraemia correlates with early IgM responses96, whereas in fatal cases, there is little evidence of an antibody response against CCHFV91,97, suggesting that a failure to mount anti-CCHFV humoral immunity may result in lethal outcomes.
The contributions of viral determinants to disease severity are unknown and it is unclear whether the genetic diversity of CCHFV contributes to the varied case fatality rates reported throughout endemic regions. A highly divergent lineage of CCHFV first recognized in Greece99 may account for high seroprevalence without associated clinical cases in this region, suggestive of reduced human pathogenic potential100,101. Disease severity is probably also a function of route of exposure, amount of inoculating virus and level of public health resources available to treat CCHFV infections. Host genetics may also contribute to disease outcome102,103,104,105,106,107. Subclinical infections with CCHFV are probably widely underappreciated, and better recognition of milder disease cases may alter case fatality rates100,108.
Insights into CCHF from animal models
Rodent models
Although initially used mainly for viral propagation, suckling mice exhibit lethal disease on infection with CCHFV and have been used to evaluate efficacy of antivirals and monoclonal antibodies33,109. More recently, adult mice deficient in type I interferon through genetic deficiency in the IFNα receptor (Ifnar−/−), transient suppression of IFNAR signalling or genetic deficiency in signal transducer and activator of transcription 1 (Stat1−/−) have been used as models of lethal CCHFV21,110,111. Hamsters deficient in STAT2 are also susceptible to lethal CCHFV infection112. In these models, disease is typically associated with uncontrolled viral replication, inflammatory immune responses, liver pathology and eventually death21,110,111,112,113. Lethal disease in these rodent models has similar correlates to lethal disease in humans, suggesting that similar disease mechanisms may result in lethal outcomes in infected mice, hamsters and human patients. These models have been valuable tools for preclinical evaluation of antivirals112,114,115,116, monoclonal antibodies40,117, vaccines118 and host-directed therapies119.
Mouse models have also provided valuable insight into CCHFV pathogenesis. In mice, hepatocytes and endothelial cells are targets of CCHFV infection21,110,114,120, and viral replication in these tissues may account for the liver damage and vascular dysfunction seen in CCHF cases. Infection of monocytes by CCHFV may also contribute to disease progression21,120,121. Infection of innate immune cells such as monocytes and macrophages by EBOV leads to a dysregulated and overwhelming inflammatory response (reviewed in ref. 122), a central feature of several VHFs123. Type I interferon-deficient mice infected with CCHFV exhibit many features of a dysregulated inflammatory immune response110,119,120, suggesting that mechanisms of immunopathogenesis in CCHF are similar to those seen in other VHFs. Transient suppression of type I interferon signalling through administration of the IFNAR-blocking antibody MAR1-5A3 has been used to investigate CCHFV infection in knockout mice to evaluate the host contribution to disease. Using this transient suppression, CCHFV-infected mice deficient in adaptive immunity (Rag2−/−) or perforin showed severe liver pathology, demonstrating that liver damage occurred in the absence of cytotoxic T cells21. Instead, activation of the tumour necrosis factor (TNF) superfamily and extensive apoptosis in infected livers suggest that liver pathology may occur through induction of apoptosis in infected and uninfected bystander cells21. Further studies showed that CCHFV infection of mice deficient in mitochondrial antiviral signalling protein (MAVS) and treated with the IFNAR-blocking antibody were protected from disease, suggesting that in the absence of type I interferon, MAVS signalling may contribute to poor outcomes119. In addition, in this model, blockade of TNF through neutralizing antibody treatment could protect against death119. Together, these data suggest that host inflammatory responses contribute to morbidity and mortality on infection with CCHFV. In a mouse model recapitulating the convalescent phase of disease120, depletion of CD4+ and/or CD8+ T cells or blockade of IFNγ signalling resulted in decreased survival, suggesting that cellular immunity and type II interferon exert control on CCHFV infection124. Furthermore, humanized mice (NSG-SGM3) engrafted with human stem cells developed CCHFV strain-specific disease outcomes ranging from mild, self-limiting disease to progressive lethal disease125. Fatal disease was associated with viral replication in glial cells and severe neurological disease125. This model may be useful for studying the potential neurological involvement of CCHFV.
The conversion of an asymptomatic infection with CCHFV in immunocompetent mice to a rapidly lethal infection in type I interferon-deficient mice demonstrates that type I interferon is a key restriction factor for CCHFV in the mammalian host. How the host senses CCHFV and initiates the innate immune response in vivo is unclear. Examining how innate immunity controls CCHFV and how CCHFV antagonizes innate immunity to cause disease in humans is difficult in type I interferon-deficient models. Furthermore, although type I interferon deficiency results in profound defects in innate immunity, this deficiency probably also extends to adaptive immunity (reviewed in refs. 126,127), confounding studies of both innate and adaptive immunity to CCHFV. Recently, a mouse-adapted strain of CCHFV was isolated that is able to cause disease in immunocompetent wild type laboratory strains of mice43. Infection of mice with this mouse-adapted strain of CCHFV resulted in inflammatory cytokine production, high viral loads in multiple tissues, pathology in the liver and spleen, and convalescence was associated with robust humoral and cellular immunity43. Studies in Ifnar−/− and Rag1−/− mice demonstrated that both type I interferon and adaptive immune responses exert control of CCHFV in this model43, enabling investigations into how host immune responses control CCHFV. Sequencing of this virus identified five coding mutations: two in the viral NP with one also mutating the viral NSs, one in the NSm and two in the L protein43. Accumulation of mutations in these viral proteins suggests that these proteins are involved in CCHFV pathogenesis in immunocompetent hosts. Unexpectedly, a sex-linked bias in disease severity was observed, with female mice largely resistant to severe disease. The more severe disease observed in male mice was associated with similar correlates of poor outcome in human CCHF cases such as greater inflammatory cytokine production, prolonged viraemia and greater tissue pathology43. Although sex-linked differences have been occasionally reported for CCHFV128,129,130,131, further studies that account for cultural practices that place men at greater risk for exposure to CCHFV61 will be needed to determine whether similar sex-linked differences in disease outcome are present in infected humans.
Non-human primate models
In addition to rodent models, non-human primate (NHP) models of CCHF have been developed. Cynomolgus macaques infected with CCHFV recapitulate many aspects of human CCHFV, including varied disease outcome. In an initial report, infection of cynomolgus macaques with a human clinical isolate, CCHFV strain Hoti, resulted in severe disease in all animals infected via the intravenous or combined intravenous and subcutaneous routes132. Four of eight animals across these groups were euthanized by day seven post-infection because they had reached humane end point criteria132. Severe disease was associated with liver pathology, inflammatory cytokines, high viral loads and coagulation disorders, similar hallmarks to severe human disease132.
However, continued reports on the model have demonstrated more variable disease outcomes. In studies evaluating the antiviral favipiravir in CCHFV-infected macaques, only one of eight animals in the placebo group reached euthanasia criteria by day eight and disease was generally moderate in the remaining placebo-treated animals133. Similarly, cynomolgus macaques infected with strain Hoti or another human clinical isolate strain, Afghan-09, developed mild-to-moderate disease characterized mainly by transient viraemia and thrombocytopenia, and no animals reached euthanasia criteria134. In a separate study evaluating infection with the same strains, more severe disease was reported, with animals developing clinical disease such as fever, viraemia, increased liver enzymes, thrombocytopenia and occasional rash and vaginal bleeding135. Again, no animals reached euthanasia criteria135. Interestingly, evidence of CCHFV persistence was found in the testes and latent tuberculosis granulomas of some CCHFV-infected macaques135, suggesting that CCHFV may persist in immune-privileged sites.
It is currently unclear what accounts for the disease variability, but the outbred genetics of cynomolgus macaques, differences in virus strains used and institutional variation in euthanasia criteria may account for variable disease and ultimate outcome. Nevertheless, the cynomolgus macaque model accurately recapitulates many aspects of human disease and represents an essential model for preclinical evaluation of anti-CCHFV therapeutics and vaccines.
Tick models
To date, animal models have overwhelmingly used needle-delivered CCHFV and omitted the contribution of the tick and tick-derived factors to CCHFV pathogenesis4. Like many other arthropod-borne viruses, CCHFV must circulate and be maintained in both tick and vertebrate hosts. This life cycle places constraints on viral evolution (reviewed in ref. 136) and may affect virulence137. Although models of tick feeding in high containment have been developed42, much remains unknown about the role and contribution of the tick to CCHF and CCHFV pathogenesis.
Treatments for CCHF
Therapeutic options for CCHFV remain limited and most have focused on interfering with viral replication or modulating the host response to the infection (Table 1). Although many candidates have shown promising preclinical data, clinical efficacy data for most remain limited.
Antivirals
To date, the nucleoside analogue ribavirin is the only direct-acting antiviral that has been widely used clinically in patients with CCHF. However, the use of ribavirin is controversial, with continued debate about whether treatment improves outcome63,77,138,139,140,141,142,143,144. Systematic meta-analyses indicate that the data supporting the efficacy of ribavirin against CCHFV are poor owing to confounding factors in reported data sets144,145, and any benefit probably requires early treatment146. Animal studies have also shown conflicting efficacy of ribavirin against CCHFV infection. Although ribavirin was effective in lethally infected Stat1−/− mice110 and Stat2−/− hamsters112, two studies in lethally infected Ifnar−/− mice showed no protection against death even with prompt treatment114,116. Cumulatively, the data from humans infected with CCHFV and from animal models suggest that ribavirin has poor efficacy against CCHFV and that any benefit probably requires prompt treatment following known exposure. This is difficult to achieve outside recognized laboratory or health-care exposure.
In contrast to ribavirin, favipiravir or a derivative (H44) showed significant protective effects in lethally infected mice114,116,147, preventing death and significantly reducing viral loads in key target tissues of CCHFV. Favipiravir or H44 treatment could even be initiated days after infection114,147, including when mice were exhibiting advanced signs of disease, and still provide significant protective effects116. These data suggest that favipiravir and related compounds may be effective in patients presenting to health-care systems with advanced CCHF. Interestingly, lethal recrudescent CCHFV infection was observed weeks after cessation of favipiravir treatment in infected mice116, suggesting that early favipiravir treatment may not completely control the virus. Favipiravir was also effective in CCHFV-infected cynomolgus macaques, reducing viraemia and viral burden in several tissues133. Although favipiravir has shown promise in preclinical animal models, efficacy data in humans infected with CCHFV is limited148 and clinical trials are needed to determine whether favipiravir can improve CCHF patient outcomes. 2′-Deoxy-2′-fluorocytidine has also shown promising results in vitro115, suggesting that this may be another effective antiviral against CCHFV. Molnupiravir, recently used to treat SARS-CoV-2 infection in humans149, exhibits efficacy against CCHFV in vitro with similar inhibitory concentrations as favipiravir147. Nevertheless, molnupiravir failed to protect against CCHFV infection in lethally infected mice even when treatment was started before infection147.
Although ribavirin, favipiravir and 2′-deoxy-2′-fluorocytidine are all thought to exert antiviral activity through catastrophic mutagenesis or inhibition of the viral replicase150, additional small molecules acting through distinct mechanisms have been reported effective against CCHFV in vitro151. TH3289, a compound with broad antiviral activity in vitro, has been shown to suppress CCHFV replication, probably by modulating interactions between viral proteins and cellular chaperone proteins151. Blockade of the catalytic activity of the CCHFV OTU domain with a synthetic ubiquitin variant was able to block CCHFV replication in vitro through interference with viral RNA synthesis50. However, further validation of these potential antivirals against CCHFV in vivo is needed.
Antibody-based therapies
In addition to small molecules, antibody-based therapies have also been evaluated for treatment of CCHFV (Table 1). Although large-scale trials have not been performed, limited evidence suggests that administration of plasma or antibodies from survivors of CCHF can confer a benefit in seriously ill patients (reviewed in refs. 152,153). However, the scalability of convalescent plasma treatments is limited, and continued research has shown efficacy of mouse and human monoclonal antibodies against CCHFV in lethally infected mice. Evaluation of a panel of mouse monoclonal antibodies to the viral GPC showed that several neutralizing and non-neutralizing antibodies could protect lethally infected neonatal mice33. However, some antibodies that showed efficacy in the neonatal model failed to protect lethally infected adult type I interferon-deficient mice40. Potently neutralizing antibodies derived from human CCHF survivors failed to protect lethally infected mice in a post-exposure setting, although a hybrid bicistronic antibody was able to protect 80% of mice when administered 24 h post-infection117. As protection was incomplete even when mice were treated with a large amount (1 mg) of this antibody just 24 h post-infection, further studies are needed to define the therapeutic window of neutralizing antibodies for treatment of CCHF. Although the CCHFV GPC is the most diverse segment, a subset of antibodies was found to exhibit broad neutralization activity, demonstrating that there are conserved neutralizing epitopes across divergent strains of CCHFV154. Interestingly, antibody-mediated protection against CCHFV does not require neutralizing activity, and several antibodies targeting non-neutralizing epitopes in the CCHFV GP38 protein were found to be protective in animal models33,40. The protective efficacy of the GP38-targeting monoclonal antibody was found to require complement activity, suggesting that antibody effector functions such as complement-mediated lysis or viral opsonization and phagocytosis may be critical for antibody-mediated protection against CCHFV40.
Anti-inflammatory drugs
Severe CCHF, like many haemorrhagic fevers, involves a dysregulated inflammatory response and cytokine storms leading to substantial immunopathology. Thus, limited attempts have been made to use anti-inflammatory drugs in patients with CCHF to suppress the hyperinflammatory host response. In a comparative study of patients with confirmed CCHF, high-dose methylprednisolone with ribavirin improved outcomes compared with ribavirin alone155. Corticosteroids also seemed to have a benefit in severely ill patients84. However, the cohort size of these studies is limited. A recent study in lethally infected type I interferon-blockaded mice showed that infection of mice lacking the TNF receptor or treatment with an antibody to block TNF signalling could protect against lethal disease119. The availability of clinically approved TNF therapeutics156 and therapeutics against other host cytokines157 may warrant evaluation of this approach to treat CCHF.
Prevention and vaccines
Although antivirals and antibody-based therapies for CCHFV have shown promise in preclinical models, the utility of these treatments is limited to well-developed health-care systems with the ability to rapidly recognize and diagnose CCHFV infections, access to the drugs and ability to promptly begin treatment. Therapies for patients in countries with limited health-care resources or presenting to health-care systems when exhibiting advanced disease are likely to remain limited. Consequently, public health education to prevent exposure to CCHFV and vaccines are critically needed to address the public health threat of CCHFV infections in areas with limited access to health care.
Prevention
Preventing CCHFV infection involves addressing the many risk factors for CCHFV exposure (Fig. 3). For farmers, wearing appropriate clothing such as long sleeves and pants (Fig. 3a), reducing activities in tick-infested areas and use of integrated pest management strategies (Fig. 3b) to reduce tick populations in the farm environment can minimize risk of CCHFV infection via tick bites (reviewed in ref. 158). Furthermore, using protective equipment when slaughtering tick-invested livestock either in backyard slaughter processes or in abattoirs may reduce exposure via contaminated animal products (Fig. 3a). In the health-care setting, personal protective equipment is essential to prevent transmission during care of patients infected with CCHFV67,159 (Fig. 3a). Educational campaigns to inform people in endemic areas of the risk factors for CCHF (such as tick bites and workplace dangers) may prompt at-risk populations to reduce their risk of exposure and to recognize and report early symptoms of CCHF (Fig. 3d). Quarantine of livestock potentially carrying CCHFV or CCHFV-infected ticks before transport or slaughter may also prevent exposure and limit the introduction of CCHFV into new areas (Fig. 3e).
Prevention of Crimean–Congo haemorrhagic fever (CCHF) requires a multifactorial approach addressing public and veterinary health. a, Farm workers should wear long sleeves and pants to limit tick bites. Transmission of CCHF in the health-care and abattoir settings can be limited by wearing of proper personal protective equipment (PPE). Standard barrier PPE such as a laboratory gown, gloves, a face shield and a mask can limit exposure of health-care personnel to contaminated bodily fluids. Abattoir workers can be similarly protected by wearing PPE such as gowns and gloves and receiving proper training on how to butcher animals. b, Control of the tick vector is also important and may include deployment of acaracides to control tick populations on the farm and on livestock. c, Vaccines are also critically needed to prevent disease on CCHF virus (CCHFV) infection and could also be deployed in livestock populations to interrupt the CCHFV life cycle. d, Public health-directed educational efforts can help to inform at-risk populations to reduce activities that expose them to CCHFV-infected ticks or livestock, to recognize the risk of tick bites in transmission of CCHFV and to promptly recognize the early symptoms of CCHFV. e, Inspection and quarantine of animals moving from CCHFV-endemic areas can reduce exposure of humans to CCHFV-infected ticks and prevent introduction of CCHFV to new areas.
Vaccines
To date, multiple vaccine platforms have been evaluated in animal models for CCHFV such as inactivated virus preparations160, subunit vaccines161, VLPs162,163, recombinant live-attenuated viruses164,165, replication-deficient viral-vectored vaccines166 and nucleic acid-based vaccines167,168,169,170,171 (Fig. 4a), many with promising efficacy. These vaccine approaches to CCHFV have been extensively reviewed elsewhere118,172. However, the vaccine landscape for CCHFV is complex: vaccine-induced neutralizing antibodies seem neither necessary nor sufficient for protection, several viral antigens can confer protection, and yet the same viral antigens expressed from different vaccine platforms can confer distinctly different levels of efficacy.
Multiple vaccine platforms for Crimean–Congo haemorrhagic fever virus (CCHFV) have reached various stages of preclinical testing and include nucleic acid-based vaccines such as plasmid DNA and mRNA, inactivated virus preparations, live-attenuated vaccines (LAVs) such as recombinant vesicular stomatitis virus, virally vectored vaccines such as adenovirus-vectored vaccines, virus-like particles (VLPs) and subunit vaccines with purified viral proteins (part a). The viral antigen expressed by these vaccines is likely to be important (part b), with protective efficacy demonstrated by vaccines expressing nucleoprotein (NP) and/or the glycoprotein precursor (GPC). Importantly, the correlates of protection for vaccines against CCHFV are unknown (part c) and neutralizing antibody is dispensable. Thus, vaccine-mediated protection may require effector functions such as viral opsonization, complement activation or antibody-dependent cellular cytotoxicity by natural killer (NK) cells. In studies demonstrating the protective efficacy of antibodies to the CCHFV NP alone, it remains unclear how antibodies to NP can protect. The role of cellular immunity such as cytotoxic T cell-mediated cytotoxicity is similarly unknown. CTL, cytotoxic T lymphocyte.
Preclinical studies have shown protection with vaccines expressing the CCHFV NP, GPC or just the glycoproteins Gn and Gc (Fig. 4b), demonstrating that there are multiple protective epitopes within CCHFV. Within these antigens, the specific protective epitopes are unclear. Mice vaccinated with a DNA plasmid expressing just the CCHFV Gn and Gc but lacking the MLD and GP38 developed anti-CCHFV antibodies as measured by enzyme-linked immunosorbent assay (ELISA) but were not protected against lethal CCHFV infection173. Yet, immunity to GP38 alone was poorly protective173. The epitopes targeted by NP-directed immunity are unknown.
To avoid the need for developing strain-specific or region-specific vaccines, a vaccine for CCHFV must confer protection against genetically diverse strains of CCHFV. The genetic diversity of CCHFV is a concern as a DNA vaccine expressing the CCHFV GPC showed incomplete protection when mice were challenged with a heterologous strain of CCHFV173, indicating that viral escape due to the genetic diversity of CCHFV is possible. By contrast, vaccines based on replicating RNA (repRNA), vesicular stomatitis virus and VLP have shown heterologous protection162,164,171. However, many vaccines evaluated in preclinical models have only evaluated homologous challenge, and it is unclear how broad or narrow the protection is that is conferred by these vaccines. Alternatively, most of the genetic diversity of CCHFV is located within the M segment encoding for the GPC. Vaccines including the more conserved viral NP have shown significant efficacy162,163,166,168,169, and vaccines expressing just NP can confer robust protection166,169,171,174, suggesting that inclusion of NP may avoid viral escape of vaccine-conferred immunity.
The correlates of vaccine-mediated protection against CCHFV are poorly understood and seem to be less straightforward than the levels of vaccine-induced neutralizing antibodies (Fig. 4c). Notably, vaccines based on repRNA, mRNA and DNA showed significant protection in mice or NHPs in the absence of detectable neutralizing antibodies following vaccination163,168,169,171. By contrast, subunit and VLP-based vaccines induced high levels of neutralizing antibodies but failed to protect lethally infected mice161,163, and protection did not correlate with neutralizing titres in comparisons between VLP-vaccinated and DNA-vaccinated mice163. Although robust ELISA titres were measured in mice vaccinated with a DNA vaccine expressing Gn and Gc but lacking MLD and GP38, mice were not protected against lethal challenge173. Yet, in mice vaccinated with a repRNA vaccine, studies in B cell-deficient mice have demonstrated that protection required humoral immunity171. Together, these data demonstrate that although humoral immunity is important, neutralizing antibodies are neither necessary nor sufficient for vaccine-mediated protection against CCHFV. These data also demonstrate that ELISA and neutralizing titres may not be sufficient to predict vaccine efficacy and that a better understanding of the correlates of protection for CCHFV is needed, an important consideration as vaccine platforms move into clinical trials.
These vaccine data add to observations that human survivors often do not develop neutralizing antibodies until well after resolution of clinical disease91 and that non-neutralizing antibodies can be effective therapeutics in preclinical models33. Thus, the functional requirement of vaccine-induced antibodies to CCHFV for protection seems complex and may require effector functions such as complement activation or antibody-dependent cellular cytotoxicity (Fig. 4c). In studies with repRNA-vaccinated mice, robust protection against CCHFV was correlated with antibody responses against the CCHFV NP, yet this antigen was not accessible on intact infected cells in vitro171, arguing against mechanisms such as antibody-dependent cellular cytotoxicity and complement activation for vaccine-mediated control of the infection. NHPs vaccinated with a plasmid expressing just the NP had significant protection following CCHFV challenge174, demonstrating that the protective efficacy of the NP antigen is not an artefact of the mouse models. How antibodies to an internal CCHFV protein can confer protection requires further study (Fig. 4c). Similarly, the role of vaccine-induced cellular immunity in protection against CCHF is unclear. Protection required transfer of both antibody and T cells from modified vaccinia Ankara-vaccinated mice175, suggesting that both humoral and cellular immunity contribute to vaccine-mediated protection. By contrast, in mice vaccinated with repRNA, depletion of T cells at the time of viral challenge did not alter survival, suggesting that cellular immunity is dispensable for protection171. Although multiple vaccines have been shown to elicit cellular immunity in animal models118, the requirement of cellular immunity for protection remains largely untested and may be vaccine-specific. It is also unknown what effector functions are required for protection.
The vaccine platform may also be an important consideration for CCHFV. A modified vaccinia Ankara vaccine expressing the CCHFV NP failed to protect mice despite eliciting both humoral and cellular immunity176. By contrast, an adenovirus vector expressing the NP was partially protective166, whereas an mRNA or repRNA expressing the NP conferred 100% protection169,171. These data demonstrate that the same vaccine-expressed antigen can have differing protective effects depending on the vaccine platform and that varied efficacy may be due to distinct vaccine-induced immune responses. Mechanistic studies investigating how CCHFV vaccine candidates protect in animal models are urgently needed to define the immune responses and their effector functions required for protection. These data are needed to refine candidate vaccines to drive protective immunogenicity and define the immune responses that must be measured in human clinical trials to monitor and predict vaccine efficacy.
Conclusions
Despite a wide geographical range and large populations at risk for infection with CCHFV, much remains to be determined regarding the host and viral determinants of CCHFV pathogenesis. Novel functions of viral proteins probably remain to be discovered, and the development of molecular virology tools and improved small-animal models will enable further mechanistic insight into how CCHFV causes disease. For at-risk populations, preventive measures such as education, reduced tick contact, treatment of livestock to control tick infestations, livestock quarantine and protection for high-risk exposure activities need to be implemented in endemic areas. Importantly, rapid and reliable diagnostics along with efficacious vaccines and antivirals are needed to limit the burden of CCHF on patients and public health-care systems. Given that vaccines may protect against CCHFV through mechanisms other than classical antibody-mediated neutralization, investigating how vaccines protect against CCHFV will provide insight into how the host can control the infection and inform treatment strategies that promote effective immune responses while limiting immunopathology. Cumulatively, continued contributions from the fields of molecular virology, immunology, vaccinology, entomology, veterinary health and public health will be needed to address the substantial risk of CCHFV infection and disease in endemic areas.
References
Simpson, D. I. et al. Congo virus: a hitherto undescribed virus occurring in Africa. I. Human isolations–clinical notes. East Afr. Med. J. 44, 86–92 (1967).
Casals, J. Antigenic similarity between the virus causing Crimean hemorrhagic fever and Congo virus. Proc. Soc. Exp. Biol. Med. 131, 233–236 (1969).
Messina, J. P. et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans. R. Soc. Trop. Med. Hyg. 109, 503–513 (2015).
Gargili, A. et al. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies. Antivir. Res. 144, 93–119 (2017).
Lindeborg, M. et al. Migratory birds, ticks, and Crimean-Congo hemorrhagic fever virus. Emerg. Infect. Dis. 18, 2095–2097 (2012).
Rainey, T., Occi, J. L., Robbins, R. G. & Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 55, 757–759 (2018).
Grandi, G. et al. First records of adult Hyalomma marginatum and H. rufipes ticks (Acari: Ixodidae) in Sweden. Ticks Tick Borne Dis. 11, 101403 (2020).
Spengler, J. R., Bergeron, É. & Rollin, P. E. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl. Trop. Dis. 10, e0004210 (2016).
Gordon, S. W., Linthicum, K. J. & Moulton, J. R. Transmission of Crimean-Congo hemorrhagic fever virus in two species of Hyalomma ticks from infected adults to cofeeding immature forms. Am. J. Trop. Med. Hyg. 48, 576–580 (1993).
Garrison, A. R. et al. ICTV virus taxonomy profile: Nairoviridae. J. Gen. Virol. 101, 798–799 (2020).
Burt, F. J., Spencer, D. C., Leman, P. A., Patterson, B. & Swanepoel, R. Investigation of tick-borne viruses as pathogens of humans in South Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol. Infect. 116, 353–361 (1996).
Guu, T. S. Y., Zheng, W. & Tao, Y. J. in Viral Molecular Machines (eds Rossmann, M. G. & Rao, V. B.) 245–266 (Springer, 2012).
Carter, S. D. et al. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J. Virol. 86, 10914–10923 (2012).
Guo, Y. et al. Crimean–Congo hemorrhagic fever virus nucleoprotein reveals endonuclease activity in bunyaviruses. Proc. Natl Acad. Sci. USA 109, 5046–5051 (2012).
Jeeva, S., Cheng, E., Ganaie, S. S. & Mir, M. A. Crimean-Congo hemorrhagic fever virus nucleocapsid protein augments mRNA translation. J. Virol. https://doi.org/10.1128/jvi.00636-17 (2017).
Surtees, R. et al. Heat shock protein 70 family members interact with Crimean-Congo hemorrhagic fever virus and Hazara virus nucleocapsid proteins and perform a functional role in the nairovirus replication cycle. J. Virol. 90, 9305–9316 (2016).
Karlberg, H., Tan, Y.-J. & Mirazimi, A. Induction of caspase activation and cleavage of the viral nucleocapsid protein in different cell types during Crimean-Congo hemorrhagic fever virus infection. J. Biol. Chem. 286, 3227–3234 (2011).
Karlberg, H., Tan, Y.-J. & Mirazimi, A. Crimean–Congo haemorrhagic fever replication interplays with regulation mechanisms of apoptosis. J. Gen. Virol. 96, 538–546 (2015).
Barnwal, B., Karlberg, H., Mirazimi, A. & Tan, Y.-J. The non-structural protein of Crimean-Congo hemorrhagic fever virus disrupts the mitochondrial membrane potential and induces apoptosis. J. Biol. Chem. 291, 582–592 (2016).
Rodrigues, R., Paranhos-Baccalà, G., Vernet, G. & Peyrefitte, C. N. Crimean-Congo hemorrhagic fever virus-infected hepatocytes induce ER-stress and apoptosis crosstalk. PLoS ONE 7, e29712 (2012).
Lindquist, M. E. et al. Exploring Crimean-Congo hemorrhagic fever virus-induced hepatic injury using antibody-mediated type I interferon blockade in mice. J. Virol. https://doi.org/10.1128/jvi.01083-18 (2018).
Engin, A., Aydin, H., Cinar, Z., Buyuktuna, S. A. & Bakir, M. Apoptosis and its relation with clinical course in patients with Crimean-Congo hemorrhagic fever. J. Med. Virol. 91, 1385–1393 (2019).
Güven, A. S. et al. Evaluation of serum perforin, caspase-3, sFasL and M-30 levels as apoptotic markers in children with Crimean-Congo hemorrhagic fever. Pediatr. Infect. Dis. J. 34, 208–213 (2015).
Fuller, J. et al. Rescue of infectious recombinant Hazara nairovirus from cDNA reveals the nucleocapsid protein DQVD caspase cleavage motif performs an essential role other than cleavage. J. Virol. 93, e00616-19 (2019).
Wang, Y. et al. Structure of Crimean-Congo haemorraghic fever virus nucleoprotein: superhelical homo-oligomers and the role of caspase-3 cleavage. J. Virol. https://doi.org/10.1128/jvi.01627-12 (2012).
Salata, C. et al. The DEVD motif of Crimean-Congo hemorrhagic fever virus nucleoprotein is essential for viral replication in tick cells. Emerg. Microbes Infect. 7, 190 (2018).
Fuller, J. et al. Hazara nairovirus elicits differential induction of apoptosis and nucleocapsid protein cleavage in mammalian and tick cells. J. Gen. Virol. 100, 392–402 (2019).
Sanchez, A. J., Vincent, M. J. & Nichol, S. T. Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J. Virol. https://doi.org/10.1128/jvi.76.14.7263-7275.2002 (2002).
Sanchez, A. J., Vincent, M. J., Erickson, B. R. & Nichol, S. T. Crimean-Congo hemorrhagic fever virus glycoprotein precursor is cleaved by furin-like and SKI-1 proteases to generate a novel 38-kilodalton glycoprotein. J. Virol. https://doi.org/10.1128/jvi.80.1.514-525.2006 (2006).
Freitas, N. et al. The interplays between Crimean-Congo hemorrhagic fever virus (CCHFV) M segment-encoded accessory proteins and structural proteins promote virus assembly and infectivity. PLoS Pathog. 16, e1008850 (2020).
Hulswit, R. J. G., Paesen, G. C., Bowden, T. A. & Shi, X. Recent advances in Bunyavirus glycoprotein research: precursor processing, receptor binding and structure. Viruses https://doi.org/10.3390/v13020353 (2021).
Garrison, A. R. et al. Crimean-Congo hemorrhagic fever virus utilizes a clathrin- and early endosome-dependent entry pathway. Virology 444, 45–54 (2013).
Bertolotti-Ciarlet, A. et al. Cellular localization and antigenic characterization of Crimean-Congo hemorrhagic fever virus glycoproteins. J. Virol. 79, 6152–6161 (2005).
Erickson, B. R., Deyde, V., Sanchez, A. J., Vincent, M. J. & Nichol, S. T. N-linked glycosylation of Gn (but not Gc) is important for Crimean Congo hemorrhagic fever virus glycoprotein localization and transport. Virology https://doi.org/10.1016/j.virol.2006.11.023 (2007).
Vincent, M. J. et al. Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1. J. Virol. https://doi.org/10.1128/jvi.77.16.8640-8649.2003 (2003).
Bente, D. A. et al. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 100, 159–189 (2013).
Deyde, V. M., Khristova, M. L., Rollin, P. E., Ksiazek, T. G. & Nichol, S. T. Crimean-Congo hemorrhagic fever virus genomics and global diversity. J. Virol. https://doi.org/10.1128/jvi.00752-06 (2006).
Yang, Z.-y et al. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6, 886–889 (2000).
Francica, J. R., Matukonis, M. K. & Bates, P. Requirements for cell rounding and surface protein down-regulation by Ebola virus glycoprotein. Virology 383, 237–247 (2009).
Golden, J. W. et al. GP38-targeting monoclonal antibodies protect adult mice against lethal Crimean-Congo hemorrhagic fever virus infection. Sci. Adv. 5, eaaw9535 (2019).
Welch, S. R. et al. The Crimean-Congo hemorrhagic fever virus NSm protein is dispensable for growth in vitro and disease in Ifnar−/− mice. Microorganisms 8, 775 (2020).
Xia, H. et al. Transstadial transmission and long-term association of Crimean-Congo hemorrhagic fever virus in ticks shapes genome plasticity. Sci. Rep. 6, 35819 (2016).
Hawman, D. W. et al. Immunocompetent mouse model for Crimean-Congo hemorrhagic fever virus. eLife 10, e63906 (2021).
Honig, J. E., Osborne, J. C. & Nichol, S. T. Crimean-Congo hemorrhagic fever virus genome L RNA segment and encoded protein. Virology https://doi.org/10.1016/j.virol.2003.09.042 (2004).
Holm, T. et al. Biochemical and structural studies reveal differences and commonalities among cap-snatching endonucleases from segmented negative-strand RNA viruses. J. Biol. Chem. 293, 19686–19698 (2018).
Honig, J. E., Osborne, J. C. & Nichol, S. T. Crimean–Congo hemorrhagic fever virus genome L RNA segment and encoded protein. Virology 321, 29–35 (2004).
Spengler, J. R. et al. RIG-I mediates an antiviral response to Crimean-Congo hemorrhagic fever virus. J. Virol. 89, 10219–10229 (2015).
Scholte, F. E. M. et al. Crimean-Congo hemorrhagic fever virus suppresses innate immune responses via a ubiquitin and ISG15 specific protease. Cell Rep. 20, 2396–2407 (2017).
Perng, Y.-C. & Lenschow, D. J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 16, 423–439 (2018).
Scholte, F. E. M. et al. Stable occupancy of the Crimean-Congo hemorrhagic fever virus-encoded deubiquitinase blocks viral infection. mBio https://doi.org/10.1128/mBio.01065-19 (2019).
Devignot, S., Kromer, T., Mirazimi, A. & Weber, F. ISG15 overexpression compensates the defect of Crimean-Congo hemorrhagic fever virus polymerase bearing a protease-inactive ovarian tumor domain. PLoS Negl. Trop. Dis. 14, e0008610 (2020).
Capodagli, G. C., Deaton, M. K., Baker, E. A., Lumpkin, R. J. & Pegan, S. D. Diversity of ubiquitin and ISG15 specificity among nairoviruses’ viral ovarian tumor domain proteases. J. Virol. 87, 3815–3827 (2013).
Dzimianski, J. V. et al. Probing the impact of nairovirus genomic diversity on viral ovarian tumor domain protease (vOTU) structure and deubiquitinase activity. PLoS Pathog. 15, e1007515 (2019).
Leventhal, S. S., Wilson, D., Feldmann, H. & Hawman, D. W. A look into Bunyavirales genomes: functions of non-structural (NS) proteins. Viruses 13, 314 (2021).
Zhou, Z. et al. Reassortment and migration analysis of Crimean–Congo haemorrhagic fever virus. J. Gen. Virol. 94, 2536–2548 (2013).
Balinandi, S. et al. Clinical and molecular epidemiology of Crimean-Congo hemorrhagic fever in humans in Uganda, 2013–2019. Am. J. Trop. Med. Hyg. 106, 88–98 (2021).
Wampande, E. M. et al. Phylogenetic characterization of Crimean-Congo hemorrhagic fever virus detected in African blue ticks feeding on cattle in a Ugandan abattoir. Microorganisms https://doi.org/10.3390/microorganisms9020438 (2021).
Estrada-Peña, A. et al. Crimean-Congo hemorrhagic fever virus in ticks, southwestern Europe, 2010. Emerg. Infect. Dis. 18, 179–180 (2012).
Hewson, R. et al. Evidence of segment reassortment in Crimean-Congo haemorrhagic fever virus. J. Gen. Virol. https://doi.org/10.1099/vir.0.80121-0 (2004).
Midilli, K. et al. The first clinical case due to AP92 like strain of Crimean-Congo hemorrhagic fever virus and a field survey. BMC Infect. Dis. 9, 90 (2009).
Gunes, T. et al. Crimean-Congo hemorrhagic fever virus in high-risk population, Turkey. Emerg. Infect. Dis. 15, 461–464 (2009).
Msimang, V. et al. Risk factors associated with exposure to Crimean-Congo haemorrhagic fever virus in animal workers and cattle, and molecular detection in ticks, South Africa. PLoS Negl. Trop. Dis. 15, e0009384 (2021).
Elaldi, N. et al. Efficacy of oral ribavirin treatment in Crimean-Congo haemorrhagic fever: a quasi-experimental study from Turkey. J. Infect. 58, 238–244 (2009).
Hoogstraal, H. Review article: the epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 15, 307–417 (1979).
Mallhi, T. H., Khan, Y. H., Sarriff, A. & Khan, A. H. Crimean-Congo haemorrhagic fever virus and Eid-ul-Adha festival in Pakistan. Lancet Infect. Dis. 16, 1332–1333 (2016).
Leblebicioglu, H. et al. Consensus report: preventive measures for Crimean-Congo hemorrhagic fever during Eid-al-Adha festival. Int. J. Infect. Dis. 38, 9–15 (2015).
Leblebicioglu, H. et al. Healthcare-associated Crimean-Congo haemorrhagic fever in Turkey, 2002–2014: a multicentre retrospective cross-sectional study. Clin. Microbiol. Infect. 22, 387.e1–387.e4 (2016).
Khan, J. A. et al. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet 346, 472–475 (1995).
Pshenichnaya, N. Y. & Nenadskaya, S. A. Probable Crimean-Congo hemorrhagic fever virus transmission occurred after aerosol-generating medical procedures in Russia: nosocomial cluster. Int. J. Infect. Dis. 33, 120–122 (2015).
Pshenichnaya, N. Y., Sydenko, I. S., Klinovaya, E. P., Romanova, E. B. & Zhuravlev, A. S. Possible sexual transmission of Crimean-Congo hemorrhagic fever. Int. J. Infect. Dis. 45, 109–111 (2016).
Rugarabamu, S. et al. Forty-two years of responding to Ebola virus outbreaks in sub-Saharan Africa: a review. BMJ Glob. Health 5, e001955 (2020).
Kar, S. et al. Crimean-Congo hemorrhagic fever virus in tortoises and Hyalomma aegyptium ticks in East Thrace, Turkey: potential of a cryptic transmission cycle. Parasit. Vectors 13, 201 (2020).
Ergonul, O. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. https://doi.org/10.1016/s1473-3099(06)70435-2 (2006).
Swanepoel, R. et al. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 11, S794–S800 (1989).
Swanepoel, R. et al. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am. J. Trop. Med. Hyg. 36, 120–132 (1987).
Bakir, M. et al. Crimean-Congo haemorrhagic fever outbreak in Middle Anatolia: a multicentre study of clinical features and outcome measures. J. Med. Microbiol. 54, 385–389 (2005).
Ergonul, O., Celikbas, A., Baykam, N., Eren, S. & Dokuzoguz, B. Analysis of risk-factors among patients with Crimean-Congo haemorrhagic fever virus infection: severity criteria revisited. Clin. Microbiol. Infect. 12, 551–554 (2006).
Çevik, M. A. et al. Clinical and laboratory features of Crimean-Congo hemorrhagic fever: predictors of fatality. Int. J. Infect. Dis. 12, 374–379 (2008).
Kleib, A. S. et al. Crimean-Congo hemorrhagic fever with acute subdural hematoma, Mauritania, 2012. Emerg. Infect. Dis. 22, 1305–1306 (2016).
Hatami, H., Qaderi, S. & Omid, A. M. Investigation of Crimean-Congo hemorrhagic fever in patients admitted in Antani Hospital, Kabul, Afghanistan, 2017–2018. Int. J. Prev. Med. 10, 117 (2019).
Qaderi, S., Hatami, H., Omid, A. M. & Sayad, J. Vaginal bleeding as a sign of Crimean-Congo hemorrhagic fever infection: a case report. J. Med. Case Rep. 16, 76 (2022).
Bastug, A. et al. Crimean-Congo hemorrhagic fever: prognostic factors and the association of leukocyte counts with mortality. Jpn J. Infect. Dis. 69, 51–55 (2016).
Aksoy, F., Yilmaz, G., Kaya, S., Karahan, S. C. & Koksal, I. The prognostic importance of platelet indices in patients with Crimean-Congo hemorrhagic fever. Open Forum Infect. Dis. 4 (Suppl. 1), S352–S353 (2017).
Dokuzoguz, B. et al. Severity scoring index for Crimean-Congo hemorrhagic fever and the impact of ribavirin and corticosteroids on fatality. Clin. Infect. Dis. 57, 1270–1274 (2013).
Papa, A. et al. Cytokines as biomarkers of Crimean-Congo hemorrhagic fever. J. Med. Virol. 88, 21–27 (2015).
Saksida, A. et al. Interacting roles of immune mechanisms and viral load in the pathogenesis of Crimean-Congo hemorrhagic fever. Clin. Vaccin. Immunol. 17, 1086–1093 (2010).
Ergonul, O., Tuncbilek, S., Baykam, N., Celikbas, A. & Dokuzoguz, B. Evaluation of serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-α in patients with Crimean-Congo hemorrhagic fever. J. Infect. Dis. 193, 941–944 (2006).
Papa, A. et al. A case of Crimean-Congo haemorrhagic fever in Greece, June 2008. Eurosurveillance 13, 18952 (2008).
Jamil, B. et al. Crimean-Congo hemorrhagic fever: experience at a tertiary care hospital in Karachi, Pakistan. Trans. R. Soc. Trop. Med. Hyg. 99, 577–584 (2005).
Ergönül, Ö. et al. Characteristics of patients with Crimean-Congo hemorrhagic fever in a recent outbreak in Turkey and impact of oral ribavirin therapy. Clin. Infect. Dis. 39, 284–287 (2004).
Shepherd, A. J., Swanepoel, R. & Leman, P. A. Antibody response in Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 11, S801–S806 (1989).
Goedhals, D., Paweska, J. T. & Burt, F. J. Long-lived CD8+ T cell responses following Crimean-Congo haemorrhagic fever virus infection. PLoS Negl. Trop. Dis. 11, e0006149 (2017).
Çevik, M. A. et al. Viral load as a predictor of outcome in Crimean-Congo hemorrhagic fever. Clin. Infect. Dis. 45, e96–e100 (2007).
Duh, D. et al. Viral load as predictor of Crimean-Congo hemorrhagic fever outcome. Emerg. Infect. Dis. 13, 1769–1772 (2007).
Papa, A. et al. Cytokine levels in Crimean-Congo hemorrhagic fever. J. Clin. Virol. 36, 272–276 (2006).
Ergunay, K. et al. Antibody responses and viral load in patients with Crimean-Congo hemorrhagic fever: a comprehensive analysis during the early stages of the infection. Diagn. Microbiol. Infect. Dis. 79, 31–36 (2014).
Kaya, S. et al. Sequential determination of serum viral titers, virus-specific IgG antibodies, and TNF-α, IL-6, IL-10, and IFN-γ levels in patients with Crimean-Congo hemorrhagic fever. BMC Infect. Dis. 14, 416 (2014).
Burt, F. J., Leman, P. A., Abbott, J. C. & Swanepoel, R. Serodiagnosis of Crimean-Congo haemorrhagic fever. Epidemiol. Infect. 113, 551–562 (1994).
Papadopoulos, O. T. & Koptopoulos, G. Crimean-Congo hemorrhagic fever (CCHF) in Greece: isolation of the virus from Rhipicephalus bursa ticks and a preliminary serological survey. Zentbl. Bakteriol. Hyg. Abt. 1, 189–193 (1980).
Papa, A. et al. Factors associated with IgG positivity to Crimean-Congo hemorrhagic fever virus in the area with the highest seroprevalence in Greece. Ticks Tick Borne Dis. 4, 417–420 (2013).
Papa, A. et al. A novel AP92-like Crimean-Congo hemorrhagic fever virus strain, Greece. Ticks Tick Borne Dis. 5, 590–593 (2014).
Arslan, S. & Engin, A. Relationship between NF-κB1 and NF-κBIA genetic polymorphisms and Crimean-Congo hemorrhagic fever. Scand. J. Infect. Dis. 44, 138–143 (2012).
Arslan, S., Engin, A., Özbilüm, N. & Bakır, M. Toll-like receptor 7 Gln11Leu, c.4-151A/G, and +1817G/T polymorphisms in Crimean Congo hemorrhagic fever. J. Med. Virol. 87, 1090–1095 (2015).
Elaldi, N. et al. Relationship between IFNA1, IFNA5, IFNA10, and IFNA17 gene polymorphisms and Crimean-Congo hemorrhagic fever prognosis in a Turkish population range. J. Med. Virol. 88, 1159–1167 (2016).
Engin, A. et al. Toll-like receptor 8 and 9 polymorphisms in Crimean-Congo hemorrhagic fever. Microbes Infect. 12, 1071–1078 (2010).
Kızıldağ, S., Arslan, S., Özbilüm, N., Engin, A. & Bakır, M. Effect of TLR10 (2322A/G, 720A/C, and 992T/A) polymorphisms on the pathogenesis of Crimean Congo hemorrhagic fever disease. J. Med. Virol. 90, 19–25 (2018).
Akıncı, E., Bodur, H., Muşabak, U. & Sağkan, R. I. The relationship between the human leukocyte antigen system and Crimean-Congo hemorrhagic fever in the Turkish population. Int. J. Infect. Dis. 17, e1038–e1041 (2013).
Bodur, H., Akinci, E., Ascioglu, S., Öngürü, P. & Uyar, Y. Subclinical infections with Crimean-Congo hemorrhagic fever virus, Turkey. Emerg. Infect. Dis. 18, 640–642 (2012).
Tignor, G. H. & Hanham, C. A. Ribavirin efficacy in an in vivo model of Crimean-Congo hemorrhagic fever virus (CCHF) infection. Antivir. Res. 22, 309–325 (1993).
Bente, D. A. et al. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J. Virol. 84, 11089–11100 (2010).
Bereczky, S. et al. Crimean–Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J. Gen. Virol. 91, 1473–1477 (2010).
Ranadheera, C. et al. Characterization of a novel STAT 2 knock-out hamster model of Crimean-Congo hemorrhagic fever virus pathogenesis. Sci. Rep. 10, 12378 (2020).
Zivcec, M. et al. Lethal Crimean-Congo hemorrhagic fever virus infection in interferon α/β receptor knockout mice is associated with high viral loads, proinflammatory responses, and coagulopathy. J. Infect. Dis. 207, 1909–1921 (2013).
Oestereich, L. et al. Evaluation of antiviral efficacy of ribavirin, arbidol, and T-705 (favipiravir) in a mouse model for Crimean-Congo hemorrhagic fever. PLoS Negl. Trop. Dis. 8, e2804 (2014).
Welch, S. R. et al. Identification of 2′-deoxy-2′-fluorocytidine as a potent inhibitor of Crimean-Congo hemorrhagic fever virus replication using a recombinant fluorescent reporter virus. Antivir. Res. 147, 91–99 (2017).
Hawman, D. W. et al. Favipiravir (T-705) but not ribavirin is effective against two distinct strains of Crimean-Congo hemorrhagic fever virus in mice. Antivir. Res. 157, 18–26 (2018).
Fels, J. M. et al. Protective neutralizing antibodies from human survivors of Crimean-Congo hemorrhagic fever. Cell 184, 3486–3501.e21 (2021).
Tipih, T. & Burt, F. J. Crimean-Congo hemorrhagic fever virus: advances in vaccine development. Biores. Open Access 9, 137–150 (2020).
Golden, J. W. et al. The host inflammatory response contributes to disease severity in Crimean-Congo hemorrhagic fever virus infected mice. PLoS Pathog. 18, e1010485 (2022).
Hawman, D. W. et al. A Crimean-Congo hemorrhagic fever mouse model recapitulating human convalescence. J. Virol. https://doi.org/10.1128/JVI.00554-19 (2019).
Welch, S. R. et al. Fluorescent Crimean-Congo hemorrhagic fever virus illuminates tissue tropism patterns and identifies early mononuclear phagocytic cell targets in Ifnar−/− mice. PLoS Pathog. 15, e1008183 (2019).
Zampieri, C. A., Sullivan, N. J. & Nabel, G. J. Immunopathology of highly virulent pathogens: insights from Ebola virus. Nat. Immunol. 8, 1159–1164 (2007).
Basler, C. F. Molecular pathogenesis of viral hemorrhagic fever. Semin. Immunopathol. 39, 551–561 (2017).
Hawman, D. W. et al. T-cells and interferon gamma are necessary for survival following Crimean-Congo hemorrhagic fever virus infection in mice. Microorganisms https://doi.org/10.3390/microorganisms9020279 (2021).
Spengler, J. R. et al. Crimean-Congo hemorrhagic fever in humanized mice reveals glial cells as primary targets of neurological infection. J. Infect. Dis. https://doi.org/10.1093/infdis/jix215 (2017).
Zivcec, M., Spiropoulou, C. F. & Spengler, J. R. The use of mice lacking type I or both type I and type II interferon responses in research on hemorrhagic fever viruses. Part 2: vaccine efficacy studies. Antivir. Res. 174, 104702 (2020).
Clarke, E. C. & Bradfute, S. B. The use of mice lacking type I or both type I and type II interferon responses in research on hemorrhagic fever viruses. Part 1: potential effects on adaptive immunity and response to vaccination. Antivir. Res. 174, 104703 (2020).
Monsalve-Arteaga, L. et al. Seroprevalence of Crimean-Congo hemorrhagic fever in humans in the World Health Organization European region: a systematic review. PLoS Negl. Trop. Dis. 14, e0008094 (2020).
Yagci-Caglayik, D., Korukluoglu, G. & Uyar, Y. Seroprevalence and risk factors of Crimean–Congo hemorrhagic fever in selected seven provinces in Turkey. J. Med. Virol. 86, 306–314 (2014).
Bower, H. et al. Detection of Crimean-Congo haemorrhagic fever cases in a severe undifferentiated febrile illness outbreak in the Federal Republic of Sudan: a retrospective epidemiological and diagnostic cohort study. PLoS Negl. Trop. Dis. 13, e0007571 (2019).
Chinikar, S. et al. Geographical distribution and surveillance of Crimean-Congo hemorrhagic fever in Iran. Vector Borne Zoonotic Dis. 10, 705–708 (2010).
Haddock, E. et al. A cynomolgus macaque model for Crimean-Congo haemorrhagic fever. Nat. Microbiol. 3, 556–562 (2018).
Hawman, D. W. et al. Efficacy of favipiravir (T-705) against Crimean-Congo hemorrhagic fever virus infection in cynomolgus macaques. Antivir. Res. https://doi.org/10.1016/j.antiviral.2020.104858 (2020).
Cross, R. W. et al. Crimean-Congo hemorrhagic fever virus strains Hoti and Afghanistan cause viremia and mild clinical disease in cynomolgus monkeys. PLoS Negl. Trop. Dis. 14, e0008637 (2020).
Smith, D. R. et al. Persistent Crimean-Congo hemorrhagic fever virus infection in the testes and within granulomas of non-human primates with latent tuberculosis. PLoS Pathog. 15, e1008050 (2019).
Coffey, L. L., Forrester, N., Tsetsarkin, K., Vasilakis, N. & Weaver, S. C. Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiol. 8, 155–176 (2013).
Hua, B. L. et al. A single mutation in Crimean-Congo hemorrhagic fever virus discovered in ticks impairs infectivity in human cells. eLife 9, e50999 (2020).
Ergonul, O. Debate (see Elaldi N et al., Efficacy of oral ribavirin treatment in Crimean-Congo haemorrhagic fever: a quasi-experimental study from Turkey. Journal of Infection 2009; 58: 238–244): biases and misinterpretation in the assessment of the efficacy of oral ribavirin in the treatment of Crimean–Congo hemorrhagic fever. J. Infect. 59, 284–286 (2009).
Ergonul, O. Evidence supports ribavirin use in Crimean-Congo hemorrhagic fever. Int. J. Infect. Dis. 29, 296 (2014).
Ceylan, B., Calica, A., Ak, O., Akkoyunlu, Y. & Turhan, V. Ribavirin is not effective against Crimean-Congo hemorrhagic fever: observations from the Turkish experience. Int. J. Infect. Dis. 17, e799–e801 (2013).
Espy, N. et al. Ribavirin had demonstrable effects on the Crimean-Congo hemorrhagic fever virus (CCHFV) population and load in a patient with CCHF infection. J. Infect. Dis. 217, 1952–1956 (2018).
Koksal, I. et al. The efficacy of ribavirin in the treatment of Crimean-Congo hemorrhagic fever in eastern Black Sea region in Turkey. J. Clin. Virol. 47, 65–68 (2010).
Tasdelen Fisgin, N., Ergonul, O., Doganci, L. & Tulek, N. The role of ribavirin in the therapy of Crimean-Congo hemorrhagic fever: early use is promising. Eur. J. Clin. Microbiol. Infect. Dis. 28, 929–933 (2009).
Ascioglu, S., Leblebicioglu, H., Vahaboglu, H. & Chan, K. A. Ribavirin for patients with Crimean–Congo haemorrhagic fever: a systematic review and meta-analysis. J. Antimicrob. Chemother. 66, 1215–1222 (2011).
Johnson, S. et al. Ribavirin for treating Crimean Congo haemorrhagic fever. Cochrane Database Syst. Rev. 6, CD012713 (2018).
Arab-Bafrani, Z. et al. Identification of the crucial parameters regarding the efficacy of ribavirin therapy in Crimean–Congo haemorrhagic fever (CCHF) patients: a systematic review and meta-analysis. J. Antimicrob. Chemother. 74, 3432–3439 (2019).
Wang, Q. et al. In vitro and in vivo efficacy of a novel nucleoside analog H44 against Crimean–Congo hemorrhagic fever virus. Antivir. Res. 199, 105273 (2022).
Büyüktuna, S. A. et al. [COVID-19 co-infection in a patient with Crimean Congo hemorrhagic fever: a case report]. Mikrobiyol. Bul. 55, 445–451 (2021).
Jayk Bernal, A. et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 386, 509–520 (2021).
Vanderlinden, E. et al. Distinct effects of T-705 (favipiravir) and ribavirin on influenza virus replication and viral RNA synthesis. Antimicrob. Agents Chemother. 60, 6679–6691 (2016).
Tampere, M. et al. Novel broad-spectrum antiviral inhibitors targeting host factors essential for replication of pathogenic RNA viruses. Viruses 12, 1423 (2020).
Fabara, S. P. et al. Crimean-Congo hemorrhagic fever beyond ribavirin: a systematic review. Cureus 13, e17842 (2021).
Keshtkar-Jahromi, M. et al. Crimean-Congo hemorrhagic fever: current and future prospects of vaccines and therapies. Antivir. Res. 90, 85–92 (2011).
Zivcec, M. et al. Identification of broadly neutralizing monoclonal antibodies against Crimean-Congo hemorrhagic fever virus. Antivir. Res. 146, 112–120 (2017).
Sharifi-Mood, B. et al. Efficacy of high-dose methylprednisolone in patients with Crimean-Congo haemorrhagic fever and severe thrombocytopenia. Trop. Doct. 43, 49–53 (2013).
Li, P., Zheng, Y. & Chen, X. Drugs for autoimmune inflammatory diseases: from small molecule compounds to anti-TNF biologics. Front. Pharmacol. https://doi.org/10.3389/fphar.2017.00460 (2017).
Kopf, M., Bachmann, M. F. & Marsland, B. J. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug Discov. 9, 703–718 (2010).
Kumar, B., Manjunathachar, H. V. & Ghosh, S. A review on Hyalomma species infestations on human and animals and progress on management strategies. Heliyon 6, e05675 (2020).
Gozel, M. G. et al. Recommended precaution procedures protect healthcare workers from Crimean-Congo hemorrhagic fever virus. Int. J. Infect. Dis. 17, e1046–e1050 (2013).
Pavel, S. T. I., Yetiskin, H., Kalkan, A. & Ozdarendeli, A. Evaluation of the cell culture based and the mouse brain derived inactivated vaccines against Crimean-Congo hemorrhagic fever virus in transiently immune-suppressed (IS) mouse model. PLoS Negl. Trop. Dis. 14, e0008834 (2020).
Kortekaas, J. et al. Crimean-Congo hemorrhagic fever virus subunit vaccines induce high levels of neutralizing antibodies but no protection in STAT1 knockout mice. Vector Borne Zoonotic Dis. 15, 759–764 (2015).
Scholte, F. E. M. et al. Single-dose replicon particle vaccine provides complete protection against Crimean-Congo hemorrhagic fever virus in mice. Emerg. Microbes Infect. 8, 575–578 (2019).
Hinkula, J. et al. Immunization with DNA plasmids coding for Crimean-Congo hemorrhagic fever virus capsid and envelope proteins and/or virus-like particles induces protection and survival in challenged mice. J. Virol. 91, e02076-16 (2017).
Rodriguez, S. E. et al. Vesicular stomatitis virus-based vaccine protects mice against Crimean-Congo hemorrhagic fever. Sci. Rep. 9, 7755 (2019).
Buttigieg, K. R. et al. A novel vaccine against Crimean-Congo haemorrhagic fever protects 100% of animals against lethal challenge in a mouse model. PLoS ONE 9, e91516 (2014).
Zivcec, M., Safronetz, D., Scott, D. P., Robertson, S. & Feldmann, H. Nucleocapsid protein-based vaccine provides protection in mice against lethal Crimean-Congo hemorrhagic fever virus challenge. PLoS Negl. Trop. Dis. 12, e0006628 (2018).
Garrison, A. R. et al. A DNA vaccine for Crimean-Congo hemorrhagic fever protects against disease and death in two lethal mouse models. PLoS Negl. Trop. Dis. 11, e0005908 (2017).
Hawman, D. W. et al. A DNA-based vaccine protects against Crimean-Congo haemorrhagic fever virus disease in a cynomolgus macaque model. Nat. Microbiol. 6, 187–195 (2021).
Appelberg, S. et al. Nucleoside-modified mRNA vaccines protect IFNAR−/− mice against Crimean Congo hemorrhagic fever virus infection. J. Virol. https://doi.org/10.1128/jvi.01568-21 (2021).
Tipih, T., Heise, M. & Burt, F. J. Immunogenicity of a DNA-based Sindbis replicon expressing Crimean–Congo hemorrhagic fever virus nucleoprotein. Vaccines https://doi.org/10.3390/vaccines9121491 (2021).
Leventhal, S. S. et al. Replicating RNA vaccination elicits an unexpected immune response that efficiently protects mice against lethal Crimean-Congo hemorrhagic fever virus challenge. eBioMedicine 82, 104188 (2022).
Dowall, S. D., Carroll, M. W. & Hewson, R. Development of vaccines against Crimean-Congo haemorrhagic fever virus. Vaccine 35, 6015–6023 (2017).
Suschak, J. J. et al. A CCHFV DNA vaccine protects against heterologous challenge and establishes GP38 as immunorelevant in mice. npj Vaccines 6, 31 (2021).
Hawman, D. W. et al. Accelerated DNA vaccine regimen provides protection against Crimean-Congo hemorrhagic fever virus challenge in a macaque model. Mol. Ther. https://doi.org/10.1016/j.ymthe.2022.09.016 (2023).
Dowall, S. D. et al. Protective effects of a modified vaccinia Ankara-based vaccine candidate against Crimean-Congo haemorrhagic fever virus require both cellular and humoral responses. PLoS ONE 11, e0156637 (2016).
Dowall, S. D. et al. A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum. Vaccin. Immunother. 12, 519–527 (2016).
Gruber, C. E. M. et al. Geographical variability affects CCHFV detection by RT-PCR: a tool for in-silico evaluation of molecular assays. Viruses https://doi.org/10.3390/v11100953 (2019).
Shrivastava, N. et al. Development of double antibody sandwich ELISA as potential diagnostic tool for rapid detection of Crimean-Congo hemorrhagic fever virus. Sci. Rep. 11, 14699 (2021).
Acknowledgements
This work was supported by the Intramural Research Program, NIAID/NIH. The authors thank R. Kissinger (the Rocky Mountain Laboratories Visual Medical Arts department) for preparing figure drafts and P. Stewart (NIAID/NIH) for critical reading of the manuscript. Funders had no role in writing of the manuscript, conclusions or recommendations.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
D.W.H. and H.F. are listed as inventors on US patent application number 63/365,015 ‘Replicating RNA vaccine for Crimean–Congo hemorrhagic fever virus’.
Peer review
Peer review information
Nature Reviews Microbiology thanks Éric Bergeron, who co-reviewed with Elif Karaaslan, and Aura Garrison for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hawman, D.W., Feldmann, H. Crimean–Congo haemorrhagic fever virus. Nat Rev Microbiol 21, 463–477 (2023). https://doi.org/10.1038/s41579-023-00871-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-023-00871-9
This article is cited by
-
LDLR is an entry receptor for Crimean-Congo hemorrhagic fever virus
Cell Research (2024)
-
CCHFV entry via LDLR keeps it ‘ticking’?
Cell Research (2024)
-
Molecular identification of Crimean-Congo haemorrhagic fever virus in Hyalomma rufipes and Amblyomma variegatum in the Upper East Region of Ghana
Archives of Virology (2024)
-
The new wave of Congo virus in Pakistan: emerging threat
Tropical Medicine and Health (2023)