Abstract
Background
Although it is known that resistance training can be as effective as stretch training to increase joint range of motion, to date no comprehensive meta-analysis has investigated the effects of resistance training on range of motion with all its potential affecting variables.
Objective
The objective of this systematic review with meta-analysis was to evaluate the effect of chronic resistance training on range of motion compared either to a control condition or stretch training or to a combination of resistance training and stretch training to stretch training, while assessing moderating variables.
Design
For the main analysis, a random-effect meta-analysis was used and for the subgroup analysis a mixed-effect model was implemented. Whilst subgroup analyses included sex and participants’ activity levels, meta-regression included age, frequency, and duration of resistance training.
Data Sources
Following the systematic search in four databases (PubMed, Scopus, SPORTDiscus, and Web of Science) and reference lists, 55 studies were found to be eligible.
Eligibility Criteria
Controlled or randomized controlled trials that separately compared the training effects of resistance training exercises with either a control group, stretching group, or combined stretch and resistance training group on range of motion in healthy participants.
Results
Resistance training increased range of motion (effect size [ES] = 0.73; p < 0.001) with the exception of no significant range of motion improvement with resistance training using only body mass. There were no significant differences between resistance training versus stretch training (ES = 0.08; p = 0.79) or between resistance training and stretch training versus stretch training alone (ES = − 0.001; p = 0.99). Although “trained or active people” increased range of motion (ES = 0.43; p < 0.001) “untrained and sedentary” individuals had significantly (p = 0.005) higher magnitude range of motion changes (ES = 1.042; p < 0.001). There were no detected differences between sex and contraction type. Meta-regression showed no effect of age, training duration, or frequency.
Conclusions
As resistance training with external loads can improve range of motion, stretching prior to or after resistance training may not be necessary to enhance flexibility.
Similar content being viewed by others
Resistance training with external loads can improve range of motion to a moderate magnitude |
Improvements in range of motion are not significantly different between resistance training and stretch training |
Additional stretching prior to or after resistance training may not be necessary to enhance flexibility |
Stretch training can still be advocated as a fitness and training component for much of the population and included as a component of a warm-up prior to competition |
1 Introduction
Stretching was long considered an essential component of warm-ups, fitness, and health [1]. However, the use of stretching as a warm-up component or training routine (i.e., stretching over several weeks) to improve range of motion (ROM), performance, and health has been subjected to a number of counter arguments over the past 20+ years [1,2,3,4,5,6,7]. For example, it has been ubiquitously reported since the late 1990s that incorporating static stretching as a pre-activity strategy can lead to performance (e.g., strength, power, speed, balance) impairments [1, 3, 4, 6, 8]. However, a number of reviews [1, 3, 4, 6, 8] have demonstrated that the study designs used in many of these studies lacked ecological validity. Hence, critical analyses of the literature were necessary to demonstrate that if static stretching of less than 60 s per muscle group is incorporated into a warm-up involving dynamic activities, the possibility of significant performance impairments is trivial [1, 3, 4, 6, 8]. However, there is little controversy regarding the effectiveness of acute and chronic (training) stretching for the improvement of joint ROM in healthy populations [1, 9,10,11]. However, stretch training may not be the only technique for improving ROM and thus it is important that other recommendations for improving ROM, such as resistance training (RT), also be critically evaluated.
Recent commentaries [2, 5] have suggested that possible benefits of stretch training such as improvements in flexibility, balance, cardiovascular measures, alleviation of pain, and decreased injury incidence among others can either be provided by other training modalities (e.g., RT) or stretching is not the most effective activity to provide such benefits (e.g., decreased injury incidence). Nuzzo [5] suggested in his Current Opinion article that chronic RT induced similar increases in ROM as stretch training. Recently, a meta-analysis [12] evaluated 11 studies and reported a non-significant difference between stretch training and RT with a small effect size (ES) in favor of stretching (Hedges’g = − 0.22; p = 0.21). However, neither the commentaries nor the meta-analysis evaluated the specific type of RT (i.e., free weights vs machine RT, vs Pilates, vs calisthenics) effects on ROM. Considering the concept of RT specificity (e.g., angle and task specificity), [13, 14] it might be expected that RT with a restricted ROM such as calisthenics or some machine-based RT might not provide similar flexibility improvements as with free weight training through a full ROM.
Typical RT movements involve concentric, isometric, and eccentric muscle contractions. As eccentric contractions can provide higher resistive forces or loads [15, 16] of a lengthening muscle–tendon unit, eccentric contractions may provide a greater stimulus for increasing ROM. There are several reports of substantial ROM increases following eccentric RT [17,18,19]. More clarity is necessary to ascertain whether there are significant differences in ROM with the different types of contractions.
While many studies do highlight that chronic RT in general can increase ROM [20,21,22,23,24,25,26], further in-depth analysis is needed to validate whether the improvement is relatively similar between RT and stretch training. Furthermore, any significant effects of RT on ROM may be moderated by variables such as the sex and trained state of the individual or the frequency and duration [27, 28] of RT. If RT in general or specific types of RT can provide similar improvements in ROM as reported in prior stretching studies, then additional stretching exercises may be removed from the typical training session. Moreover, it is not known whether there is an additive effect of combining RT and stretch training on ROM. Hence, the objective of this research was to conduct a comprehensive systematic review with a meta-analysis to evaluate the effect of chronic RT on ROM compared to controls, stretch training, as well as any possible additive ROM effects of RT and stretch training, with consideration of moderating variables such as the type, frequency, and duration of RT, as well as participants’ sex, age, and activity level (i.e., trained state).
2 Methods
This systematic review with meta-analysis was conducted according to the suggestions from Moher and colleagues and meets the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [29].
2.1 Search Strategy
A literature search following PRISMA review guidelines was performed by six of the co-authors in pairs of two using PubMed, SPORTDiscus, Web of Science, Scopus, and Google Scholar databases. After identifying the eligible studies, data were extracted by two co-authors and if consensus was not reached then a third author provided additional advice. The topic was systematically searched in February 2022 using a Boolean search strategy with the operators “AND”, “OR”, and a combination of the following title keywords: resistance exercise, resistance training, strength, strength training, endurance, endurance training, range of motion, flexibility, stretch, stretching. For example, the following query was used in PubMed database: [“resistance exercise” OR “resistance training” OR strength OR “strength training” OR endurance OR “endurance training” AND “range of motion” OR flexibility OR stretch OR stretching].
Based on our knowledge of the area, we also contributed additional studies from our own computer libraries. Furthermore, we conducted searches of our personal computer databases for related articles and conducted additional ‘snowballing’ searches throughout the process of conducting the review and analysis, which located some newer studies not available when we conducted the initial systematic search. The search ended in May 2022.
2.2 Inclusion and Exclusion Criteria
This review included studies that separately compared the training effects of RT exercises with a control group, stretching group, or combined stretch and RT group on ROM in healthy participants. We included controlled and randomized controlled trials written in English with a longitudinal training design (i.e., pre- to post-training comparison). Moreover, we excluded studies that investigated the combined effects of RT with other treatments such as aerobic training. We further excluded conference papers or theses.
2.3 Extraction of the Data
From all the included papers, the characteristics of the participants (i.e., sex, trained state, age), sample size number, study design, characteristics of the intervention (frequency and duration of RT, exercise type), muscles tested by the ROM test, and the pre- and post-intervention values plus standard deviation of the main variable ROM were extracted. If the full paper did not provide all the data required for the meta-analysis, the corresponding authors were contacted via e-mail and Research Gate.
2.4 Statistics and Data Synthesis
The meta-analysis was conducted using the Comprehensive Meta-Analysis software according to the suggestions of Borenstein et al. [30]. Consequently, a random-effect meta-analysis was used to assess the ES (standardized mean difference) for the ROM effects. If any study reported more than one ES, as suggested by Borenstein et al. the mean of all the outcomes (ESs) within one study was used for the analysis and defined with the term “combined” [30]. Although there is no general rule of thumb [30], we only performed a meta-analysis when three or more studies could be included in the respective analysis. Moreover, to assess possible relations in the moderating variables, we conducted a meta-regression (i.e., age of the participants, weeks of intervention, and training sessions per week). Additionally, by using a mixed-effect model, we conducted various subgroup analyses with the activity level of the participants (untrained and sedentary vs trained and active), sex (male vs female vs mixed), type of contraction (eccentric, concentric, and isometric), type of exercise (body weight vs free weight vs machine vs Pilates vs resistance bands vs mixed), and the joint tested (elbow vs hip vs knee vs shoulder vs trunk vs trunk and hip). Trained and active individuals were defined as people who regularly participated in exercise and sports on a weekly basis. Q-statistics were applied [30] to determine if there were differences between the ESs of the subgroups. Hopkins suggested to define the standardized mean difference of < 0.2, 0.2–0.6, 0.6–1.2, 1.2–2.0, 2.0–4.0, and > 4.0 as trivial, small, moderate, large, very large, and extremely large, respectively [31]. To assess the heterogeneity, I2 statistics were calculated among the ESs, and thresholds of 25%, 50%, and 75% were defined as having a low, moderate, and high level of heterogeneity, respectively [32]. An alpha level of 0.05 was defined for the statistical significance of all the tests.
2.5 Risk of Bias Assessment and Methodological Quality
To assess the methodological quality of the included studies, the PEDro scale was used. Two independent researchers assessed 11 methodological issues by assigning either one or no point. Note that studies with a higher score represent a higher methodological quality. If any conflict between the ratings of the two researchers was found, the methodological issues were reassessed and discussed. Moreover, to assess a possible publication bias, visual inspection of the funnel plot and the statistics of the Egger’s regression intercept test were used.
3 Results
3.1 Results of the Search
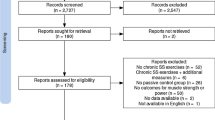
Overall, after removal of the duplicates, 14,851 papers were screened, from which 52 papers were found to be eligible for this review. However, following the additional search of the references (search through the reference list) and citations (search through Google Scholar) of the 52 already included papers, three more papers were identified as relevant. Therefore, in total, 55 papers were included in this systematic review and meta-analysis. The search process is illustrated in Fig. 1. Overall, 222 ESs could be extracted from these studies. In summary, 2756 participants with a mean age of 23.9 ± 6.3 years (range 8.1–78.8 years) participated in the included studies. The characteristics and outcomes of the 55 studies are provided in Table S1 (characteristics table) of the Electronic Supplementary Material (ESM).
3.2 Risk of Bias Assessment and Methodological Quality
Figure 2 shows the funnel plots of all the main meta-analyses. A visual inspection of the funnel plot and the Egger’s regression intercept test (intercept − 1.89; p = 0.002) indicated reporting bias for RT versus controls but not for RT versus stretch training (intercept 0.32; p = 0.95) and for RT and stretch training versus stretch training (intercept 0.54; p = 0.85). The methodological quality, as assessed with the PEDro scale, revealed a range of scores between 4 and 9 points (out of 10) for all the included studies. The average PEDro score was 6.5 (± 0.94), indicating a low risk of bias. The two assessors agreed with 96% of the 605 criteria (55 studies × 11 scores). The mismatched outcomes were discussed, and the assessors agreed on the scores presented in Table 1.
3.3 Main Analysis
The meta-analysis on joint ROM revealed a moderate ES in favor of RT compared with the control condition (ES = − 0.729; Z = − 7.763; 95% confidence interval − 0.913 to − 0.545; p < 0.001; I2 = 73.76; number of ES = 183; number of studies = 52). Figure 3 presents the forest plot of the meta-analysis, sorted by the standard difference in means beginning with the lowest value (− 4.166: [76]) up to the highest value (1.712: [38]). Figures 4 and 5 illustrate the lack of difference in ROM improvements when comparing RT to stretch training (Fig. 4: ES = 0.084; Z = 0.256; 95% confidence interval − 0.558 to 0.725; p = 0.79; I2 = 79.12; number of ES = 23; number of studies = 7) and stretch training to a combination of RT and stretch training (Fig. 5 ES = − 0.001; Z = − 0.005; 95% confidence interval − 0.422 to 0.420; p = 0.996; I2 = 0.00; number of ES = 14; number of studies = 4).
A sensitivity analysis indicated that by removing the lowest ES (− 4.166 by Phrompaet et al. [76]), there was still a significant moderate effect in favor of RT whilst heterogeneity decreased slightly (ES = − 0.669; p < 0.001, I2 = 67.37%). Similarly, the removal of the highest ES (1.712 by Christou et al. [38]) still provided a significant moderate effect in favor of RT with a similar heterogeneity (ES = − 0.759; p < 0.001, I2 = 71.78%). Finally, by removing both the highest and lowest ES [38, 76], we also found a significant moderate effect in favor of RT while heterogeneity decreased (ES = − 0.696; p < 0.001, I2 = 64.07%).
3.4 Moderating Variables
A summary of the subgroup analyses is provided in Table 2. Q statistics of the subgroup analyses revealed no significant differences between sex (male vs female vs mixed), the type of contraction (concentric vs eccentric), and the joint tested (elbow vs hip vs knee vs shoulder vs trunk vs trunk and hip). However, a further subgroup analysis showed a significant (p = 0.005) and greater magnitude of change with the increases in ROM for “untrained and sedentary” (ES = 1.042; p < 0.001) compared with “trained or active people” (ES = 0.43; p < 0.001). Furthermore, Q statistics revealed a significant difference between the types of exercise (p = 0.02). While RT with resistance bands, free weights, machines, Pilates, or mixed exercises (e.g., combination of free weights and machines) showed an increase in ROM, body weight exercises showed no such change (p = 0.11). Meta-regression showed no significant 2p > 0.05) between the ESs to age (R2 = − 0.07), weeks of RT intervention (R2 = 0.02), or training sessions per week (R2 = − 0.01), respectively.
4 Discussion
The major findings of this meta-analysis were that RT (free weights, machines, Pilates) significantly improves joint ROM (ES = 0.73; p < 0.001) with the exception of no significant ROM improvement with RT using body mass. Furthermore, the beneficial effects of RT on ROM were not significantly different from stretch training or the combination of RT and stretch training versus stretch training alone. Although both groups improved ROM with RT, “untrained and sedentary” individuals had a significantly (p = 0.005) higher magnitude of ROM change (ES = 1.042; p < 0.001) compared with “trained or active people” (ES = 0.43; p < 0.001). There were no significant differences between sex or contraction type (e.g., concentric vs eccentric). A meta-regression showed no effect of age, training duration, or frequency.
A Current Opinion article by Nuzzo [5] based on 15 publications (nine articles measured sit and reach, six articles measured ROM with tests other than sit and reach) led him to propose that stretching does not need to be a standard component of exercise because RT and other activities are sufficient for promoting flexibility increases. Nuzzo reported individual study percentage changes in ROM with RT but did not provide information on overall mean changes, ESs (magnitude of change that incorporates standard deviation), or a full meta-analysis (includes ESs as well as measures of sensitivity, bias, and heterogeneity). Subsequently, Afonso et al. [12] did perform a meta-analysis based on 11 studies and reported no significant differences between RT and stretch training for improving ROM. However, they did observe a small ES in favor of stretching (Hedges’g = − 0.22; p = 0.21) over RT for improving ROM. The current meta-analysis generally concurs with the findings of Nuzzo [5] and Afonso et al. [12] based on an expanded analysis of 55 studies. Compared with controls, RT induced a moderate magnitude (standard difference in means: 0.73) increase in ROM, which was not significantly different from the increases incurred with stretch training or a combination of RT and stretch training. The current meta-analysis delved deeper than the prior reviews by differentiating between free weight, machine, body mass, and Pilates RT. Interestingly, only RT with body mass did not provide a significant increase in ROM. However, caution should be taken not to overemphasize these results, as this finding is based on only four ESs (see Table 2).
When contemplating the mechanics of free weight, machine, and Pilates RT on ROM, one might propose that the actions are similar to dynamic stretching albeit with an additional external load. Dynamic stretching has been described as an action that involves controlled movement through the active joint ROM [3, 8, 80]. Free weights and machines (including Pilates) RT typically permits the joints to reach their endpoint ROM or the individual’s point of maximum discomfort at a controlled pace. In contrast, RT with body mass activities may not always permit such an expansive ROM. For example, while a push-up is restricted by chest circumference and the surface (i.e., floor or ground), free weights and machines can permit the shoulder to surpass this more restricted ROM. There are a dearth of studies comparing the effects of partial versus full ROM RT on ROM. Kawama et al. [81] reported significantly greater decreases in the shear modulus of the semimembranosus with eccentric RT through a wide ROM versus either eccentric RT through a narrow ROM or concentric RT with a wide ROM. Furthermore, dynamic stretching involves repeated cyclical muscle loading and unloading [80]. The addition of an external load with RT would augment the stress on musculotendinous and connective tissue and minimize the unloading component. As there are no studies comparing dynamic stretch training and RT on ROM, possible differential effects of these two activities on ROM should be investigated.
Dynamic stretching in some studies has been reported to produce similar [82, 83] as well as greater [84, 85] acute increases in ROM when compared to static stretching. While one study reported more than double the ROM improvements with static versus dynamic stretch training [86], another did not report any significant difference [87]. Zhou and colleagues [88], however, reported that while all dynamic stretching modes in their study improved hip extension ROM in the elderly, the greatest ROM was achieved with dynamic stretching with no additional load versus dynamic stretching with low (0.2-kg) or high (0.5-kg) loads. Hence, it is possible that the full or nearly full ROM used in isoinertial RT is more important for increasing ROM than the external load. As isoinertial RT might be described as dynamic stretching with load, the attributed mechanisms underlying ROM improvements with dynamic stretching may be similar. Generally, these stretching adaptations have been attributed to neural, morphological, and psychological adaptations.
Although all joints exhibited significant moderate-to-large magnitude ROM increases with RT, there were no significant differences in the extent of ROM improvement between the joints. This finding is partially in accord with Afonso et al. [89] who also reported significant improvements with RT in all joints analyzed; however, they did not make direct comparisons between joints. Although it is commonly known that some joints have a substantially greater ROM than others (e.g., hip flexion vs dorsiflexion) [1], the present results demonstrate that the relative RT-induced increases were similar.
In terms of possible neural adaptations, there have been reports with static stretch training (3 and 6 weeks) of reductions in tonic Ia (facilitatory) afferent feedback from muscle spindles (T-reflexes and H-reflexes), which could reduce reflex-induced contractions inducing a more relaxed muscle (disfacilitation) [90, 91]. However, dynamic stretching and isoinertial RT would tend to excite rather than disfacilitate muscle spindle activity and thus would be an unlikely chronic training-induced mechanism for increased ROM. Golgi tendon organ inhibition is more likely to occur with large amplitude stretches [92] and higher muscle tension; however, Golgi tendon organ inhibition tends to subside almost immediately (60–100 ms post-stretching) after the stimulus discontinues [93], thus it is also an unlikely candidate for chronic dynamic stretching or isoinertial RT mechanisms. Recurrent or Renshaw cell inhibition is more prevalent with acute dynamic rather than tonic contractions [94] and can induce stabilizing effects on motoneuron discharge variability, and motor unit synchronization [95]. However, there is no research to confirm whether any of these possible acute neural responses lead to chronic training adaptations.
Morphologically, there is some evidence for dynamic ballistic stretch training to decrease tendon stiffness [96]. There are also reports of acute dynamic stretch-induced decreases in passive resistive torque [10] and muscle stiffness [10, 97], suggesting a more compliant musculotendinous unit following a single session of dynamic and ballistic stretching. However, a 6-week ballistic stretch training program did not detect any significant change in muscle morphology [98]. Magnusson and colleagues [99] contend that in response to loading, tendon metabolic activity is relatively high and can undergo significant length changes allowing the tendon to adapt to changing demands (i.e., changes in tensile force, length, compliance). Furthermore, repeated loading of the tendon with stretching can shift the stress–strain curve to promote an elevated elastic modulus [100].
In contrast, a review by Thomas et al. [101] indicated that the loading of a tendon with RT increases its stiffness by modifying elastic properties versus morphological adaptations such as an increased cross-sectional area. This increase in tendon stiffness was not dependent on muscle contraction type, trained state, or age. The review also summarized that there are reports of both increases and decreases in muscle tissue stiffness with RT and thus there is a lack of clarity regarding the effects of RT on muscle stiffness. Hence, it is unknown whether the increases and possible increases in tendon and muscle stiffness, respectively, with RT are counterbalanced by increased compliance with dynamic stretching.
Eccentric actions demand that the muscle produce force at extended positions and thus might be expected to increase ROM [12]. Training studies (4–15 weeks) emphasizing either eccentric [102, 103] or concentric [103, 104] contractions have reported increases in fascicle length. However, Reeves et al. [103] reported significantly greater increases in fascicle length (20% vs 8%) and lower increases in the pennation angle (5% vs 35%) with 14 weeks (80% of 5 repetition maximum) of eccentric versus conventional (concentric and eccentric) training. A meta-analytical review of the literature demonstrated limited-to-moderate evidence that eccentric training induces significant increases in fascicle length [105]. Hence, while there is lack of clarity regarding RT-induced changes in tendon and muscle stiffness, RT may augment ROM with alterations in fascicle length and the pennation angle.
There is also strong evidence with stretching for an increase in stretch (pain) tolerance (sensory theory) [106, 107]. The discomfort associated with the external torques on the muscles and joints with isoinertial RT would contribute to this increase in pain (stretch) tolerance permitting the individual to push beyond prior limits of discomfort. Hence, the mechanisms for increasing ROM with dynamic stretching with load (isoinertial RT) would likely be related to musculotendinous unit changes in stiffness and compliance as well as augmented stretch tolerance. As there were no significant differences between sex or contraction type and the meta-regression showed no effect of age, training duration, or frequency, these reported ROM changes and mechanisms may be similar across varied populations and training parameters.
“Untrained and sedentary individuals” displayed a significantly higher magnitude of ROM change compared with “trained or active people”. This difference is likely related to the baseline level of flexibility. Trained individuals would have already experienced an increased ROM owing to the prior chronic dynamic loading on their musculotendinous units. Hence, their scope of training-induced ROM increases would be blunted compared with previously untrained individuals [1, 108]. However, the trained individuals still experienced significant ROM improvements albeit to a lesser degree than the untrained.
The funnel plot (Fig. 2A) and the Egger’s regression intercept test (intercept − 1.89; p = 0.002) for RT versus controls (but not for the other meta-analyses) indicated a reporting bias limitation. It is clearly established that significant positive results are more likely to be published with an increased probability that they would be published in higher impact journals and thus also achieve a higher number of citations [109, 110]. Although one must always be cautious when interpreting results, especially those with a possibility of bias, the results of the main analysis (RT vs control) of 52 studies did demonstrate moderate standardized differences in means (0.72).
As with all studies, there are limitations to this meta-analysis. The computer software program used for our analysis (Comprehensive Meta-Analysis) calculates the arithmetic mean when more than one ES from one study is taken, which does not fully take into account the dependency of ESs, and which may therefore contribute to imprecise estimates (true heterogeneity is higher). While nesting dependent ESs within each study is preferable, the Comprehensive Meta-Analysis software does not possess that capability. Both a strength and weakness of this review may be related to the fact that different ROM tests were combined in the analysis. As the meta-analysis compares standard mean differences, it allows for a comparison of disparate but related tests. However, the mean difference of changes of joints with different anatomical configurations (e.g., hip flexion and dorsiflexion ROM) or different tests for the same joint (e.g., sit and reach vs supine hip flexion) may not provide as sensitive an analysis as comparing the same joints and tests. In contrast, it does allow a comparison of a much greater volume of research articles providing a broader perspective. Regarding ecological validity, the articles in this review cover a wide spectrum of the population and training parameters. For example, the review articles include age ranges of 10–70 years, training durations of 4–24 weeks, and training frequencies of 2–5 sessions per week at 40–110% of 1RM with a spectrum of sedentary, athletic, untrained, recreationally active individuals (see Table S1 in the ESM). Finally, the inclusion of articles that the authors may be aware of in addition to those found through objective search criteria could introduce bias. However, we identified these articles and a further analysis did not detect bias.
5 Conclusions
As RT with external loads can improve ROM, additional stretching prior to or after RT may not be necessary to enhance flexibility. Based on the present studies and the literature, both stretching and RT can improve ROM, improve strength [1, 111, 112], and decrease musculotendinous injury incidence [113]. When circumstances dictate (i.e., time restrictions), flexibility training benefits can be incorporated into RT; however, stretch training can still be advocated as a fitness and training component for much of the population. For example, RT would not be suitable as a component of a warm-up prior to competition and thus stretching would play an important role in certain activity or competition preparation. Stretching is also used as a form of relaxation for many practitioners, for which RT may not be as appropriate.
Availability of Data and Material
The data from articles used for this meta-analysis are available in Table S1 of the ESM.
Code Availability
Not applicable.
References
Behm DG. The science and physiology of flexibility and stretching: implications and applications in sport performance and health. London: Routledge Publishers; 2018.
Afonso J, Olivares-Jabalera J, Andrade R. Time to move from mandatory stretching? We need to differentiate “can I?” from “do I have to?” Front Physiol. 2021;12:714166.
Behm DG, Chaouachi A. A review of the acute effects of static and dynamic stretching on performance. Eur J Appl Physiol. 2011;111(11):2633–51.
Behm DG, Kay AD, Trajano GS, Blazevich AJ. Mechanisms underlying performance impairments following prolonged static stretching without a comprehensive warm-up. Eur J Appl Physiol. 2021;121(1):67–94.
Nuzzo JL. The case for retiring flexibility as a major component of physical fitness. Sports Med. 2020;50(5):853–70.
Kay AD, Blazevich AJ. Effect of acute static stretch on maximal muscle performance: a systematic review. Med Sci Sports Exerc. 2012;44(1):154–64.
Chaabene H, Behm DG, Negra Y, Granacher U. Acute effects of static stretching on muscle strength and power: an attempt to clarify previous caveats. Front Physiol. 2019;10:1468.
Behm DG, Blazevich AJ, Kay AD, McHugh M. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: a systematic review. Appl Physiol Nutr Metab. 2016;41(1):1–11.
Konrad A, Gad M, Tilp M. Effect of PNF stretching training on the properties of human muscle and tendon structures. Scand J Med Sci Sports. 2015;25(3):346–55.
Konrad A, Stafilidis S, Tilp M. Effects of acute static, ballistic, and PNF stretching exercise on the muscle and tendon tissue properties. Scand J Med Sci Sports. 2017;27(10):1070–80.
Konrad A, Tilp M. Increased range of motion after static stretching is not due to changes in muscle and tendon structures. Clin Biomech. 2014;29(6):636–42.
Afonso J, Ramirez-Campillo R, Moscao J, Rocha T, Zacca R, Martins A, et al. Strength training versus stretching for improving range of motion: a systematic review and meta-analysis. Healthcare. 2021;9(4):427.
Behm DG. Neuromuscular implications and applications of resistance training. J Strength Cond Res. 1995;9(4):264–74.
Behm DG, Sale DG. Velocity specificity of resistance training. Sports Med. 1993;15(6):374–88.
Hahn D. Stretching the limits of maximal voluntary eccentric force production in vivo. J Sport Health Sci. 2018;7(3):275–81.
Hollander DB, Kraemer RR, Kilpatrick MW, Ramadan ZG, Reeves GV, Francois M, et al. Maximal eccentric and concentric strength discrepancies between young men and women for dynamic resistance exercise. J Strength Cond Res. 2007;21(1):34–40.
Delvaux F, Schwartz C, Decréquy T, Devalckeneer T, Paulus J, Bornheim S, et al. Influence of a field hamstring eccentric training on muscle strength and flexibility. Inter J Sports Med. 2020;41(04):233–41.
Kay AD, Richmond D, Talbot C, Mina M, Baross AW, Blazevich AJ. Stretching of active muscle elicits chronic changes in multiple strain risk factors. Med Sci Sports Exerc. 2016;48(7):1388–96.
Nelson RT, Bandy WD. Eccentric training and static stretching improve hamstring flexibility of high school males. J Athl Train. 2004;39(3):254–8.
Morton SK, Whitehead JR, Brinkert RH, Caine DJ. Resistance training vs. static stretching: effects on flexibility and strength. J Strength Cond Res. 2011;25(12):3391–8.
Wyon MA, Smith A, Koutedakis Y. A comparison of strength and stretch interventions on active and passive ranges of movement in dancers: a randomized controlled trial. J Strength Cond Res. 2013;27(11):3053–9.
Kim E, Dear A, Ferguson SL, Seo D, Bemben MG. Effects of 4 weeks of traditional resistance training vs. superslow strength training on early phase adaptations in strength, flexibility, and aerobic capacity in college-aged women. J Strength Cond Res. 2011;25(11):3006–13.
Faigenbaum AD, Zaichkowsky L, Westcott W, Michelli L, Fehlandt A. The effects of twice per week strength training program on children. Pediatr Exerc Sci. 1993;5:339–46.
Faigenbaum AD, Milliken LA, Loud RL, Burak BT, Doherty CL, Westcott WL. Comparison of 1 and 2 days per week of strength training in children. Res Q Exerc Sport. 2002;73(4):416–24.
Simao R, Lemos A, Salles B, Leite T, Oliveira E, Rhea M, et al. The influence of strength, flexibility, and simultaneous training on flexibility and strength gains. J Strength Cond Res. 2011;25(5):1333–8.
Moraes E, Fleck SJ, Ricardo Dias M, Simao R. Effects on strength, power, and flexibility in adolescents of nonperiodized vs. daily nonlinear periodized weight training. J Strength Cond Res. 2013;27(12):3310–21.
Konrad A, Nakamura M, Tilp M, Donti O, Behm DG. Foam rolling training effects on range of motion: a systematic review and meta-analysis. Sports Med. 2022;52(10):2523–35.
Wilke J, Muller AL, Giesche F, Power G, Ahmedi H, Behm DG. Acute effects of foam rolling on range of motion in healthy adults: a systematic review with multilevel meta-analysis. Sports Med. 2020;50(2):387–402.
David Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. John Wiley & Sons; 2021.
Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–12.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Barbosa AR, Santarem JM, Filho WJ, Maricci MdFN. Effects of resistance training on the sit-and-reach test in elderly women. J Strength Cond Res. 2002;16(1):14–8.
Abdel-aziem AA, Soliman ES, Abdelraouf OR. Isokinetic peak torque and flexibility changes of the hamstring muscles after eccentric training: trained versus untrained subjects. Acta Orthop Traumatol Turc. 2018;52(4):308–14.
Souza AC, Melibeu BC, Freitas SB, Machado RV, Vilaça AJ, Miranda H, et al. Influence of inter-set stretching on strength, flexibility and hormonal adaptations. J Hum Kinet. 2013;36(1):127–35.
Kwak C, Kim YL, Lee SM. Effects of elastic-band resistance exercise on balance, mobility and gait function, flexibility and fall efficacy in elderly people. J Phys Ther Sci. 2016;28:3189–96.
Chinnavan E, Gopaladhas S, Kaikondan P. Effectiveness of pilates training in improving hamstring flexibility of football players. Bangladesh J Med Sci. 2015;14(3):265–9.
Christou M, Smilios I, Sotiropoulos K, Volaklis K, Pilianidis T, Tokmakidis SP. Effects of resistance training on the physical capacities of adolescent soccer players. J Strength Cond Res. 2006;20(4):783–91.
da Costa TC, Locks RR, Koppe S, Yamaguti AM, Formiga AC, Gomes ARS. Strength and stretching training and detraining on flexibility of older adults. Top Geriatr Rehabil. 2013;29(2):142–8.
da Cruz TMF, Germano MD, Crisp AH, Sindorf MAG, Verlengia R, da Mota GR, et al. Does Pilates training change physical fitness in young basketball athletes? J Exerc Physiol. 2014;17(1).
Campos de Oliveira L, Gonçalves de Oliveira R, Pires-Oliveira DAdA. Comparison between static stretching and the Pilates method on the flexibility of older women. J Bodyw Mov Ther. 2016;20(4):800–6.
Cyrino ES, de Oliveira AR, Leite JC, Porto DB, Dias RMR, Segantin AQ, et al. Flexibility behavior after 10 weeks of resistance training. Rev Bras Med Esporte. 2004;10(4).
Faigenbaum AD, Mediate P. Effects of medicine ball training on fitness performance of high-school physical education students. Phys Educ. 2006;63(3):160–7.
Fatouros IG, Taxildaris K, Tokmakidis SP, Kalapotharakos V, Aggelousis N, Athanasopoulos S, et al. The effects of strength training, cardiovascular training and their combination on flexibility of inactive older adults. Int J Sports Med. 2002;23(2):112–9.
Fatouros IG, Kambas A, Katrabasas I, Leontsini D, Chatzinikolaou A, Jamurtas AZ, et al. Resistance training and detraining effects on flexibility performance in the elderly are intensity-dependent. J Strength Cond Res. 2006;20(3):634–42.
Fourie M, Gildenhuys GM, Shaw I, Shaw BS, Toriola AL, Goon DT. Effects of a mat pilates program on flexibility in elderly women. Med Sport. 2013;66(4):545–53.
Delvaux F, Schwartz C, Decréquy T, Devalckeneer T, Paulus J, Bornheim S, et al. Influence of a field hamstring eccentric training on muscle strength and flexibility. Int J Sports Med. 2020;41(04):233–41.
Fritz NB, Juesas Á, Gargallo P, Calatayud J, Fernández-Garrido J, Rogers ME, et al. Positive effects of a short-term intense elastic resistance training program on body composition and physical functioning in overweight older women. Biol Res Nurs. 2018;20(3):321–34.
González-Gálvez N, Vaquero-Cristóbal R, Marcos-Pardo PJ. Effect of Pilates method on muscular trunk endurance and hamstring extensibility in adolescents during twelve weeks training and detraining. J Bodyw Mov Ther. 2020;24(2):11–7.
Greco G, Patti A, Cataldi S, Iovane A, Messina G, Fischetti F. Changes in physical fitness in young female volleyball players after an 8-week in-season Pilates training program. Acta Medica Mediterranea. 2019;35:3375–81.
Greco G, Messina G, Angiulli A, Patti A, Iovane A, Fischetti F. A preliminary comparative study on the effects of Pilates training on physical fitness of young female volleyball players. Acta Medica Mediterranea. 2019;35:783–9.
Guex KJ, Lugrin V, Borloz S, Millet GP. Influence on strength and flexibility of a swing phase-specific hamstring eccentric program in sprinters’ general preparation. J Strength Cond Res. 2016;30(2):525–32.
Elsangedy HM, Oliveira GTA, Machado DGS, Tavares MPM, Araújo AdO, Krinski K, et al. Effects of self-selected resistance training on physical fitness and psychophysiological responses in physically inactive older women: a randomized controlled study. Percept Mot Skills. 2020;128(1):467–91.
JOA˜O BRENO Ribeiro-Alvares JB, Marques VB, Vaz MA, Baroni BM. Four weeks of nordic hamstring exercise reduce muscle injury risk factors in young adults. J Strength Cond Res. 2017;32(5):1254–62.
Júnior R, Leite T, Reis V. Influence of the number of sets at a strength training in the flexibility gains. J Hum Kinet. 2011;29A(Special-Issue):47–52.
Vasilios I. Kalapotharakos VI, Maria Michalopoulos M, Savvas p. Tokmakidis SP, Godolias G, Gourgoulis V. Effects of a heavy and a moderate resistance training on functional performance in older adults. J Strength Cond Res. 2005;19(3):652–7.
Kao Y, Liou T, Huang Y, Tsai Y, Wang K. Effects of a 12-week Pilates course on lower limb muscle strength and trunk flexibility in women living in the community. Health Care Women Int. 2014;36(3):303–19.
Kılıç T, Hınçal S. The effects of Pilates mat exercises on self-rated health levels, body mass index and flexibility in middle age sedentary women. Educ Quart Rev. 2021;4(2).
Kloubec JA. Pilates for improvement of muscle endurance, flexibility, balance, and posture. J Strength Cond Res. 2010;24(3):661–7.
Lee H, Caguicla JMC, Park S, Kwak DJ, Won D, Park Y, et al. Effects of 8-week Pilates exercise program on menopausal symptoms and lumbar strength and flexibility in postmenopausal women. J Exerc Rehab. 2016;12(3):247–51.
Leite T, Teixeira AdS, Saavedra F, Leite RD, Rhea MR, Simao R. Influence of strength and flexibility training, combined or isolated, on strength and flexibility gains. J Strength Cond Res. 2015;29(4):1083–8.
Vécseyné Kovách M, Kopkáné Plachy J, Bognár J, Olvasztóné Balogh Z, Barthalos I. Effects of Pilates and aqua fitness training on older adults’ physical functioning and quality of life. Biomed Hum Kinet. 2013;5(1):22–7.
Manshouri M, Rahnama N, Babaei KM. Effects of pilates exercises on flexibility and volleyball serve skill in female college students. Inter J Curr Res. 2014;6(12):11188–92.
Monteiro WD, Simao R, Polito MD, Santana CA, Chaves RB, Bezerra E, et al. Influence of strength training on adult women’s flexibility. J Strength Cond Res. 2008;22(3):672–7.
Mueller D, Redkva PE, Fernando de Borba E, Barbosa SC, Krause MP, Gregorio da Silva S. Effect of mat vs. apparatus pilates training on the functional capacity of elderly women. J Bodyw Mov Ther. 2021;25:80–6.
Takeshima N, Islam MM, Rogers ME, Rogers NL, Sengoku N, Koizum D, et al. Effects of Nordic walking compared to conventional walking and band-based resistance exercise on fitness in older adults. J Sports Sci Med. 2013;12:422–30.
Ruslan N, Wan Norman WMN, Muhamad AS, Madzlan NH. Effects of eccentric training using Theraband on hamstring flexibility in elderly. Proceedings of the International Colloquium on Sports Science, Exercise, Engineering and Technology 2014 (ICoSSEET 2014). 2014:127–34.
Potier TG, Alexander CM, Seynnes OR. Effects of eccentric strength training on biceps femoris muscle architecture and knee joint range of movement. Eur J Appl Physiol. 2009;105(6):939–44.
Rayes ABR, de Lira CAB, Viana RB, Benedito-Silva AA, Vancini RL, Mascarin N, et al. The effects of Pilates vs. aerobic training on cardiorespiratory fitness, isokinetic muscular strength, body composition, and functional tasks outcomes for individuals who are overweight/obese: a clinical trial. PeerJ. 2019;7:e6022.
Reinold MM, Macrina LC, Fleisig GS, Aune K, Andrews JR. Effect of a 6-week weighted baseball throwing program on pitch velocity, pitching arm biomechanics, passive range of motion, and injury rates. Sports Health. 2018;10(4):327–33.
Vatovec R, Marušič J, Marković G, Šarabon N. Effects of Nordic hamstring exercise combined with glider exercise on hip flexion flexibility and hamstring passive stiffness. J Sports Sci. 2021;39(20):2370–7.
Santos E, Rhea MR, Simao R, Dias I, de Salles BF, Novaes J, et al. Influence of moderately intense strength training on flexibility in sedentary young women. J Strength Cond Res. 2010;24(11):3144–9.
Saraiva AR, Reis VM, Costa PB, Bentes CM, Costa e Silva GV, Novaes JS. Chronic effects of different resistance training exercise orders on flexibility in elite judo athletes. J Hum Kinet. 2014;40(1):129–37.
Sekendiz B, Altun Ö, Korkusuz F, Akın S. Effects of Pilates exercise on trunk strength, endurance and flexibility in sedentary adult females. J Bodyw Mov Ther. 2007;11(4):318–26.
Sinđić M, Mačak D, Todorović N, Purda B, Batez M. Effect of integrated neuromuscular exercise in physical education class on health-related fitness in female children. Healthcare. 2021;9(3):312.
Phrompaet S, Paungmali A, Pirunsan U, Sitilertpisan P. Effects of Pilates training on lumbo-pelvic stability and flexibility. Asian J Sports Med. 2011;2(1):16–22.
Swank AM, Funk DC, Durham MP, Roberts S. Adding weights to stretching exercise increases passive range of motion for healthy elderly. J Strength Cond Res. 2003;17(2):374–8.
Versic S, Idrizovic K, Ahmeti GB, Sekulic D, Majeric M. Differential effects of resistance- and endurance-based exercise programs on muscular fitness, body composition, and cardiovascular variables in young adult women: contextualizing the efficacy of self-selected exercise modalities. Medicina. 2021;57(7):654.
Yaprak Y. The effects of back extension training on back muscle strength and spinal range of motion in young females. Biol Sport. 2013;30(3):201–6.
Fletcher IM. The effect of different dynamic stretch velocities on jump performance. Eur J Appl Physiol. 2010;109(3):491–8.
Kawama R, Yanase K, Hojo T, Wakahara T. Acute changes in passive stiffness of the individual hamstring muscles induced by resistance exercise: effects of contraction mode and range of motion. Eur J Appl Physiol. 2022;122(9):2071–83.
Beedle BB, Mann CL. A comparison of two warm-ups on joint range of motion. J Strength Cond Res. 2007;21(3):776–9.
Perrier ET, Pavol MJ, Hoffman MA. The acute effects of a warm-up including static or dynamic stretching on countermovement jump height, reaction time, and flexibility. J Strength Cond Res. 2011;25(7):1925–31.
Duncan MJ, Woodfield LA. Acute effects of warm-up protocol on flexibility and vertical jump in children. J Exerc Physiol. 2006;9(3):9–16.
Amiri-Khorasani M, Abu Osman NA, Yusof A. Acute effect of static and dynamic stretching on hip dynamic range of motion during instep kicking in professional soccer players. J Strength Cond Res. 2011;25(6):1647–52.
Bandy WD, Irion JM, Briggler M. The effect of static stretch and dynamic range of motion training on the flexibility of the hamstring muscles. J Orthop Sports Phys Ther. 1998;27(4):295–300.
Wiemann K, Hahn K. Influences of strength, stretching and circulatory exercises on flexibility parameters of the human hamstrings. Int J Sports Med. 1997;18(5):340–6.
Zhou WS, Lin JH, Chen SC, Chien KY. Effects of dynamic stretching with different loads on hip joint range of motion in the elderly. J Sports Sci Med. 2019;18(1):52–7.
Afonso J, Ramirez-Campillo R, Moscao J, Rocha T, Zacca R, Martins A, et al. Strength training versus stretching for improving range of motion: a systematic review and meta-Analysis. Healthcare (Basel). 2021 Apr 7;9(4).
Blazevich AJ, Cannavan D, Waugh CM, Fath F, Miller SC, Kay AD. Neuromuscular factors influencing the maximum stretch limit of the human plantar flexors. J Appl Physiol. 2012;113(9):1446–55.
Guissard N, Duchateau J. Effect of static stretch training on neural and mechanical properties of the human plantar-flexor muscles. Muscle Nerve. 2004;29:248–55.
Guissard N, Duchateau J. Neural aspects of muscle stretching. Exerc Sport Sci Rev. 2006;34(4):154–8.
Houk JC, Crago PE, Rymer WZ. Functional properties of the Golgi tendon organs. In: Desmedt JE, editor. Spinal and supraspinal mechanisms of voluntary motor control and locomotion. 1980: p. 33–43.
Katz R, Pierrot-Deseilligny E. Recurrent inhibition in humans. Prog Neurobiol. 1999;57(3):325–55.
Mattei B, Schmied A, Mazzocchio R, Decchi B, Rossi A, Vedel JP. Pharmacologically induced enhancement of recurrent inhibition in humans: effects on motoneurone discharge patterns. J Physiol. 2003;548(Pt 2):615–29.
Mahieu NN, McNair P, De MM, Stevens V, Blanckaert I, Smits N, et al. Effect of static and ballistic stretching on the muscle-tendon tissue properties. Med Sci Sports Exerc. 2007;39(3):494–501.
Herda TJ, Herda ND, Costa PB, Walter-Herda AA, Valdez AM, Cramer JT. The effects of dynamic stretching on the passive properties of the muscle-tendon unit. J Sports Sci. 2013;31(5):479–87.
Konrad A, Tilp M. Effects of ballistic stretching training on the properties of human muscle and tendon structures. J Appl Physiol. 2014;117(1):29–35.
Magnusson SP, Narici MV, Maganaris CN, Kjaer M. Human tendon behaviour and adaptation, in vivo. J Physiol. 2008;586(1):71–81.
Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Sports. 2003;13(4):211–23.
Thomas E, Ficarra S, Nakamura M, Paoli A, Bellafiore M, Palma A, et al. Effects of different long-term exercise modalities on tissue stiffness. Sports Med Open. 2022;8:71.
Marusic J, Vatovec R, Markovic G, Sarabon N. Effects of eccentric training at long-muscle length on architectural and functional characteristics of the hamstrings. Scand J Med Sci Sports. 2020;30(11):2130–42.
Reeves ND, Maganaris CN, Longo S, Narici MV. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp Physiol. 2009;94(7):825–33.
Valamatos MJ, Tavares F, Santos RM, Veloso AP, Mil-Homens P. Influence of full range of motion vs. equalized partial range of motion training on muscle architecture and mechanical properties. Eur J Appl Physiol. 2018 118(9):1969–83.
Gerard R, Gojon L, Decleve P, Van Cant J. The effects of eccentric training on Biceps femoris architecture and strength: a systematic review with meta-analysis. J Athl Train. 2020;55(5):501–14.
Magnusson SP, Simonsen EB, Aagaard P, Sorensen H, Kjaer M. A mechanism for altered flexibility in human skeletal muscle. J Physiol. 1996;497(Pt 1):291–8.
Magnusson P, Renstrom P. The European college of sports sciences position statement: the role of stretching exercises in sports. Eur J Sport Sci. 2006;6(2):87–91.
Lima CDR, Behm DG, Brown LE. Acute effects of stretching on flexibility and performance: a narrative review. J Sci Sport Exerc. 2019;1:29–37.
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–72.
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320(7249):1574–7.
Yahata K, Konrad A, Sato S, Kiyono R, Yoshida R, Fukaya T, et al. Effects of a high-volume static stretching programme on plantar-flexor muscle strength and architecture. Eur J Appl Physiol. 2021;121(4):1159–66.
Warnecke K, Keiner M, Hillebrecht M, Schiemann S. Influence of one hour versus two hours of daily static stretching for six weeks using a calf-muscle-stretching orthosis on maximal strength. Inter J Environ Res Public Health. 2022;19:11621–9.
Behm DG, Kay AD, Trajano GS, Alizadeh S, Blazevich AJ. Effects of stretching on injury risk reduction and balance. J Clin Exerc Physiol. 2021;10(3):106–16.
Funding
Open access funding provided by Austrian Science Fund (FWF). This work was partially supported by the Natural Science and Engineering Research Council of Canada (NSERC) (RGPIN-2017-03728) [David Behm] as well as a grant (Project J 4484) from the Austrian Science Fund (FWF) [Andreas Konrad]. The NSERC provides overall funding (equipment, supplied and student funding) for Dr. Behm’s laboratory. The FWF pays for open access charges.
Author information
Authors and Affiliations
Contributions
AD, AZ, SHA, RG, JPH, and SA were the main contributors for article and data search and collection. SA also organized the data for the meta-analysis. AK conducted the meta-analysis and was the primary author of the results section. SA and AK were the primary authors for the methods section. DB was the primary author for the abstract, introduction, and discussion. All authors reviewed each iteration of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Shahab Alizadeh, Abdolhamid Daneshjoo, Ali Zahiri, Saman Hadjizadeh Anvar, Reza Goudini, Jared Hicks, Andreas Konrad, and David Behm have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
No ethics approval was necessary as there were no participants in this systematic review and meta-analysis.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alizadeh, S., Daneshjoo, A., Zahiri, A. et al. Resistance Training Induces Improvements in Range of Motion: A Systematic Review and Meta-Analysis. Sports Med 53, 707–722 (2023). https://doi.org/10.1007/s40279-022-01804-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-022-01804-x