Abstract
SLC7A11-mediated cystine uptake suppresses ferroptosis yet promotes cell death under glucose starvation; the nature of the latter cell death remains unknown. Here we show that aberrant accumulation of intracellular disulfides in SLC7A11high cells under glucose starvation induces a previously uncharacterized form of cell death distinct from apoptosis and ferroptosis. We term this cell death disulfidptosis. Chemical proteomics and cell biological analyses showed that glucose starvation in SLC7A11high cells induces aberrant disulfide bonds in actin cytoskeleton proteins and F-actin collapse in a SLC7A11-dependent manner. CRISPR screens and functional studies revealed that inactivation of the WAVE regulatory complex (which promotes actin polymerization and lamellipodia formation) suppresses disulfidptosis, whereas constitutive activation of Rac promotes disulfidptosis. We further show that glucose transporter inhibitors induce disulfidptosis in SLC7A11high cancer cells and suppress SLC7A11high tumour growth. Our results reveal that the susceptibility of the actin cytoskeleton to disulfide stress mediates disulfidptosis and suggest a therapeutic strategy to target disulfidptosis in cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The MS/MS raw data to support the findings of this study have been deposited in MassIVE datasets with the identifier MSV000088066. The MS/MS raw data were searched against the human proteome database from UniProt (updated 26 January 2019; 93,798 sequences) using the MaxQuant software (v1.6.5.0) with default parameters for SILAC labelling. Gene Ontology analyses were done in http://geneontology.org/ against the protein list in Supplementary Table 2 and the protein–protein interaction network was done in https://string-db.org/ (v11.0b) against the protein list in Supplementary Table 2. The network was generated by the STRING database with medium confidence (0.7) and visualized using Cytoscape (version 3.8.2). Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding authors on reasonable request.
References
Liu, X. et al. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 22, 476–486 (2020).
Joly, J. H., Delfarah, A., Phung, P. S., Parrish, S. & Graham, N. A. A synthetic lethal drug combination mimics glucose deprivation-induced cancer cell death in the presence of glucose. J. Biol. Chem. 295, 1350–1365 (2020).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
El Mjiyad, N., Caro-Maldonado, A., Ramirez-Peinado, S. & Munoz-Pinedo, C. Sugar-free approaches to cancer cell killing. Oncogene 30, 253–264 (2011).
Pereira, D. J., Schoolwerth, A. C. & Pais, V. M. Cystinuria: current concepts and future directions. Clin. Nephrol. 83, 138–146 (2015).
Elmonem, M. A. et al. Cystinosis: a review. Orphanet J. Rare Dis. 11, 47 (2016).
Xiao, H. et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180, 968–983 (2020).
Neumann, C. A., Cao, J. & Manevich, Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle 8, 4072–4078 (2009).
Musaogullari, A. & Chai, Y. C. Redox regulation by protein S-glutathionylation: from molecular mechanisms to implications in health and disease. Int. J. Mol. Sci. 21, 8113 (2020).
Schwanhausser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Colic, M. et al. Identifying chemogenetic interactions from CRISPR screens with drugZ. Genome Med. 11, 52 (2019).
Birsoy, K. et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508, 108–112 (2014).
Koppula, P., Zhuang, L. & Gan, B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12, 599–620 (2021).
Alekhina, O., Burstein, E. & Billadeau, D. D. Cellular functions of WASP family proteins at a glance. J. Cell Sci. 130, 2235–2241 (2017).
Ibarra, N., Pollitt, A. & Insall, R. H. Regulation of actin assembly by SCAR/WAVE proteins. Biochem. Soc. Trans. 33, 1243–1246 (2005).
Kunda, P., Craig, G., Dominguez, V. & Baum, B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr. Biol. 13, 1867–1875 (2003).
Miki, H., Suetsugu, S. & Takenawa, T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 (1998).
Steffen, A. et al. Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 23, 749–759 (2004).
Siebeneicher, H. et al. Identification and optimization of the first highly selective GLUT1 inhibitor BAY-876. ChemMedChem 11, 2261–2271 (2016).
Olszewski, K. et al. Inhibition of glucose transport synergizes with chemical or genetic disruption of mitochondrial metabolism and suppresses TCA cycle-deficient tumors. Cell Chem. Biol. 29, 423–435 (2022).
Tang, D., Kang, R., Berghe, T. V., Vandenabeele, P. & Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 29, 347–364 (2019).
Dai, F. et al. BAP1 inhibits the ER stress gene regulatory network and modulates metabolic stress response. Proc. Natl Acad. Sci. USA 114, 3192–3197 (2017).
Zhang, Y. et al. H2A monoubiquitination links glucose availability to epigenetic regulation of the endoplasmic reticulum stress response and cancer cell death. Cancer Res. 80, 2243–2256 (2020).
Lei, G. et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene 40, 3533–3547 (2021).
Koppula, P., Zhang, Y., Shi, J., Li, W. & Gan, B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 292, 14240–14249 (2017).
Zhang, Y. et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 20, 1181–1192 (2018).
Zhang, Y., Koppula, P. & Gan, B. Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1. Cell Cycle 18, 773–783 (2019).
Xiao, Z. D. et al. Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat. Commun. 8, 783 (2017).
Lin, A. et al. The FoxO–BNIP3 axis exerts a unique regulation of mTORC1 and cell survival under energy stress. Oncogene 33, 3183–3194 (2014).
Liu, X. & Gan, B. lncRNA NBR2 modulates cancer cell sensitivity to phenformin through GLUT1. Cell Cycle 15, 3471–3481 (2016).
Gan, B. et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature 468, 701–704 (2010).
Lee, H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234 (2020).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Zhang, Y. et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 12, 1589 (2021).
Lei, G. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30, 146–162 (2020).
Koppula, P. et al. KEAP1 deficiency drives glucose dependency and sensitizes lung cancer cells and tumors to GLUT inhibition. iScience 24, 102649 (2021).
Lin, A. et al. FoxO transcription factors promote AKT Ser473 phosphorylation and renal tumor growth in response to pharmacologic inhibition of the PI3K-AKT pathway. Cancer Res. 74, 1682–1693 (2014).
Lee, H. et al. BAF180 regulates cellular senescence and hematopoietic stem cell homeostasis through p21. Oncotarget 7, 19134–19146 (2016).
Yang, Y. Y., Grammel, M., Raghavan, A. S., Charron, G. & Hang, H. C. Comparative analysis of cleavable azobenzene-based affinity tags for bioorthogonal chemical proteomics. Chem. Biol. 17, 1212–1222 (2010).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Liu, X. et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat. Cell Biol. 18, 431–442 (2016).
Gan, B., Melkoumian, Z. K., Wu, X., Guan, K. L. & Guan, J. L. Identification of FIP200 interaction with the TSC1–TSC2 complex and its role in regulation of cell size control. J. Cell Biol. 170, 379–389 (2005).
Nie, L. et al. The landscape of histone modifications in a high-fat diet-induced obese (DIO) mouse model. Mol. Cell. Proteomics 16, 1324–1334 (2017).
Lu, S. et al. Mapping disulfide bonds from sub-micrograms of purified proteins or micrograms of complex protein mixtures. Biophys. Rep. 4, 68–81 (2018).
Chen, Y., Kwon, S. W., Kim, S. C. & Zhao, Y. Integrated approach for manual evaluation of peptides identified by searching protein sequence databases with tandem mass spectra. J. Proteome Res. 4, 998–1005 (2005).
Chauhan, A. S. et al. STIM2 interacts with AMPK and regulates calcium-induced AMPK activation. FASEB J. 33, 2957–2970 (2019).
Wu, S. et al. A ferroptosis defense mechanism mediated by glycerol-3-phosphate dehydrogenase 2 in mitochondria. Proc. Natl Acad. Sci. USA 119, e2121987119 (2022).
Koppula, P. et al. A targetable CoQ–FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 13, 2206 (2022).
Acknowledgements
We thank M. Kalocsay for providing comments on proteomic studies during our manuscript revision. This research was supported by the Institutional Research Fund and Bridge Fund from The University of Texas MD Anderson Cancer Center, Emerson Collective Cancer Research Fund as well as grant nos R01CA181196, R01CA244144 and R01CA247992 (to B.G.), R01DK107733 (to D.D.B.) and R35GM130119 (to T.H.) from the National Institutes of Health. B.G. and T.H. were Andrew Sabin Family Fellows. This research has also been supported by the National Institutes of Health Cancer Center Support Grant P30CA016672 to The University of Texas MD Anderson Cancer Center. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The manuscript was edited by S. Bronson, ELS, of the Research Medical Library at MD Anderson.
Author information
Authors and Affiliations
Contributions
B.G. and X.L. conceived and designed the study and wrote most of the manuscript with assistance from L.N. X.L. performed most of the experiments with assistance from Y.Z., C.W., Y.Y., A.H., X.C., G.L., C.M., S.W. and L.Z. L.N. conducted all the proteomic analyses under the direction of J.C. and wrote parts of the manuscript. K.O. conducted the metabolomic analyses with the support of M.V.P. M.C. conducted the data analysis of CRISPR–Cas9 screening under the direction of T.H. M.J.Y. performed histopathological analyses. D.D.B. provided WAVE-2 constructs and guided the actin cytoskeleton-related experiments. B.G. and J.C. acquired the funding support and supervised the study. J.C. reviewed and edited the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
B.G. reports receiving consultation fees from Guidepoint Global, Cambridge Solutions and NGM Bio, and is an inventor with patent applications involving targeting ferroptosis in cancer therapy. K.O. is a full-time employee of the Barer Institute and a former full-time employee of Kadmon Corporation. M.V.P. is a full-time employee of Kadmon Corporation, a Sanofi Company. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Scott Dixon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
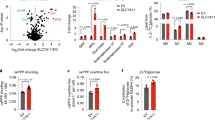
Extended Data Fig. 1 A unique form of cell death in glucose-starved SLC7A11high cells.
a, Diagrams showing cystine metabolism and glucose-derived pentose phosphate pathway (PPP) metabolism. G6P, glucose-6-phosphate; 6PG, 6-phosphogluconate; R5P, ribose-5-phosphate; F6P, fructose-6-phosphate. b, SLC7A11 western blots of different cell lines. c,d, Cell death in H460 (c) and A549 (d) cells cultured in glucose-containing (+Glc) or glucose-free (−Glc) medium with or without the indicated concentrations of DFO, Ferr-1, Z-VAD, Nec-1, Nec-2 and CQ for 5 (c) or 15 h (d). e, SLC7A11 western blots in the indicated 786-O cells. f, Cell death in UMRC6 cells treated with vehicle (DMSO) or 0.1 µM RSL3 with or without 100 µM DFO or 10 µM Ferr-1 for 8 h. g, Cell death in UMRC6 cells treated with vehicle (DMSO) or 1 µM STS with or without 20 µM Z-VAD for 16 h. h, Representative phase-contrast images and cell death measurements of UMRC6 cells cultured in the indicated medium with or without 2 mM DTT, 2 mM 2ME or 1 mM TCEP for 7 h. i, SLC7A11 western blots in WT and SLC7A11-KO (K1/K2) UMRC6 cells. j,k, Cell death in the indicated cell lines cultured in glucose-free medium for the indicated times. l, Cell death in 786-O cells cultured in the indicated medium with or without Z-VAD for 30 h. m, Western blot analysis of apoptosis markers in the indicated 786-O cells in glucose-free medium for the indicated times. n, Diagram illustrating the mechanism of the thiol-oxidizing agents diamide and diethyl-maleate. o, Cell death in the indicated 786-O cells cultured in the indicated medium for 3 h. p, Cell death in A549 cells cultured in the indicated medium for 15 h. q,r, Cell death in UMRC6 (q) and A549 (r) cells cultured in the indicated medium for 7 h (q) or 15 h (r). s,t, Intracellular cystine (s) and glutamyl-cystine (t) in UMRC6 cells cultured in the indicated medium for 2 h. u,v, Typical transmission electron microscopic images of UMRC6 cells (u) and 786-O cells overexpressing SLC7A11 (786-O SLC7A11; v) cultured in glucose-free medium for the indicated times. All P values were calculated using a two-tailed unpaired Student’s t-test. Data are the mean ± s.d.; n = 3 independent repeats. All western blotting was repeated three times, independently, with similar results.
Extended Data Fig. 2 Proteomic analyses in glucose-starved SLC7A11high cells.
a, Approach used to identify disulfide-containing peptides. -S-CAM is the cysteine blocked with IAA and -S-ALK is the cysteine blocked with IAA-alkyne, whereas -S-B is the product after the click reaction between -S-ALK and azido-azo-biotin. b, Protein–protein interaction network of the proteins with disulfide bonds increased at least 1.5-fold following the glucose starvation in Fig. 2a. The network was generated by the STRING database with medium confidence (0.7) and visualized using Cytoscape (v3.8.2). c, Flanking-sequence analysis of the ‘disulfide’ cysteine sites of the proteins with disulfide bonds increased at least 1.5-fold following the glucose starvation in Fig. 2a.
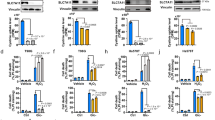
Extended Data Fig. 3 Disulfide bonding in cytoskeleton proteins in disulfidptosis.
a, Diagrams illustrating the glutathionylation. b, Western blot showing glutathionylation in the indicated UMRC6 cells cultured in glucose-containing (+Glc) or glucose-free (−Glc) medium for 3 h. c, Western blot showing destrin in UMRC6 cells cultured in glucose-free medium for 0–3 h. Red arrow indicates the slow migrated band of Destrin proteins. d, NADP+/NADPH ratios of UMRC6 cells cultured in glucose-free medium for 0–4 h. e, Workflow of SLC7A11high-mediated cystine uptake and disulfidptosis under glucose starvation. f, NADP+/NADPH ratios of UMRC6 cells cultured in the indicated medium for 1 h. g, Representative phase-contrast images and cell death of UMRC6 cells cultured in the indicated medium for 7 h. h, Western blotting analysis of UMRC6 cells cultured in the indicated medium for 3 h. i,j, The relative lipid peroxidation (i) and cell death (j) levels of UMRC6 cells cultured in the indicated medium with or without ferroptosis inhibitor (10 µM Ferr-1) for 36 (i) or 48 h (j). k, Diagram illustrating the metabolism of glucose and 2DG. HK, hexokinase; PGI, phosphoglucose isomerase; G6PD, glucose-6-phosphate dehydrogenase; 2D-6-P, 2-deoxyglucose-6-phosphate. l, NADP+/NADPH ratios of UMRC6 cells cultured in the indicated medium with or without 2 mM 2DG, 25 µM Tempol or 100 µM Trolox for 1 h. m, Representative phase-contrast images and cell death of UMRC6 cells cultured in the indicated medium with or without 2 mM 2DG, 25 µM Tempol or 100 µM Trolox for 7 h. n, Relative ROS levels of UMRC6 cells cultured in the indicated medium with or without 2 mM 2DG, 25 µM Tempol or 100 µM Trolox for 4 h. o, Western blot analysis of MYH9 and TLN1 in UMRC6 cells cultured in the indicated medium with or without 1 mM 2ME treatment for 3 h. All P values were calculated using a two-tailed unpaired Student’s t-test. Data are the mean ± s.d.; n = 3 independent repeats. NS, not significant (P > 0.05). All western blotting was repeated at least twice, independently, with similar results.
Extended Data Fig. 4 Annotated MS/MS spectra of disulfide-linked peptides.
a–f, Insets: the precursor ion mass with deviation.
Extended Data Fig. 5 Annotated MS/MS spectra of disulfide-linked peptides.
a–e, Insets: the precursor ion mass with deviation. f, Extracted ion chromatograms for the precursor ion of the quadruply charged disulfide (MYH9_C988-C1379) peptides shown in Fig. 3e in non-reduced (without DTT treatment) and reduced (with DTT treatment) samples. The MS/MS spectrum identified in these non-reduced samples was the same as shown in Fig. 3e. The extracted ion chromatograms were set with a deviation of ±5 ppm.
Extended Data Fig. 6 F-actin contraction during disulfidptosis.
a,b, Fluorescent staining of F-actin with phalloidin in H460 (a) and A549 (b) cells cultured in glucose-containing (+Glc) or glucose-free (−Glc) medium for 4 (a) and 9 h (b), respectively. Nuclei were stained by DAPI. c, Fluorescent staining of F-actin with phalloidin in 786-O cells overexpressing SLC7A11 and transfected with empty vector (EV) cultured in glucose-containing or glucose-free medium for 2 h. Nuclei were stained by DAPI. d, Fluorescent staining of F-actin (red) and membrane (green) in the 786-O cells overexpressing SLC7A11 (786-O_SLC7A11) cultured in glucose-free medium for the indicated times. e,f, Fluorescent staining of F-actin (red) in UMRC6 (e) and 786-O_SLC7A11 cells (f) cells overexpressing membrane-bound GFP (mGFP) cultured in glucose-free medium for the indicated times. g, Fluorescent staining of F-actin in UMRC6 cells cultured in glucose-containing or glucose-free medium with or without 1 mM 2ME for 4 h. Nuclei were stained by DAPI. All experiments were repeated at least twice, independently, with similar results.
Extended Data Fig. 7 The WAVE regulatory complex and Rac regulate disulfidptosis.
a, Workflow of CRISPR–Cas9 screenings. b, RPN1 western blots of the indicated UMRC6 cells. c, Cell death in WT and RPN1-knockdown (sh1-3) UMRC6 cells cultured in glucose-containing (+Glc) or glucose-free (−Glc) medium for 8 h. d, Schematic illustrating the Rac1–WRC–Arp2/3 signalling axis. e,g,i, Western blots of WT and NCKAP1-KO (K1/K2) A549 cells (e), H460 cells (g) and the indicated 786-O cells (i). f,h,j, Cell death in WT and NCKAP1-KO A549 cells (f), H460 cells (h) and 786-O cells overexpressing SLC7A11 (j) cultured in the indicated medium for 15 h (f), 5 h (h) or the indicated times (j). k, Relative NCKAP1L mRNA levels in the indicated cells. l, Cell viability in the indicated UMRC6 cells treated with vehicle or 1 µM STS for 16 h. m, Cell death in the indicated UMRC6 cells treated with vehicle or 1 µM RSL3 for 8 h. n–q, Western blots of the WT cells and their KO counterparts with deficiency of the indicated genes. r, Cell death in the indicated 786-O cells overexpressing SLC7A11 cultured in glucose-free medium for 4 h. s,t, Western blot (left) and RT-PCR (right) analysis of the indicated UMRC6 cells. u, Cell death in the indicated UMRC6 cells cultured in glucose-free medium for 7 h. v,x, Western blot analysis in the indicated cell lines. w, Cell death in the indicated 786-O_SLC7A11 cells cultured in glucose-free medium with or without 1 mM TCEP for 3 h. y, Cell death in the indicated NCKAP1-KO 786-O_SLC7A11 cells cultured in glucose-free medium for 7 h. All P values were calculated using a two-tailed unpaired Student’s t-test. Data are the mean ± s.d.; n = 3 independent repeats. NS, not significant (P > 0.05). All western blotting was repeated at least twice, independently, with similar results.
Extended Data Fig. 8 GLUT inhibitors induce disulfidptosis in SLC7A11high tumours.
a, Cell death measured by PI staining in NCI-H226 cells treated with vehicle DMSO and 5 µM BAY-876 for 6 h. b, End-point weights of NCI-H226 xenografts with the indicated treatments. Data are the mean ± s.d.; n = 6 mice. c, Haematoxylin and eosin staining of NCI-H226 tumours with the indicated treatments. Tumour areas with necrotic cell death are indicated. d–f, Representative immunochemical images (d) from NCI-H226 tumours with the indicated treatments and corresponding immunoreactive scores of cleaved caspase-3 (e) and 4-HNE (f). g, End-point tumour weights of TC551 xenografts with the indicated treatments. Data are the mean ± s.d.; n = 5 mice. h–j, Representative immunochemical images (h) from TC551 and TC494 PDX models with the indicated treatments and corresponding immunoreactive scores of cleaved caspase-3 (i) and 4-HNE (j). k–m, Weight over time of mice with NCI-H226 (k), TC551 (l) and TC494 (m) xenografts with the indicated treatments. Data are the mean ± s.d.; n = 6 (k), 5 (l) or 8 (m) mice. n, Representative haematoxylin and eosin staining of major organs from mice treated with vehicle or BAY-876. The experiment was repeated twice, independently, with similar results. o,p, Working model depicting how glucose and SLC7A11 coordinately regulate the disulfide homeostasis of the actin cytoskeleton. See Discussion for detailed description. ABPs, actin-binding proteins; small red arc, disulfide bond. All P values were calculated using a two-tailed unpaired Student’s t-test. Data are the mean ± s.d.; n = 3 independent repeats unless otherwise specified. NS, not significant (P > 0.05).
Extended Data Fig. 9 The induction of disulfidptosis in SLC7A11low cells.
a, NADP+/NADPH ratios of 786-O cells cultured in glucose-containing or glucose-free medium with the indicated concentrations of cystine for 4 h. b, Non-reducing and reducing western blot analysis of MYH9 and TLN1 in 786-O cells cultured in glucose-containing or glucose-free medium with the indicated concentrations of cystine for 7 h. c, Fluorescent staining of F-actin in 786-O cells cultured in glucose-containing or glucose-free medium with the indicated concentrations of cystine for 8 h. Nuclei were stained by DAPI. d, Cell death measured by propidium iodide (PI) staining in 786-O cells cultured in glucose-containing or glucose-free medium with the indicated concentrations of cystine for 15 h. e, Cell death measured by PI staining in 786-O cells cultured in glucose-containing or glucose-free medium containing 400 µM cystine with or without the indicated concentrations of DFO, Ferr-1, Z-VAD, Nec-1, Nec-2, CQ, DTT, 2ME and TCEP for 15 h (DTT, 2ME and TCEP were replenished at the 7-h time point). All P values were calculated using a two-tailed unpaired Student’s t-test. Data are the mean ± s.d.; n = 3 independent repeats. All western blotting was repeated at least twice, independently, with similar results.
Extended Data Fig. 10 An example for the gating strategy for the flow cytometry.
Initial cell population gating (FSC-Height versus SSC-Height) was adopted to make sure only single cells were used for analysis.
Supplementary information
Supplementary Tables 1–5
Supplementary Table 1. All disulfide-containing peptides identified from the bio-orthogonal chemical proteomic analyses in UMRC6 cells. Supplementary Table 2. The 90 cysteine-containing peptides with disulfide bonds increased at least 1.5-fold following glucose starvation from both forward and reverse labelling analyses in UMRC6 cells. Supplementary Table 3. The relative fold changes (as normZ scores) for gRNAs in SLC7A11-overexpressing 786-O cells following glucose starvation. The data were analysed using the drugZ algorithm. Supplementary Table 4. Oligonucleotide sequences of the gRNAs, shRNA and primers used in this study. Supplementary Table 5. Information on the human research participants of PDXs.
Source data
Source Data Fig. 1
Unprocessed western blots for Fig. 1.
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Unprocessed western blots for Fig. 3.
Source Data Fig. 5
Unprocessed western blots for Fig. 5.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Unprocessed western blots for Fig. 6.
Source Data Fig. 6
Statistical source data for Fig. 6.
Source Data Extended Data Fig. 1
Unprocessed western blots for Extended Data Fig. 1.
Source Data Extended Data Fig. 1
Statistical source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 3
Unprocessed western blots for Extended Data Fig. 3.
Source Data Extended Data Fig. 3
Statistical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 7
Unprocessed western blots for Extended Data Fig. 7.
Source Data Extended Data Fig. 7
Statistical source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Statistical source data for Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Unprocessed western blots for Extended Data Fig. 9.
Source Data Extended Data Fig. 9
Statistical source data for Extended Data Fig. 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Nie, L., Zhang, Y. et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat Cell Biol 25, 404–414 (2023). https://doi.org/10.1038/s41556-023-01091-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01091-2
This article is cited by
-
Development and validation of a disulfidptosis and disulfide metabolism-related risk index for predicting prognosis in lung adenocarcinoma
Cancer Cell International (2024)
-
Identification of sulfur metabolism-related gene signature in osteoarthritis and TM9SF2’s sustenance effect on M2 macrophages' phagocytic activity
Journal of Orthopaedic Surgery and Research (2024)
-
Integrated analysis of disulfidptosis-related immune genes signature to boost the efficacy of prognostic prediction in gastric cancer
Cancer Cell International (2024)
-
Prognostic and immune infiltration features of disulfidptosis-related subtypes in breast cancer
BMC Women's Health (2024)
-
Disulfidptosis-related signature predicts prognosis and characterizes the immune microenvironment in hepatocellular carcinoma
Cancer Cell International (2024)