Abstract
A green advanced oxidation (AO) strategy to destroy dye pollutants and remove them from aquatic environments is to utilize sunlight and employ thin-film semiconducting photo-reactors. In this light-driving AO method, besides the type of dye and semiconductor material, attention to the electrostatic interactions between dye and electrode is of great importance. In this paper, a couple of nanostructured, narrow-bandgap, semiconducting photoelectrodes, i.e., hematite (n-type) and pyrite (p-type) were fabricated electrochemically and employed for the elimination of two cationic (malachite green) and anionic (methyl orange) dyes inside a single-electrode photoreactor. It was shown that without applying a faradic potential bias and consuming electricity or changing the pH of medium, the decolorization ability of the fabricated photoelectrodes can be substantially boosted just by their connection to an electrostatic (non-faradic) bias source. Regardless of the type of photoelectrode, in the case of cationic dye, the application of a negative polarity and for the anionic dye, a positive polarity remarkably promoted the reactor activity. These observations were discussed in detail through electrostatic attractive/repulsive forces between ionic dyes and charged photoelectrodes, and finally a mechanistic perspective was put forward for the photo-electrostatic dye removal process.
Similar content being viewed by others
Introduction
Ionic dyes are widely-used water-soluble ecologically harmful chemicals which their presence as a residue in untreated effluents of textile industries endanger aquatic ecosystems by changing their oxygen demand (BOD/COD), disrupting underwater photosynthesis, causing genetic mutations, spoiling landscapes, etc.1,2,3. One of the green sustainable methods to destroy these organic pollutants is the application of semiconducting electrodes in a light-induced oxidizing/reducing reactor4,5. In this solar-assisted remedy of the environment, powdery semiconductors provide a larger surface area and thus exhibit a higher activity. But the separation of these light-harnessing materials from the reaction solution is often a trouble of the system at the end of process6. This problem, however, can be practically solved by integrating the individual particles (powdery photocatalyst) and fabricating a thin-film (porous) photoelectrode7. The application of semiconducting photoelectrodes for degradation of dye pollutants can be conducted in two ways, called PEC (photoelectrocatalysis or photoelectrochemical; two/three-electrode) and single-electrode (wireless) approaches8,9,10. In this article, the authors have focused on the second route, because it is the simplest form of a PEC, without needing to employ a counter/reference electrode (CE/RE) and consuming electricity.
To improve the degradation performance of AO systems, although some advances have been achieved in recent years, and strategies such as coupled reactors and synthesis of new semiconductor materials have been successfully employed for photoelectrocatalytic degradation of dye pollutants11,12,13, in the present article, the authors are going to introduce a new alternative for effortless boosting the reactor activity. Here we answer this key question that “without changing the pH of medium, type of semiconductor or consuming electricity, how can we boost the degradation/decolorization ability of a fabricated photoelectrode?” Before answering this question, we should clarify that in a PEC system by applying an electrical potential to the photoelectrode (V; faradic bias), electron transfer occurs at the surface of both working (WE) and counter (CE) electrodes4,5. By passing current through the PEC reactor, electrical energy (\(W = {\int} {VI{{{\mathrm{d}}}}t}\)) is consumed14. In non-faradic processes (the case of present study), however, the potential bias is electrostatically applied to the WE vs. Earth [the reference outside the reaction medium]. Due to single-electrode nature of the setup (absence of CE/RE in the reaction medium), no faradic current passes through the cell and thus no electricity is consumed during the photo-degradation process.
In this article, the authors demonstrate for a fabricated photoelectrode, how its dye decolorization ability can be electrostatically boosted by applying a non-faradic potential bias to the photoelectrode. The investigations are systematically conducted upon two cationic and anionic dyes over a couple of positively and negatively polarized p- and n-type semiconducting photoelectrodes. Herein, through a facile electrodeposition route, we first fabricated two p and n narrow-bandgap, stable, affordable semiconducting thin-film photoelectrodes, viz. pyrite (FeS2) on Cu and hematite (α-Fe2O3) on FTO (fluorine-doped tin oxide) glass. The aforementioned photoelectrodes were employed in the proposed photo-electrostatic reactor for degradation of two widely used, water-soluble cationic (malachite green; triphenylmethyl family15) and anionic (methyl orange; azo compound16,17) dyes.
Taking notice of electrostatic interactions is not a new matter in dye removal systems18,19. Based on electrostatic attractive/repulsive forces, it has been discussed why, e.g., the decolorization ability of a system is generally enhanced for cationic dyes by raising the alkalinity but declined with decreasing pH of the medium20,21. Besides rationalizing the pH effect, a particular attention has been also paid to electrostatic interactions in introducing and synthesizing effective dye adsorbing materials. Through Coulombic forces, the superior activity of adsorbents such as reduced graphene oxide (rGO), carbon nanotube (CNT), chitosan, clay, metal organic framework, cellulose-based bio-adsorbents, sulfonate lignin-based hydrogel, and resins has been attributed to the existence of charged functional groups (adsorbing centers) in the mentioned substrates, facilitating the adsorption of particular ionic dyes to the sorbent surface22,23,24,25,26,27,28,29. Unlike the conventional approach, instead of using chemicals, altering the pH of medium, attempting to synthesize new functionalized materials, or applying a faradic bias and consuming electricity, the present article intends to introduce a new straightforward non-chemical route and unveils how we could easily boost the dye removing capacity of a given system through a facile in situ charging of the reaction substrate by means of electrostatic interactions and applying a non-faradic potential bias to the photo-electrode/reactor. This photo-electrostatic boosting strategy proposed in the present article is disparate from that of conventional two- or three-electrode PEC reactors—which work on the basis of applying a potential bias in a closed electrical circuit.
Results and discussion
Photoelectrodes: morphology and photo-response
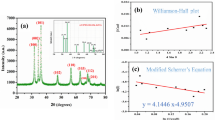
The cross-sectional FE-SEM images and surface morphology of the fabricated electrodes are shown in Fig. 1. Examination of Fig. 1 revealed that the thin-film semiconductors under consideration have a porous structure which is suitable for dye adsorption and removal from the aqueous medium30. The thickness of the semiconductor film was about 320 nm for hematite and ~430 nm for pyrite (Supplementary Fig. 1). The elemental mapping taken from the photoelectrodes confirmed the presence of the constituting atoms and their dispersion in the electrodeposited thin films. Moreover, EDS and XRD data approved the formation of pyrite and hematite on the Cu and FTO substrates, respectively (Supplementary Figs. 2 and 3).
a FeS2 thin film electrodeposited on Cu substrate (the inset is elemental mapping image of Fe (red), S (green) and Cu (blue) atoms; the scale bar is 500 nm). b Fe2O3 thin film electrodeposited on FTO glass (the inset is an elemental mapping image of Fe (red), Sn (blue), Si (green) and O (yellow) atoms; the scale bar is 500 nm). c, d are top-view of the FeS2 and Fe2O3 thin films, respectively (the scale bar is 1 µm).
The ability of semiconducting thin films to harness photons along with their band gap–determined through Tauc’s approach31 is shown in Fig. 2. This figure indicates that both semiconductors can absorb incident light in the visible region and their band gaps are 2.2 and 0.7 eV for hematite and pyrite films, respectively. The values measured here are in good agreement with those reported in the literature32,33.
To determine the conductivity type of the electrodeposited semiconducting thin films, the measurements of Mott–Schottky (MS) and light-induced OCP shift (called photogenerated potential or photovoltage in short) were performed on the fabricated photoelectrodes; see Fig. 3. The observation of a positive slope for MS line of hematite and a negative slope for pyrite clearly indicated that the fabricated thin-film electrodes had n- and p-type semiconducting nature, respectively34,35. Moreover, a negative photovoltage observed for hematite (n-type) and a positive one for pyrite (p-type) are further evidence confirming their semiconducting properties. Such a shift in OCP can be explained in terms of the semiconductor band bending direction and type of redox reaction occurring upon the photoelectrode surface14,36. For example, for n-type electrodes, as we know, their band bending is normally depicted upward and under illuminated condition, an anodic process occurs on the electrode surface35. Therefore by irradiation of photons to an n-type electrode and generation of \({{{\mathrm{e}}}}^ - /{{{\mathrm{h}}}}^ +\) pairs upon the semiconductor thin film, the photogenerated holes are consumed through an anodic process but their counterparts (i.e., photogenerated electrons) are repelled from the semiconductor surface and accumulated at the electrode terminal. Hence, the negative OCP shift of hematite is justified. For p-type electrodes, however, the band bending is plotted downward37. Therefore under light irradiation, the photogenerated electrons are consumed in a cathodic process whereas the holes are pushed toward the electrode terminal, thereby causing a positive shift in the OCP of the p-type (pyrite) electrode.
Photo-electrostatic interaction and boosting the dye removal
In order to photo-electrostatically remove dye pollutants using semiconducting photoelectrodes, like any heterogeneous catalytic process, the dye species need first to be adsorbed on the surface of semiconductor (SC) thin film. By absorption of photons and generation of electron/hole pairs under illuminated condition, a couple of redox (reduction/oxidation) phenomena occur upon the SC surface, which eventually results in the decolorization/degradation of dye pollutant in the aquatic medium38:
In addition to Eq. (2), the reported mechanistic investigations indicate that the photogenerated \({{{\mathrm{e}}}}^ - /{{{\mathrm{h}}}}^ +\) pairs can alternatively participate in the reduction reaction of O2 molecules in the aqueous medium (Eq. (3)) and water/hydroxide oxidation process as well (Eq. (4)), leading to the generation of reactive oxygen species (ROS) on the SC surface. These radical species are reactive and serve as hydrogen abstracting agents, destabilizing dye molecules and ultimately converting them to other colorless products39,40,41,42,43:
As mentioned above, to achieve the dye decolorization/degradation objective, it is necessary for the dye species to be first adsorbed on the photo-electrode/reactor surface. Since ionic dyes are charged in aqueous environments, polarity and charge of the electrode could thus affect dye removal by altering electrostatic interactions between charged electrode surface and dissolved dye species in the medium.
The ability of p- and n-type semiconducting thin films to remove the cationic and anionic dyes was scrutinized in zero bias and presence of different electrostatic biases applied to the photoelectrodes. The results are plotted in Figs. 4 and 5 for pyrite (p-type) and hematite (n-type) electrodes, respectively. The optimal biases observed for the mentioned dye-removing systems are also summarized in Table 1.
DP “decolorization percentage” data plotted for (a) cationic malachite green and (b) anionic methyl orange dyes (the error bars are standard deviation). The insets are schematic illustration of the electrostatic (attractive/repulsive) forces exerting between ionic dye and polarized/charged electrode.
Figures 4a and 5a show for the cationic malachite green, regardless of the type of photoelectrode, the reactor’s decolorization performance is generally promoted by changing the bias potential from zero toward negative values. This empirical evidence can be explained through facilitation of the adsorption process by increasing the electrostatic attractive force, exerting between the cationic dye and the negatively polarized electrode. Similarly in the case of anionic dye (Figs. 4b and 5b), the decolorization enhancement occurs at positive values (see Table 1), where the electrodes get positively charged and the interactions become attractive. Under this circumstance, the electrostatic adsorption of the anionic dye is facilitated, thereby boosting dye elimination process.
Facile improvement of the decolorization performance of the reactor by applying an appropriate electrostatic bias to the photoelectrode is an intriguing finding of this research. Due to single-electrode nature of the system (lack of counter electrode), the circuit is not complete and no external current passes through the system. This means that without consuming electricity, just by applying an electrostatic bias to the photoelectrode, the reactor’s performance increases significantly. Other interesting results which can be deduced by examining Figs. 4 and 5 are as follows:
-
(1)
At the bias extremes (±300 V), the decolorization ability of the reactor (DP) declined, reaching their limiting value, i.e., DP in the absence of electrode but presence of photon and oxygen (Supplementary Fig. 4, control experiments). In other words, by applying very large biases, the reactor behaves like a setup without photoelectrode. This behavior can be rationalized through repulsive forces exerting between dye species and electrode surface: e.g., in the case of positive bias (+300 V), owing to the strong electrostatic repulsion between the cationic dye and extremely positively charged photoelectrode, the dye species cannot be adsorbed on the photoelectrode surface (no adsorption equals the absence of electrode; the lack of adsorption means that the presence or absence of electrode does not make a difference in the system behavior and the decolorization occurs in the bulk of reaction medium, rather than on the surface of the photoelectrode). A similar argument can also be made in the case of negative-bias/anionic-dye, causing the reactor to behave like an electrode-free setup.
-
(2)
In addition to the matter of electrostatic interaction between dye species and electrode surface, it should not be forgotten that the application of the bias to the photoelectrode can also shift the semiconductor’s Fermi level and displace its conduction and valance band (CB/VB) energy levels14. This is an important fact because using it we can explain the plateau (limiting) behavior–observed in the extreme biased conditions (see #3).
-
(3)
During photo-electrostatic removal of dye species, besides dye adsorption, a set of redox (reduction/oxidation) processes occur inside the decolorization reactor (Eqs. (2)–(4)). Depending on the polarity and value of the applied bias, the ability of photogenerated \({{{\mathrm{e}}}}^ - /{{{\mathrm{h}}}}^ +\) pairs to participate in the redox processes can be altered. For example, in the case of cationic dye (Figs. 4a and 5a) under highly negatively polarized condition, owing to the accumulation of electrons on the photoelectrode, the oxidation process cannot occur on the electrode surface. During photoredox processes, since both oxidation (hole consumption) and reduction (electron consumption) reactions must synchronously occur at the same rate44, by suppressing one of them, the other and thus the overall (redox) reaction is automatically stopped. This is a reason why for the aforementioned system, despite the existence of an attractive force, the degradation of the cationic dye does not occur upon the highly negatively polarized electrode and the reactor behaves like an electrode-free setup. Similar argument can also be made about anionic dye and positive bias, stopping the electrodic degradation of dye species by suppressing its reduction counterpart due to the highly oxidative nature of the positively biased electrode.
-
(4)
In short, the application of the non-faradic bias to the photoelectrode can alter the reactor performance in terms of strengthening/weakening the electrostatic forces (exerting between ionic dye and charged surface) and facilitating/impeding the redox phenomena involved in the dye elimination process. Based on these boosting/hindering effects, we could explain the bell-shaped behavior witnessed in Figs. 4 and 5. For example, in the case of cationic dye (Figs. 4a and 5a), we mentioned earlier that by applying a negative polarity, the electrostatic attraction is boosted and by facilitating dye adsorption on the electrode surface, its removal is justified. Using this argument, we could interpret the efficiency improvement from bias zero up to the optimum values of −85 VEarth and −80 VEarth for the hematite and pyrite photoelectrodes, respectively. However, at more negative values, due to the accumulation of electrons on the electrode surface, the oxidation process becomes unfavorable, thereby diminishing the reactor performance. Similar arguments can be made for the anionic dye. The optimal biases observed in Figs. 4b and 5b (+15 and +22 VEarth) are indeed the potentials where a balance is reached between enhancing and diminishing effects of the bias on dye electrosorption19 and electron/hole consumption14.
Besides the UV–visible spectroscopic investigations, to ensure the degradation and mineralization of dye pollutants, additional tests were conducted on the dye-polluted water samples, before and after their treatment in the photo-electrostatic reactor. Table 2 shows that in line with the results of spectroscopic approach, by application of the electrostatic bias to the photoelectrode, both COD and DM percentages are increased and the reactor decolorization ability is boosted.
A mechanistic perspective and concluding remarks
In short we could say that decolorization of dye-polluted water inside a single-electrode photo-electrostatic reactor consists of two main steps: (1) adsorption of pollutant molecules onto the electrode surface and (2) annihilation/mineralization of the adsorbed molecules through redox reactions with photogenerated electron/hole pairs upon semiconductor thin film. Since the pollutant molecules under study are ionic species, their adsorption (step 1) is electrostatically facilitated by applying an appropriate bias to the photoelectrode, thereby improving DP by virtue of electrosorption19. Besides pollutant electrosorption, the application of electrostatic bias could influence on step 2 (photo-degradation of adsorbed dye) by altering the semiconductor band bending, Fermi level, charge separation and redox phenomena11,14,45. In the present photo-electrostatic dye removal system, both steps 1 and 2 are important; without considering them, the bell-shaped behavior of Figs. 4 and 5 cannot be thoroughly interpreted.
Regarding the importance of pollutant electrosorption highlighted in this article, we should also remark that in the absence of electrostatic bias, the pollutant species arrive to the semiconductor surface through a diffusion phenomenon46,47 that occurs naturally due to the existence of a concentration gradient (∇C) between pollutant molecules in the bulk solution and the molecules at the electrode surface. At low dye concentration, because the gradient is small, the diffusion and hence dye removal process occur with difficulty48. This bottleneck issue of conventional systems can be easily resolved through the present approach by applying an appropriate electrostatic bias to the photoelectrode, leading to an accelerated decolorization by forming an attractive Coulombic force between dye species and oppositely charged semiconductor surface. Based on the present study, the authors briefly conclude that:
-
Instead of the strategy of finding and fabricating new sophisticated electrodes, in practical approaches we could employ the available robust affordable electrodes (made of eco-friendly earth abundant elements) in simplified single-electrode setups and enhance their activity by applying non-faradic (electrostatic) potential biases (with proper polarity and value) to the photoelectrode.
-
Independent of the semiconductor type, the application of negative bias for cationic and positive bias for anionic dyes could promote the photoelectrode activity and improve its decolorization (degradation) performance.
-
Unlike faradic, the application of electrostatic (non-faradic) potential bias to the present single-electrode reactor is not accompanied by electricity consumption (Supplementary Fig. 5 and Supplementary Table 1); in the current photo-electrostatic approach, light is indeed the main energy source for the dye elimination process.
Methods
Photoelectrodes fabrication
To fabricate the pyrite photoelectrode, i.e., FeS2/Cu, we employed a modified version of the procedure reported in our previous study14. The electrolyte bath was an aqueous solution containing ammonium ferrous sulfate ((NH4)2Fe(SO4)2(H2O)6; 0.01 M) and sodium thiosulfate (Na2S2O3.6H2O; 0.02 M) and the pH of solution was adjusted to 3.8 using a dilute sulfuric acid. In order to prevent the oxidation of ferrous to ferric, dissolved oxygen in the medium was purged by argon gas. Using a conventional three-electrode setup (Pt counter, SCE reference, and Cu working electrodes) at room temperature, the electrodeposition of pyrite on copper substrate (1 × 3 cm2) was cathodically conducted at a constant current (400 mA cm−2; 400 s) through a three-step procedure. To achieve a film with good attachment to the substrate, at the end of each step, the loosely attached deposits were wiped with a tissue paper. Furthermore, the last step was conducted at a lower (200 mA cm−2) current density without any wiping. At the end of electrodeposition process, the electrode was washed with distilled water and ethanol, and finally heated at 200 °C for 25 min in an argon atmosphere.
For hematite (α-Fe2O3) electrodeposition on a FTO glass (1 × 3 cm2; Solaronix: 10 ohm/square), a similar three-electrode setup containing ammonium ferrous sulfate solution (0.02 M; pH = 4.8) was employed and during the process, the temperature of electrolyte was kept between 70 and 80 °C49. The electrodeposition was carried out anodically for 15 min (current density: 170 μA cm−2). After completing the electrodeposition process, the working electrode (FTO glass) was removed from the electrolyte bath, washed several times with distilled water and finally annealed under atmospheric condition at 550 °C for 5 h.
Instrumental and characterization
Field emission scanning electron micrographs (FE-SEMs) with energy dispersive X-ray (EDX) and elemental mapping analyses, X-ray diffraction (XRD) patterns, and bandgaps (Tauc’s approach) of the semiconducting photoelectrodes were obtained by employing a Mira3 TESCAN, a Philips X’Pert Pro X-ray diffractometer (λ = 1.54 Å; Cu Kα beam), and a Pharmacia Biotech Ultrospec 4000 UV–visible spectrophotometer, respectively. Open Circuit Potential (OCP) and Mott–Schottky measurements (1 kHz) as well as the electrodeposition of semiconducting thin films were carried out using an Ivium-Vertex Potentio/Galvanostat. Electrostatic (non-faradic) potential biases were applied by utilizing a high-voltage dc power source, viz. EPS 600 (Pharmacia Biotech).
Reactor setup and photo-electrostatic dye degradation tests
Degradation tests were conducted in a handmade, double-walled, temperature-controlled, tubular glass reactor filled by the aqueous dye solution (3.4 × 10−5 M; volume: 50 cc). The ionic dyes under consideration were malachite green (C52H54N4O12 (oxalate salt); Sigma-Aldrich, 90%) and methyl orange (C14H14N3NaO3S; Sigma-Aldrich, 85%). After placing the photoelectrode inside the reaction chamber, the reactor was illuminated by a Xenon light source with intensity of 100 mW cm−2 50,51. During the test, a gentle flow of oxygen gas (~40 ml/min) was blown into the reactor. To apply the electrostatic bias, we used the scheme illustrated in Fig. 6. The dye degradation experiments were performed at the same time intervals (1 h) and at the end of test, the degradation [decolorization] performance (DP) of the reactor was calculated using the following formula and UV–visible spectroscopic data of the reaction medium52,53:
where \(A_ \circ\) and A represent the absorbance of the dye solution recorded at the beginning and at the end of each test, respectively. The absorption data of the malachite green and methyl orange solutions were obtained at 615 and 460 nm, respectively (Supplementary Fig. 6). The dye degradation experiments were repeated at least three times and the mean values were calculated and reported as final data.
The percentage of dye mineralization (DM %) was obtained using Eq. (7)13:
where \({{{\mathrm{TOC}}}}_ \circ\) and \({{{\mathrm{TOC}}}}\) are total organic carbon concentrations of the dye-polluted water, recorded instrumentally using an Analytik Jena multi N/C 3100 TOC analyzer, before and after the photo-electrostatic dye degradation tests, respectively.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Al-Tohamy, R. et al. A critical review on the treatment of dye-containing wastewater: ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 231, 113160 (2022).
Nath, I., Chakraborty, J. & Verpoort, F. Degradation of environmental contaminants by topical heterogeneous photocatalysts. In Handbook of Smart Photocatalytic Materials (eds Hussain, C. M. & Mishra, A. K.) 151–182 (Elsevier, 2020).
Salleh, M. A. M., Mahmoud, D. K., Karim, W. A. W. A. & Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280, 1–13 (2011).
Liu, D. et al. Photoelectrocatalytic degradation of methylene blue using F doped TiO2 photoelectrode under visible light irradiation. Chemosphere 185, 574–581 (2017).
Zhai, C. et al. Enhanced photoelectrocatalytic performance of titanium dioxide/carbon cloth based photoelectrodes by graphene modification under visible-light irradiation. J. Hazard. Mater. 263, 291–298 (2013).
Abdelsamad, A. M., Gad-Allah, T. A., Mahmoud, F. A. & Badawy, M. I. Enhanced photocatalytic degradation of textile wastewater using Ag/ZnO thin films. J. Water Process Eng. 25, 88–95 (2018).
Andronic, L. & Duta, A. TiO2 thin films for dyes photodegradation. Thin Solid Films 515, 6294–6297 (2007).
Miao, H., Yang, J., Peng, G., Li, H. & Zhu, Y. Enhancement of the degradation ability for organic pollutants via the synergistic effect of photoelectrocatalysis on a self-assembled perylene diimide (SA-PDI) thin film. Sci. Bull. 64, 896–903 (2019).
Zarei, E. & Ojani, R. Fundamentals and some applications of photoelectrocatalysis and effective factors on its efficiency: a review. J. Solid State Electrochem. 21, 305–336 (2017).
Khanahmadlou, T. & Lashgari, M. Wireless photoelectrochemical degradation of methylene blue dye using a NiCo2O4/Cu photoelectrode. In 22nd Iranian Physical Chemistry Conference (2019).
Alulema-Pullupaxi, P. et al. Fundamentals and applications of photoelectrocatalysis as an efficient process to remove pollutants from water: a review. Chemosphere 281, 130821 (2021).
Castillo-Cabrera, G. et al. Bismuth oxyhalide-based materials (BiOX: X= Cl, Br, I) and their application in photoelectrocatalytic degradation of organic pollutants in water: a review. Front. Chem. 10, 900622 (2022).
Sigcha-Pallo, C. et al. Photoelectrocatalytic degradation of diclofenac with a boron-doped diamond electrode modified with titanium dioxide as a photoanode. Environ. Res. 212, 113362 (2022).
Lashgari, M. & Zeinalkhani, P. Electrostatic promotion of the catalyst activity for ammonia photosynthesis upon a robust affordable nanostructured uni-electrode photodevice/reactor. Catal. Sci. Technol. 10, 7998–8004 (2020).
Salamat, S., Hadavifar, M. & Rezaei, H. Preparation of nanochitosan-STP from shrimp shell and its application in removing of malachite green from aqueous solutions. J. Environ. Chem. Eng. 7, 103328 (2019).
Ma, J. et al. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 4, 5749–5760 (2012).
El-Hakam, S. A. et al. Synthesis of sulfamic acid supported on Cr-MIL-101 as a heterogeneous acid catalyst and efficient adsorbent for methyl orange dye. RSC Adv. 8, 20517–20533 (2018).
Sakkayawong, N., Thiravetyan, P. & Nakbanpote, W. Adsorption mechanism of synthetic reactive dye wastewater by chitosan. J. Colloid Interface Sci. 286, 36–42 (2005).
Lissaneddine, A. et al. A critical review on the electrosorption of organic compounds in aqueous effluent–influencing factors and engineering considerations. Environ. Res. 204, 112128 (2022).
Ghoniem, M. G. et al. Highly selective removal of cationic dyes from wastewater by MgO nanorods. Nanomaterials 12, 1023 (2022).
Bée, A., Obeid, L., Mbolantenaina, R., Welschbillig, M. & Talbot, D. Magnetic chitosan/clay beads: a magsorbent for the removal of cationic dye from water. J. Magn. Magn. Mater. 421, 59–64 (2017).
Gupta, K. & Khatri, O. P. Reduced graphene oxide as an effective adsorbent for removal of malachite green dye: plausible adsorption pathways. J. Colloid Interface Sci. 501, 11–21 (2017).
Mashkoor, F. & Nasar, A. Carbon nanotube-based adsorbents for the removal of dyes from waters: a review. Environ. Chem. Lett. 18, 605–629 (2020).
Huang, C. et al. Adsorption performance of chitosan Schiff base towards anionic dyes: electrostatic interaction effects. Chem. Phys. Lett. 780, 138958 (2021).
Yang, R., Li, D., Li, A. & Yang, H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 151, 20–28 (2018).
Zhang, W., Zhang, R. Z., Huang, Y. Q. & Yang, J. M. Effect of the synergetic interplay between the electrostatic interactions, size of the dye molecules, and adsorption sites of MIL-101 (Cr) on the adsorption of organic dyes from aqueous solutions. Cryst. Growth Des. 18, 7533–7540 (2018).
Liu, L. et al. Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustain. Chem. Eng. 3, 432–442 (2015).
Li, J. et al. Role of sulfonation in lignin-based material for adsorption removal of cationic dyes. Int. J. Biol. Macromol. 135, 1171–1181 (2019).
Hu, Y. et al. Dye adsorption by resins: effect of ionic strength on hydrophobic and electrostatic interactions. Chem. Eng. J. 228, 392–397 (2013).
Yagub, M. T., Sen, T. K., Afroze, S. & Ang, H. M. Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid Interface Sci. 209, 172–184 (2014).
Lashgari, M., Shafizadeh, N. & Zeinalkhani, P. A nanocomposite p-type semiconductor film for possible application in solar cells: photo-electrochemical studies. Sol. Energy Mater. Sol. Cells 137, 274–279 (2015).
Shinde, S. S., Bansode, R. A., Bhosale, C. H. & Rajpure, K. Y. Physical properties of hematite α-Fe2O3 thin films: application to photoelectrochemical solar cells. J. Semicond. 32, 013001 (2011).
Han, K. et al. Marcasite-FeS2@carbon nanodots anchored on 3D cell-like graphenic matrix for high-rate and ultrastable potassium ion storage. J. Power Sources 469, 228429 (2020).
Lashgari, M. Fundamental aspects of CO2 transformation into C/H/O based fuels/chemicals. In Nanomaterials for CO2 Capture, Storage, Conversion and Utilization (eds Tri, P. N., Wu, H., Nguyen, T. A., Barnabé, S. & Bénard, P.) 283–305 (Elsevier, 2021).
Memming, R. Semiconductor Electrochemistry Ch. 5 (Wiley-VCH, 2015).
Lashgari, M. & Ghanimati, M. Pollutant photo-conversion strategy to produce hydrogen green fuel and valuable sulfur element using H2S feed and nanostructured alloy photocatalysts: Ni-dopant effect, energy diagram and photo-electrochemical characterization. Chem. Eng. Res. Des. 162, 85–93 (2020).
Bockris, J. O’M. & Reddy A. K. N. Modern Electrochemistry 2B: Electrodics in Chemistry, Engineering, Biology and Environmental Science Ch. 10 (Kluwer Academic Publishers, 2004).
Konstantinou, I. K. & Albanis, T. A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl. Catal. B 49, 1–14 (2004).
Gaya, U. I. & Abdullah, A. H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. C. 9, 1–12 (2008).
Kumar, S., Kaushik, R. D. & Purohit, L. P. Novel ZnO tetrapod-reduced graphene oxide nanocomposites for enhanced photocatalytic degradation of phenolic compounds and MB dye. J. Mol. Liq. 327, 114814 (2021).
Oh, W. D., Lok, L. W., Veksha, A., Giannis, A. & Lim, T. T. Enhanced photocatalytic degradation of bisphenol A with Ag-decorated S-doped g-C3N4 under solar irradiation: performance and mechanistic studies. Chem. Eng. J. 333, 739–749 (2018).
Wu, B. et al. The photochemistry of carbon nanotubes and its impact on the photo-degradation of dye pollutants in aqueous solutions. J. Colloid Interface Sci. 439, 98–104 (2015).
Fuku, X., Thovhogi, N. & Maaza, M. Photocatalytic effect of green synthesised CuO nanoparticles on selected environmental pollutants and pathogens. AIP Conf. Proc. 1962, 040006 (2018).
Lashgari, M. & Zeinalkhani, P. Photocatalytic N2 conversion to ammonia using efficient nanostructured solar-energy-materials in aqueous media: a novel hydrogenation strategy and basic understanding of the phenomenon. Appl. Catal. A 529, 91–97 (2017).
Jiang, Z., Wang, H., Huang, H. & Cao, C. Photocatalysis enhancement by electric field: TiO2 thin film for degradation of dye X-3B. Chemosphere 56, 503–508 (2004).
Atkins, P. W., De Paula, J. & Keeler, J. Atkins’ Physical Chemistry Ch. 16 (Oxford University Press, 2018).
Alardhi, S. M., Albayati, T. M. & Alrubaye, J. M. Adsorption of the methyl green dye pollutant from aqueous solution using mesoporous materials MCM-41 in a fixed-bed column. Heliyon 6, e03253 (2020).
Wang, W. et al. Mesoporous silicate/carbon composites derived from dye-loaded palygorskite clay waste for efficient removal of organic contaminants. Sci. Total Environ. 696, 133955 (2019).
Spray, R. L. & Choi, K. S. Photoactivity of transparent nanocrystalline Fe2O3 electrodes prepared via anodic electrodeposition. Chem. Mater. 21, 3701–3709 (2009).
Lashgari, M. & Zeinalkhani, P. Ammonia photosynthesis under ambient conditions using an efficient nanostructured FeS2/CNT solar-energy-material with water feedstock and nitrogen gas. Nano Energy 48, 361–368 (2018).
Lashgari, M. & Ghanimati, M. A highly efficient nanostructured quinary photocatalyst for hydrogen production. Int. J. Energy Res. 39, 516–523 (2015).
Kaviyarasu, K. et al. Photocatalytic activity of ZrO2 doped lead dioxide nanocomposites: investigation of structural and optical microscopy of RhB organic dye. Appl. Surf. Sci. 421, 234–239 (2017).
Fuku, X., Matinise, N., Masikini, M., Kasinathan, K. & Maaza, M. An electrochemically active green synthesized polycrystalline NiO/MgO catalyst: use in photo-catalytic applications. Mater. Res. Bull. 97, 457–465 (2018).
Acknowledgements
The authors would like to acknowledge the anonymous Referees as well as the Editor of this journal for their useful comments to enhance the quality of work. Special thanks are also due to Prof. Rasoulifard and Dr. Atrak for their assistance in COD measurement.
Author information
Authors and Affiliations
Contributions
M.L.: conceptualization, methodology, project supervising/administration, photoelectrochemical tests, writing and editing, interpretation and analysis of data S.N.-M.: methodology, data acquisition/curing, T.K.: original setup design, literature survey, data acquisition/curing, R.H.: co-thinking, commenting, review and editing. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lashgari, M., Naseri-Moghanlou, S., Khanahmadlou, T. et al. Electrostatic boosting of ionic dye pollutant removal from aquatic environment using a single electrode photoreactor. npj Clean Water 6, 10 (2023). https://doi.org/10.1038/s41545-023-00230-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-023-00230-4