Abstract
Long-term active restoration is often employed to restore degraded grasslands. The establishment of a viable soil seed bank is the key to successful restoration, as it enhances the resilience of vegetation. However, little is known of how the soil seed bank affects vegetation resilience following long-term active restoration of degraded grasslands. We determined seed abundance and species composition of the soil seed bank and soil properties and vegetation resilience of intact, degraded, and long-term (>10 years) actively restored grasslands on the Tibetan plateau (3900–4200 m a.s.l.). The plant-soil-seed bank quality index and structural equation modelling (SEM) were used to assess the effect of the soil seed bank on vegetation resilience. After long-term (>10 years) active restoration of degraded grasslands by sowing seeds of native plant species, the densities of transient and persistent seeds increased by 5%, but seed richness (number of species) decreased by 25% when compared with degraded grasslands. This occurred largely as a result of an increase in grass but decrease in forb seeds. Persistent seeds of grasses play an important role in the productivity of restored grasslands, while the density of persistent seeds serves as an indicator of the resilience of vegetation. A combination of the plant community and soil properties determined seed density. Here, we show for the first time that long-term active restoration enhances vegetation resilience of grasslands by altering the soil seed bank. A high seed density of sown Gramineae and a low seed density of forbs in the soil seed bank is a key to the successful active restoration of degraded grasslands.
Similar content being viewed by others
1 Introduction
The restoration of degraded grasslands relies largely on the re-establishment of the soil seed bank (European Commission 2015; Guittar et al. 2020; Freitag et al. 2021). The soil seed bank functions as a genetic memory of plant species in reserve (LaForgia et al. 2018; Moreno-de las Hera et al. 2016; Plue et al. 2021) and also plays a crucial role in the restoration of grasslands (Ma et al. 2010). As seeds are potential vegetation buried in the soil, the impact of ecosystem restoration on soil seed banks is of major interest since seeds are important in maintaining communities and preventing local extinction (Plue et al. 2021). In addition, soil seed banks represent the regenerative potential of above-ground vegetation, act as a buffer to offset annual fluctuations in seed yield and quality, indicate the restoration capability of degraded grasslands, are major determinants of ecosystem stability (Plue et al. 2021; van Moorsel et al. 2021), and are usually tolerant of adverse conditions (Bakker et al. 1996).
The sowing of seeds from native plants may be the most effective strategy for recovery of vegetation and local plant diversity of degraded grasslands (Crouzeilles et al. 2016; Ludewig et al. 2021; Philipson et al. 2020). This strategy allows the choice of plant species, and the management of functions and services of degraded ecosystems; however, restoring degraded grasslands to its prior state may be difficult or nearly impossible (Hobbs et al. 2009; 2014). In a study on short-term active restoration, it could not be determined whether sowing seeds of native plants re-established the seed bank of degraded alpine grasslands (Dong et al. 2020). Compared with passive restoration, active restoration, such as sowing seeds, active planting, transplanting, and burning, could accelerate the rate and extent of recovery of degraded grasslands (Crouzeilles et al. 2017; Philipson et al. 2020).
Assessments of the resilience of vegetation and succession of active restoration of degraded grasslands have employed a number of measurements, including changes in above-ground biomass (Carlsson et al. 2017), below-ground biomass (Sabatier et al. 2017), species richness, plant coverage (Li et al. 2013a), seed and seedling traits (Leger et al. 2019), soil total carbon (Bai et al. 2020), nitrogen (Li et al. 2013b) and phosphorus (Müller et al. 2016) contents, and microbial carbon and nitrogen biomasses (Li et al. 2013b). However, the status of the soil seed bank was reported to be a better indicator for assessing restoration success, especially for potential vegetation succession (Kiss et al. 2018; Plue et al. 2021). Persistent seeds, which remain viable for more than 1 year, played a crucial role in the restoration of degraded wetland ecosystems (Ma et al. 2018). Transient seeds remain viable for less than 1 year (Thompson et al. 1997). However, little is known on the impact of re-vegetation on seasonal dynamics of soil seed banks, and this gap needs to be filled if active restoration is employed in restoring degraded grasslands.
Despite widespread reports of the impacts of restoration on plant community structure and soil properties, studies on soil seed banks in degraded and restored grasslands are lacking and, when examined, are generally limited to a single sampling period (Engst et al. 2017). It was reported that management regimes, above-ground vegetation, and soil properties affected the soil seed bank composition and diversity in a saline-alkaline grasslands (Mohammed and Denboba 2020). Deferred grazing from spring to autumn and rotational grazing increased the soil seed bank of perennial and annual grasses (Nie et al. 2015). However, soil seed banks made limited contributions to the maintenance of plant biodiversity in both mown and abandoned sites on the Great Hungarian Plain (Valko et al. 2020).
It is difficult to establish soil seed banks for targeted plant species in alpine areas by sowing seeds of native plant species in short-term active restoration programs, as was noted in our previous studies (Li et al. 2012; Shang et al. 2013; 2016). Gao et al. (2019) concluded that more than 10 years were required to restore the above-ground vegetation in degraded alpine grasslands. Consequently, we reasoned that the re-establishment of the soil seed bank of native species may also require more than 10 years of active restoration in the harsh environment of the degraded alpine grasslands. However, there is little information on long-term effects of active restoration on the soil seed bank and the resilience of the vegetation. To fill this gap, the soil seed bank of a long-term (>10 years) restored grasslands was compared with soil seed banks of intact and degraded grasslands (Fig. 1), and three questions were addressed: (1) is there a change in the soil seed bank after long-term active restoration?; (2) is the persistent or transient seed bank more important in long-term restoration of degraded grasslands?; and (3) which components of the soil seed bank (grasses, sedges and forbs) contribute to the resilience of vegetation in long-term active restoration? Results from this study could enhance our understanding of the impact of long-term active restoration on soil seed banks and could aid in developing programs for the active restoration of degraded grasslands.
2 Materials and methods
2.1 Study sites
Grasslands, which cover more than 60% of the Tibetan Plateau, are of great importance in water, nutrient, and carbon cycles and also provide ecological services and functions. However, approximately 30% of the grasslands is degraded (Miehe et al. 2019), mainly due to climate change and anthropogenic activities (e.g., grazing, land use change). It has been estimated that non-climatic factors accounted for 66% of the degradation and climatic factors accounted for 34% (Pan et al. 2017). Sowing seeds from native plants, rotational grazing of livestock and grazing exclusion have been employed in attempts to actively restore degraded grasslands (Dong et al. 2020).
The study sites in Maqin, Dari, and Gande counties (Fig. 2; Table S1) were located in the Sanjiangyuan region, Qinghai Province, Tibetan plateau (31°39′N–36°12′N, 89°45′E–102°23′E). There were at least a 100 km between any two sites (Fig. 2; Table S1). Altitudes ranged between 3900 and 4200 m above sea level, mean annual air temperature ranged between −5.6 °C and 3.8 °C and annual precipitation ranged between 262 and 773 mm, with approximately 80% falling in the growing season from May to September. The grasslands were alpine pasture and the soil was alpine meadow in the Chinese Soil Taxonomy (Li. 2001), and classified as Fluvaquentic Cryaquepts from alluvium in the USDA soil taxonomy system (Soil Survey Staff. 2014). Dominant plant species were grasses (Poa pratensis, Stipa przewalskii, Elymus nutans, and Festuca ovina), sedges (Carex atrofusca, Kobresia tibetica, K. pygmaea, and K. humilis), and forbs (Saussurea superba, Astragalus fenzelianus, Herba potentillae, Ajania tenuifolia, Chenopodium glaucum, and Polygonum viviparum). Yaks (Poephagus grunniens) grazed at all sites in winter and spring.
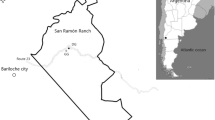
The location of the sampling sites, Maqin, Gande, and Dari counties, on the Tibetan plateau. Locations of Maqin, Gande, and Dari counties (a). Locations of intact, restored, and degraded grasslands in each county (b–d) based on the result of zoomed map (a), different locations represent distance among them. A scale is presented in b–d. Elevation is indicated by color shading.
2.2 Experimental design
For true replications of intact, degraded, and restored grasslands, all three types of grasslands were selected in each county in 2013 (Zhang et al. 2019; Bai et al. 2020). To minimize differences in climate and topography, three plots (100 m × 100 m) of each grassland were selected in each county. The intact grasslands were dominated by Kobresia spp. and Gramineae, and the degraded grasslands (bare land, i.e., “black soil land”) were dominated by poisonous and ruderal plants, mainly Oxytropis ochrocephala, Carum carvi, Aconitum pendulum, and Heteropappus altaicus, in the warm season (Shang et al. 2016; Table S1). The active restored grasslands were degraded grasslands that were sown with native seeds in June 2000 (see Bai et al. 2020 for full details on the study sites). The land, consisting of black soil, was plowed to a depth of 20 to 30 cm, and yak and Tibetan sheep dung (3000 kg/ha) with inorganic fertilizer (carbamide, 150 kg/ha) were applied. A mixture of Elymus nutans, Festuca sinensis, and Poa pratensis cv. Qinghai seeds (1:1:1) were sown (20–23 kg/ha) at a depth of 10 to 20 mm. The grasslands were not grazed by livestock for 5 years after seeding, and then were grazed at a stocking rate of 3.1 to 5.5 sheep units ha-1. To determine the plant community (above-ground biomass and above-ground richness), physical and chemical properties of the soil (total nitrogen, soil organic carbon, soil pH, soil moisture content and soil bulk density), and parameters of the soil seed bank (seed density and seed richness), three subplots were selected randomly within each plot. In total, there were 81 subplots (3 types of grasslands × 3 seasons × 3 plots × 3 subplots).
2.3 Soil seed bank sampling and characterization
Three subplots (30 m × 30 m) were chosen randomly in each of the three plots, and twenty soil core (3.6 cm diameter) samples were collected randomly from each at depths of 0–5 cm and 5–10 cm (Erfanzadeh et al. 2022; Gasempour et al. 2022). To follow seasonal dynamics of the soil seed bank and to identify both transient and persistent seeds, samples were collected in April before seed germination for the transient and persistent seeds, in August after the spring seed germination flush and before seed dispersal for the persistent seeds, and in December after seed dispersal for the transient and persistent seeds. Twenty samples from each depth were taken randomly with a soil auger (3.6 cm diameter) and pooled into a single sample. The total number of soil samples was 18 per grasslands type (9 samples at 2 depths) and the total area sampled was 1831 cm2 for each. Soil samples were air-dried, sieved through a 6-mm screen to remove stones, debris, and coarse material, and stored at 2–4 °C in darkness for 2 months before processing (An et al. 2020; Ma et al. 2017b; Miller and Cummins 2003).
Identification of seeds followed the seedling emergence method described by Thompson et al. (1997). Each soil sample was spread out over a sand and gravel mixture in a germination tray (300 mm × 300 mm × 53 mm). The layer of soil was less than 2-cm thick and the gravel mixture had been sterilized at 150 °C for 24 h. In addition, 30 control trays with the sand and gravel mixture were used to check for seed contamination. All trays were placed randomly in the greenhouse and maintained under 16-h light: 8-h dark, with an average air temperature of 11.5 °C. The trays were watered regularly from below. Seedlings that emerged were removed weekly after identification to the level of species. This part of the study was completed in 13 months.
2.4 Vegetation sampling
The frequency, cover, and above-ground biomass were recorded in three quadrats (each 50 cm × 50 cm) in each subplot, where soil seed bank samples were collected during August (the peak growing season; Table S2). Cover was estimated by the needles method following Zhang et al. (2019). Above-ground biomass was harvested, separated into species (An et al. 2020; Ma et al. 2020), and oven-dried at 65 °C to constant weight. Frequency was estimated by presence or absence (Zhang et al. 2019).
2.5 Soil physical and chemical properties
Following above-ground vegetation sampling, three additional soil core samples (3.6-cm diameter, 10-cm deep) were collected in each quadrat and combined into a single sample for each quadrat to measure soil physical and chemical properties (Table S3). After removal of roots and litter, the samples were air-dried, passed through a 2-mm sieve to ensure uniformity, and then stored at room temperature for analyses. Soil moisture content was determined by oven-drying fresh soil at 105 °C to constant weight; soil pH was measured by a glass electrode meter in a soil: water ratio of 1:2.5, w/v (PB-10, Sartorius, Goettingen, Germany); and soil bulk density (SBD) was measured by the ring sampler weighing technique (Wu et al. 2010). Total nitrogen (TN) concentration (g/kg dry weight) was determined by a flow injection auto-analyzer (FIAstar 5000 Analyzer, Foss Analytical, Hilleroed, Denmark) after digestion with sulfuric acid (Ferreira et al. 1996), and soil organic carbon (SOC) concentration (g/kg dry weight) was determined by colorimetry after oxidation with a mixture of potassium dichromate and sulfuric acid (Delgado-Baquerizo et al. 2013).
2.6 Plant-soil-seed bank quality index assessment
Five soil (TN, SOC, soil pH, soil moisture content, and SBD), two plant (above-ground biomass and above-ground richness), and two soil seed bank (seed density and seed richness) variables were used in a total and minimum data set (TDS) to assess the plant-soil-seed bank system quality indices in degraded, intact, and restored grasslands. These variables were selected due to their effect on soil fertility, vegetation growth, the soil seed bank, and soil aggregate stability (Nabiollahi et al. 2018; Gao et al. 2019; Yang et al. 2021). Principal components analysis (PCA) reduced dimensionality in the data with vegan R-packages and identified the most important properties in the multi-dimensional scaling (MDS) (Nabiollahi et al. 2018).
Each variable from TDS was transformed into a unitless score between 0 and 1. Standard scoring functions (SSF) indicated whether the variable had either a “positive,” “negative,” or “optimum” effect on the plant-soil-seed bank quality index. For these variables from TDS, scores were assigned by the “more is better” or the “low is better” function, depending on whether the indicator value was below or above the optimal threshold value. The “more is better” function (Eq. 1) was used for TN, SOC, above-ground biomass, above-ground richness, seed density, and seed richness (Nabiollahi et al. 2018), the “low is better” function (Eq. 2) was used for SBD, and the “optimum range” function (Eq. 3, 4, 5) was used for soil pH and soil moisture content (Nakajima et al. 2015). The thresholds for soil pH and soil moisture content were 7 (Nabiollahi et al. 2018).
where SF is the indicator score between 0 and 1, X is the measured variable value, L is the minimum value, H is the maximum value, and O is the optimum threshold (Gao et al. 2019).
Selected indicators from MDS were used to evaluate the plant-soil-seed bank quality index (QI) as (Gao et al. 2019):
where Wi is the weighting factor for the indicator from PCA analysis, SFi is the indicator score, and n is the number of selected variables.
2.7 Statistical analyses
Generalized linear mixed models with the Poisson error distribution and a log link function were employed to analyze the data, using the glmer functions of the lme4 R-packages. Grasslands’ type and season were fixed effects and plot was a random effect for seed density and richness. Non-parametric Kruskal-Wallis test or Wilcoxon test tested for significance in seed density, seed richness, functional group in the soil seed bank, and plant-soil-seed bank system quality index profiles among grasslands and among seasons. Pearson correlation (two-tailed) and principal component analysis (PCA) screened the TDS and determined the weights for the indicators of the plant-soil-seed bank system quality indices.
The dissimilarity between above-ground vegetation and soil seed banks were compared by Bray-Curtis distance in R-package vegan. To visualize the variations in soil seed banks across types of grasslands (degraded, restored and intact), composition data were ordinated by non-metric multi-dimensional scaling (NMDS) based on the Bray-Curtis distance (Erfanzadeh et al. 2020a, b). Analyses used the meta-MDS function in R-package vegan on untransformed values. P values were determined by comparing correlation coefficients with those generated from 9999 random permutations and adonis analysis.
To further assess the effects of soil properties and the plant community on soil seed banks, structural equation modelling (SEM) was employed after the data were log-transformed to achieve normality. To release the degrees of freedom, Pearson correlation analysis tested relationships between all predictors in the models, and the weakest correlation between two variables was removed. The fit of the model was determined by the χ2 (P > 0.05), low AIC, and low RMSEA (< 0.05, root-mean-square error of approximation) (Grace. 2006). We used standardized path coefficients to compare the strength of paths in the final model.
3 Results
3.1 Seed density and richness of soil seed bank
There were significant effects of grasslands type and season on seed density (number of seeds m-2) and grasslands type on seed richness (number of species) (P < 0.001; Table 1). There were 8386 seeds m-2 in the degraded grasslands, 5108 seeds m-2 in the restored grasslands, and 2578 seeds m-2 in the intact grasslands. The proportions of seeds from annual, biennial, and perennial plant species and of life-forms of vegetation did not differ among types of grasslands (P > 0.05, Fig. 3). Species richness was lower in restored than degraded grasslands in August and December, while there were more species in the soil seed bank in April than in August and December in the degraded and restored grasslands (Fig. 4). The topsoil layer contained more species than the lower layer (10.1 vs 6.1 species).
Species richness of the soil seed banks in the 0–10-cm soil layer in degraded, restored, and intact grasslands in different months. The species richness among grassland types (a) and among months (b). Purple represents intact grasslands, blue represents restored grasslands, and green represents degraded grasslands. *P < 0.05, **P < 0.01, ***P < 0.001.
3.2 Plant functional groups in the soil seed bank
There was a significant effect (P < 0.001) of grasslands type on soil seed bank densities of the functional groups (grasses, sedges, and forbs) across seasons (Fig. 5). In the degraded grasslands, the seed density of forbs was greater than grasses in April, while the density of sedges was lowest. A similar pattern emerged in the intact grasslands in April and December, but in August, seed density of grasses was greatest. In restored grasslands, the seed densities of grasses and forbs were greater than of sedges, but there was no difference between grasses and forbs in April, August, and December.
3.3 Relationship between vegetation and soil seed bank
According to the Bray-Curtis distance, the similarity between the soil seed bank and above-ground vegetation was greatest in restored, intermediate in intact, and lowest in degraded grasslands (Fig. S1). Non-metric multi-dimensional scaling (NMDS) demonstrated that species compositions in above-ground vegetation and soil seed bank were significantly distinct and differed among grasslands type in April (adonis analysis: r2 = 0.25, P < 0.001), August (adonis analysis: r2 = 0.33, P < 0.001), and December (adonis analysis: r2 = 0.29, P < 0.001) (Figs. 6 and 7). The grasslands type was correlated significantly (adonis analysis: r2 = 0.10, P < 0.001) with the soil seed bank composition (Fig. 7). There were 32 above-ground plant species in the degraded grasslands, and 15, 12, and 13 species in the soil seed banks in April, August, and December, respectively; 29 above-ground plant species in the restored grasslands and 13, 8, and 9 species in the soil seed banks, respectively; and 35 above-ground plant species in the intact grasslands and 14, 10, and 11 species in the soil seed banks, respectively.
Two-dimensional non-metric multi-dimensional scaling (NMDS) ordination of soil seed bank and above-ground vegetation in different grassland types in April (a), August (b), and December (c). Similarity between soil seed bank and above-ground plant species is presented in Fig. S1. Closed symbols represent the soil seed bank values, open symbols represent the above-ground vegetation values. Purple represents intact grasslands, blue represents restored grasslands, and green represents degraded grasslands.
Two-dimensional non-metric multi-dimensional scaling (NMDS) ordination of soil seed banks in all grassland types (adonis analysis: R2 = 0.10, P < 0.001). Adonis analysis and permutational multivariate analysis of variance (PERMANOVA) were used for statistical testing of grasslands’ type similarities. The dotted ellipse borders represent the 95% confidence interval. Purple represents intact grasslands, blue represents restored grasslands, and green represents degraded grasslands.
3.4 Relationship of soil seed bank to soil physical and chemical properties and above-ground vegetation
The plant-soil-seed bank system quality index was higher in restored than in degraded grasslands (Fig. 8). Structural equation modeling (SEM) determined the direct and indirect effects of soil properties and plant community on the soil seed bank (Fig. 9, Fig. S2). In intact grasslands, a decrease in AGB (above-ground biomass) and AGR (above-ground richness) increased SOC and soil pH, but these soil factors did not affect the soil seed bank density. An increase in TN and a decrease in soil moisture content (SM) increased the SBD directly (Fig. 9a). In degraded grasslands, a decrease in soil pH increased soil seed density, while an increase in soil pH and a decrease in SOC increased the soil seed bank richness directly. An increase in AGB increased TN, but AGB was not correlated with the soil seed bank density (Fig. 9b). In restored grasslands, AGB had a positive effect, while AGR had a negative effect on SOC, but they were not related to soil seed bank density (Fig. 9c). Soil seed bank density increased with soil seed bank richness in all types of grasslands. The plant community did not have a direct effect on the soil seed bank richness or density in any grasslands type, indicating that soil properties did not have an indirect effect on the soil seed bank through plant properties.
Structural equation models (SEM) with significant relationships (P < 0.05) between soil properties, plant communities, and their influence on the soil seed bank in intact (a), degraded (b), and restored (c) grasslands. Soil properties include soil pH, soil moisture (SM), soil bulk density (SBD), total nitrogen (TN), and soil organic carbon (SOC); plant community includes above-ground biomass (AGB) and above-ground richness (AGR); and soil seed banks include seed bank density (SBD) and seed bank richness (SDR). Pearson correlations between variables are presented in Fig. S2. The strength of the relationship is presented by the thickness of the arrows. The dashed arrow indicates an insignificant effect (P > 0.05). The percentages of the variance explained by the variables in the model are provided by the R2 (a. χ2 = 18.29, P = 0.44, χ2/df = 1.02, RMSEA = 0.02; b. χ2 = 14.06, P = 0.45, χ2/df = 1.04, RMSEA = 0.01; c. χ2 = 20.44, P = 0.43, χ2/df = 1.02, RMSEA = 0.03). RMSEA, root-mean-square error of approximation. *P < 0.05, **P < 0.01, ***P < 0.001.
4 Discussion
4.1 Soil seed bank changes with grasslands restoration
This study demonstrated an improvement in the soil seed banks after 13 years of active restoration of degraded grasslands. Seed density in the restored grasslands (5108 seeds m-2) was lesser than in the degraded grasslands (8386 seeds m-2), but greater than in the intact grasslands (2578 seeds m-2). The rapid recovery of seed density in the restored grasslands was due, at least in part, to the large number of free seeding ruderal species, with more grass (2238 vs 2122 m-2) and sedge (181 vs 163 m-2) seeds than in degraded grasslands. “Ruderal strategies” often occur after restoration, as was reported for calcareous and fen meadow plant communities (Matus et al. 2003; Bisteau and Mahy 2005). Least vulnerable ecosystems in terms of diversity of above-ground vegetation should be those that combine high soil seed bank diversity with high density, whereas least resilient ecosystems should be those with low seed densities (Yang et al. 2021). The long-term active restoration resulted in substantial alterations in seed densities of the species. The higher grass and sedge but lower forb seed densities might improve the resilience of the restored grasslands. These shifts in seed densities can affect the genetic diversity and the functioning of the grasslands ecosystem (Basto et al. 2015a) and improve above-ground plant diversity.
More grass than forb seeds were present in the intact grasslands in August, when most grasses had started seed dispersal, while most forbs were flowering. These trends were not observed in the restored grasslands as there was no difference between grasses and forbs in their contributions to the soil seed bank in any month. This demonstrated that sowing seeds from native perennial grasses in long-term active restoration established a persistent soil seed bank that was not limited by the abiotic and biotic conditions in these highly modified grasslands. These results also indicated that most seeds in the soil bank were related to sown grasses after 10 years and that it is possible to enhance the soil seed bank with native perennial grasses by long-term active restoration of degraded grasslands.
4.2 Species richness of seeds in response to grasslands disturbance
It was suggested that the soil seed bank is a key factor in the resilience of an ecosystem in face of major disturbances linked to climate or land use changes (Ma et al. 2021; Yang et al. 2021). Species richness of the soil seed bank in the restored grasslands was lower than in the degraded grasslands in August and December, but did not differ from the degraded grasslands in April, which could be attributed to the regrowth of plants and improved resilience of the restored grasslands. This implies that the transient soil seed bank in the restored grasslands was reduced due to the germination of more grass and sedge seeds, but was increased in December due to seed dispersal. Therefore, these soil seed banks are part of the regenerative mechanism whereby dead plants are replaced during a favorable season (Thompson and Grime 1979). The reduction of species richness after long-term active restoration was due to less forbs and short-lived species in the restored than in the degraded grasslands. Grassland degradation increases seed richness by more short-lived species (e.g., Cardamine hirsuta, Elsholtzia eriostachya, Draba nemorosa, Aconitum gymnandrum) in above-ground vegetation that are prolific seed producers (Shang et al. 2013). Most seed species in the three types of grasslands were perennials that relied on above-ground vegetation reproduction, although perennials produce less seeds than annuals (Ma et al. 2018). These seeds remained in the soil seed bank until conditions were favorable for germination and enhanced the resilience of the grasslands. Although species richness in the soil seed bank did not differ among the three types of grasslands in April, the composition of species and functional groups differed, with more forbs and grasses. Hence, the soil seed bank was a positive factor in the restoration of degraded grasslands.
The present study demonstrated that species richness of the soil seed bank fluctuated across seasons in the grasslands types, with greater species richness in April, which might be due to the deposition and dormancy of seeds from the previous year. On the Tibetan Plateau, low temperature severely restricts plant growth during the non-growing season. The soil seed bank in April had experienced cold stratification during winter to break dormancy, and the most favorable period for germination occurred in spring (Thompson et al. 1997). Most seeds collected in December failed to germinate because they did not undergo prolonged cold stratification, which is required for germination (Shang et al. 2016). Therefore, these seasonal variations in the soil seed bank could result from the delayed germination until a favorable period (e.g., growing season). The decrease in seed density in August in restored grasslands can be explained by the germination of a large number of transient seeds during the preceding summer. Many transient seeds in December delayed germination until the beginning of the next growing season, while some seeds remained buried and did not germinate during extended dormancy. These seeds were then incorporated into the persistent soil seed bank, as was noted previously (Thompson and Grime 1979). Therefore, we concluded that active restoration enhanced the accumulation of transient and persistent soil seed banks, which is vital because the persistent soil seed bank plays an important role in the productivity of restored grasslands. The persistent soil seed bank allows alpine species to germinate under favorable conditions and, consequently, improves the rate of vigorous seedlings that are established (Ma et al. 2018).
4.3 Relationship between above-ground vegetation and soil seed bank
The response of the soil seed bank to disturbance of grasslands sometimes mirrors above-ground vegetation changes and, under these circumstances, composition alterations at the community level are reflected in the soil seed bank (Basto et al. 2018). Specifically, in the present study, both above-ground vegetation and the soil seed bank responded to grassland degradation by increasing the abundance of forbs. There was evidence that the long-term active restoration increased the similarity in species between above-ground vegetation and the soil seed bank (Dong et al. 2020). There was less similarity in degraded grasslands due to the low nutrient contents in the soil, which caused low seed germination (Dong et al. 2020). This suggests that the contribution of the soil seed bank to above-ground vegetation in actively restored grasslands was greater than in degraded grasslands, which was consistent with previous results in degraded and re-vegetated alpine grasslands (Li et al. 2012).
The relationship between the soil seed bank and above-ground vegetation was closest in the restored grasslands, which is consistent with a previous study (Shang et al. 2013). A low similarity of species composition between the soil seed bank and above-ground vegetation was reported in grazed grasslands (Ma et al. 2018). In the current study, there was low similarity between the soil seed bank and above-ground vegetation in all types of grasslands and in all seasons, which was related to the dominance of perennial species. These results are not in agreement with those of Ma et al. (2013), who reported that differences between the soil seed bank and above-ground vegetation occurred only in the least disturbed sites. The soil seed bank in the degraded grasslands was dominated by annual and perennial forbs; however, the restored and intact grasslands were dominated by clonal species, including Poaceae (Poa crymophila, Elymus dahuricus, Festuca sinensis) and Cyperaceae (Kobresia humilis, Carex atrofusca). Clonal species propagate through vegetative growth and produce less seeds and, as a consequence, there is a low similarity between vegetation and the soil seed bank (Ma et al. 2020). The low similarity was mainly a consequence of the short growing season (from May to September) on the Tibetan plateau when plants use primarily clonal reproduction. Perennial species dominated the above-ground vegetation in the study sites and these species reproduced asexually by producing vegetative offspring and contributed little to the soil seed bank (Erfanzadeh et al. 2020b, 2021; Hadinezhad et al. 2021).
4.4 Direct and indirect factors affect vegetation restoration quality
In this study, soil pH, a key regulator of the soil seed bank (Yang et al. 2021), was correlated positively with seed density in intact grasslands and negatively in degraded grasslands. The negative effect on seed density in degraded grasslands was due to a reduction in grasses and sedges with increasing soil pH (Basto et al. 2015b). An increase in soil pH reduced the viability of seeds and inhibited seed germination, which increased seed density in the soil seed bank but decreased species diversity in the above-ground vegetation (Ma et al. 2017a; Yang et al. 2021). In addition, the dicotyledon to grass ratio increased with an increase in soil pH (Dupre et al. 2010) and, with higher pH, degraded grasslands had more forbs in the soil seed bank than intact grasslands. Consequently, manipulation of soil pH could be a useful option in the restoration of grasslands.
The vegetation and seeds were sampled in close vicinity to each other, but not at the same spot. However, the random sampling represented the above-ground vegetation and soil seed bank in each of the grasslands. Seed dynamics are complex and mediated by interactive factors from both above- and below-ground after long-term active restoration. In the present study, seed density and richness in restored grasslands were not affected by a soil or vegetation variable when compared with degraded and intact grasslands. Palatable grasses, such as E. dahuricus, F. sinensis, and P. pratensis cv. Qinghai, have extensive fibrous root systems, whereas, forbs have tap and less fibrous roots. Fibrous roots provide more organic carbon because of their faster growth and decomposition rates and greater biological activity (Li et al. 2014b). Studies in the alpine regions reported that SOC plus other nutrients decreased in re-vegetated grassland after 5–7 years but increased after 9–10 years (Li et al. 2014a, b; Gao et al. 2019). Consequently, a combination of plant species and soil properties determine seed density after long-term active restoration (Fry et al. 2017). The quality index of the plant-soil-seed bank system of restored grasslands was greater than degraded grasslands. This was a consequence of the combination of soil properties, plant species, and soil seed bank after the long-term active restoration. In intact, restored, and degraded grasslands, both species richness and biomass in above-ground vegetation did not affect seed density and seed richness, which supported the low similarity between above-ground vegetation and the soil seed bank. Low air temperature in the present study reduced the decomposition rate of the seeds, resulting in stable soil seed bank densities of long-lived seeds, which could explain the higher seed densities in the three sites when compared to a previous study (Bueno et al. 2011), and suggested high resilience to degradation in alpine grasslands.
5 Conclusions
In our study, we show that long-term (more than 10 years) active restoration had a positive effect on the richness and density of the soil seed bank, which enhanced the resilience of vegetation on the alpine grasslands. The higher grass and sedge but lower forb seed densities improved the resilience of the restored grasslands. These shifts in seed densities can affect the genetic diversity and the functioning of the grasslands ecosystem. Soil seed bank composition was largely dependent on changes in the plant community in the restored grasslands for both the transient and persistent seeds. An increase in the persistent soil seed bank serves as an indicator of the resilience of re-vegetation and implies that long-term active restoration could be effective in increasing the soil seed bank. These results also indicated that it is possible to enhance the soil seed bank with native perennial grasses by long-term active restoration of degraded grasslands. Results from this study could enhance our understanding of the impact of long-term active restoration on soil seed banks and could be beneficial in developing programs for the active restoration of degraded grasslands.
Data availability
Data are available from the corresponding author upon reasonable request.
Code availability
Not applicable
References
An H, Zhao YP, Ma MJ (2020) Precipitation controls seed bank size and its role in alpine meadow community regeneration with increasing altitude. Global Change Biol 00:1–11. https://doi.org/10.1111/gcb.15260
Bai YF, Ma LN, Degen AA et al (2020) Long-term active restoration of extremely degraded alpine grassland accelerated turnover and increased stability of soil carbon. Global Change Biol 26:7217–7228. https://doi.org/10.1111/gcb.15361
Bakker JP, Poschlod P, Strykstra RJ, Bekker RM, Thompson K (1996) Seed banks and seed dispersal: important topics in restoration ecology. Acta Bot Neerl 45:461–490. https://doi.org/10.1111/j.1438-8677.1996.tb00806.x
Basto S,Thompson K, Grime JP, Fridley JD, Calhim S, Askew AP, Rees M (2018) Severe effects of long-term drought on calcareous grassland seed banks. npj Clim Atmos Sci 1:1. https://doi.org/10.1038/s41612-017-0007-3
Basto S, Thompson K, Phoenix G, Sloan V, Leake J, Rees M (2015a) Long-term nitrogen deposition depletes grassland seed banks. Nat Commun 6:6185. https://doi.org/10.1038/ncomms7185
Basto S, Thompson K, Rees M (2015b) The effect of soil pH on persistence of seeds of grassland species in soil. Plant Ecol 216:1163–1175. https://doi.org/10.1007/s11258-015-0499-z
Bisteau E, Mahy G (2005) Vegetation and seed bank in a calcareous grassland restored from a Pinus forest. Appl Veg Sci 8:167–174. https://doi.org/10.1111/j.1654-109X.2005.tb00642.x
Carlsson M, Merten M, Kayser M, Isselstein J, Wrage-Monnig N (2017) Drought stress resistance and resilience of permanent grasslands are shaped by functional group composition and N fertilization. Agr Ecosyst Environ 236:52–60. https://doi.org/10.1016/j.agee.2016.11.009
Crouzeilles R, Curran M, Ferreira MS, Lindenmayer DB, Grelle CEV, Rey BJM (2016) A global meta-analysis on the ecological drivers of forest restoration success. Nat Commun 7:11666. https://doi.org/10.1038/ncomms11666
Crouzeilles R, Ferreira MS, Chazdon RL et al (2017) Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Sci Adv 3:e1701345. https://doi.org/10.1126/sciadv.1701345
Delgado-Baquerizo M, Maestre FT, Gallardo A et al (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502:672–676. https://doi.org/10.1038/nature12670
Dong SK, Shang ZH, Gao JX, Boone RB (2020) Enhancing sustainability of grassland ecosystems through ecological restoration and grazing management in an era of climate change on Qinghai-Tibetan Plateau. Agr Ecosyst Environ 287:106684. https://doi.org/10.1016/j.agee.2019.106684
Dupre EC, Stevens C, Ranke T et al (2010) Changes in species richness and composition in European acidic grasslands over the past 70 years: the contribution of cumulative atmospheric nitrogen deposition. Global Change Biol 16:344–357. https://doi.org/10.1111/j.1365-2486.2009.01982.x
Engst K, Baasch A, Bruelheide H (2017) Predicting the establishment success of introduced target species in grassland restoration by functional traits. Ecol Evol 7:7442–7453. https://doi.org/10.1002/ece3.3268
European Commission (2015) Towards and EU research and innovation policy agenda for nature-based solutions and re-naturing cities. Final Report of the Horizon 2020 Expert Group on “Nature-Based Solutions and Re-naturing cities”, 74
Erfanzadeh R, Barzegaran F, Hazhir S, Amoli SS (2021) Role of Different Shrubs in Soil Seed Bank Conservation in Different Climates of Iran. International Grassland Congress Proceedings 29(1-2):1-4. https://uknowledge.uky.edu/igc/24/1-2/29
Erfanzadeh R, Hazhir S, Jafari M (2020a) Effect of cushion plants on the soil seed bank in overgrazed semiarid regions. Land Degrad Dev 31(8):990–1000. https://doi.org/10.1002/ldr.3517
Erfanzadeh R, Shayesteh Palaye AA, Ghelichnia H (2020b) Shrub effects on germinable soil seed bank in overgrazed rangelands. Plant Ecol Divers 13(2):199–208. https://doi.org/10.1080/17550874.2020.1718233
Erfanzadah R, Barzegaran F, Amoli SS, Petillon J (2022) The effect of shrub community on understory soil seed bank with and without livestock grazing. Community Ecol 23(1):75–85. https://doi.org/10.1007/s42974-021-00074-3
Ferreira AMR, Lima J, Rangel A (1996) Potentiometric determination of total nitrogen in soils by flow injection analysis with a gas-diffusion unit. Aust J Soil Res 34:503–510. https://doi.org/10.1071/sr9960503
Freitag M, Klaus VH, Bolliger R et al (2021) Restoration of plant diversity in permanent grassland by seeding: Assessing the limiting factors along land-use gradients. J Appl Ecol 00:1–12. https://doi.org/10.1111/1365-2664.13883
Fry EL, Pilgrim ES, Tallowin JRB et al (2017) Plant, soil and microbial controls on grassland diversity restoration: a long-term, multi-site mesocosm experiment. J Appl Ecol 54:1320–1330. https://doi.org/10.1111/1365-2664.12869
Gao XX, Dong SK, Xu YD et al (2019) Resilience of revegetated grassland for restoring severely degraded alpine meadows is driven by plant and soil quality along recovery time: a case study from the Three-river Headwater Area of Qinghai-Tibetan Plateau. Agr Ecosyst Environ 279:169–177. https://doi.org/10.1016/j.agee.2019.01.010
Ghasempour M, Erfanzadeh R, Torok P (2022) Fire effects on soil seed banks under different woody plant species in Mazandaran province. Iran. Ecol Eng 183:106762. https://doi.org/10.1016/j.ecoleng.2022.106762
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Guittar J, Goldberg D, Klanderud K, Berge A, Boixaderas MR, Meineri E, Topper J, Vandvik V (2020) Quantifying the roles of seed dispersal, filtering, and climate on regional patterns of grassland biodiversity. Ecology 101(10):e03061. https://doi.org/10.1002/ecy.3061
Hadinezhad M, Erfanzadeh R, Ghelichnia H (2021) Soil seed bank characteristics in relation to different shrub species in semiarid regions. Land Degrad Dev 32(5):2025–2036. https://doi.org/10.1002/Idr.3856
Hobbs RJ, Higgs E, Hall CM et al (2014) Managing the whole landscape: historical, hybrid, and novel ecosystems. Front Ecol Environ 12:557–564. https://doi.org/10.1890/130300
Hobbs RJ, Higgs E, Harris JA (2009) Novel ecosystems: implications for conservation and restoration. Trends Ecol Evol 24:599–605. https://doi.org/10.1016/j.tree.2009.05.012
Kiss R, Deak B, Torok P, Tothmeresz B, Valko O (2018) Grassland seed bank and community resilience in a changing climate. Restor Ecol 26:141–150. https://doi.org/10.1111/rec.12694
LaForgia ML, Spasojevic MJ, Case EJ, Latimer AM, Harrison SP (2018) Seed banks of native forbs, but not exotic grasses, increase during extreme drought. Ecology 99(4):896–903. https://doi.org/10.1002/ecy.2160
Leger EA, Atwater DZ, James JJ (2019) Seed and seedling traits have strong impacts on establishment of a perennial bunchgrass in invaded semi-arid systems. J Appl Ecol 56:1343–1354. https://doi.org/10.1111/1365-2664.13367
Li F (2001) Chinese Soil Taxonomy. In: Science Press. Beijing
Li XL, Perry GLW, Brierley G, Sun HQ, Li CH, Lu GX (2014a) Quantitative assessment of degradation classifications for degraded alpine meadows (Heitutan), Sanjiangyuan, western China. Land Degrad Dev 25:417–427. https://doi.org/10.1002/ldr.2154
Li YY, Dong SK, Wen L, Wang XX, Wu Y (2012) Soil seed banks in degraded and revegetated grasslands in the alpine region of the Qinghai-Tibetan Plateau. Ecol Eng 49:77–83. https://doi.org/10.1016/j.ecoleng.2012.08.022
Li YY, Dong SK, Wen L, Wang XX, Wu Y (2013a) Three-dimensional framework of vigor, organization, and resilience (VOR) for assessing rangeland health: a case study from the alpine meadow of the Qinghai-Tibetan Plateau, China. EcoHealth 10:423–433. https://doi.org/10.1007/s10393-013-0877-8
Li YY, Dong SK, Wen L, Wang XX, Wu Y (2013b) Assessing the soil quality of alpine grasslands in the Qinghai-Tibetan Plateau using a modified soil quality index. Environ Monit Assess 185:8011–8022. https://doi.org/10.1007/s10661-013-3151-1
Li YY, Dong SK, Wen L, Wang XX, Wu Y (2014b) Soil carbon and nitrogen pools and their relationship to plant and soil dynamics of degraded and artificially restored grasslands of the Qinghai-Tibetan Plateau. Geoderma 213:178–184. https://doi.org/10.1016/j.geoderma.2013.08.022
Ludewig K, Hansen W, Klinger YP, Otte ERL, A, (2021) Seed bank offers potential for active restoration of mountain meadows. Restor Ecol 29(1):e13311. https://doi.org/10.1111/rec.13311
Ma MJ, Baskin CC, Yu K, Ma Z, Du GZ (2017a) Wetland drying indirectly influences plant community and seed bank diversity through soil pH. Ecol Indic 80:186–195. https://doi.org/10.1016/j.ecolind.2017.05.027
Ma MJ, Collins SL, Du GZ (2020) Direct and indirect effects of temperature and precipitation on alpine seed banks in the Tibetan Plateau. Ecol Appl 30(5):e02096. https://doi.org/10.1002/eap.2096
Ma MJ, Collins SL, Ratajczak Z, Du GZ (2021) Soil seed banks, alternative stable state theory, and ecosystem resilience. Bioscience 71:697–707. https://doi.org/10.1093/biosci/biab011
Ma MJ, Dalling JW, Ma Z, Zhou XH (2017b) Soil environmental factors drive seed density across vegetation types on the Tibetan Plateau. Plant Soil 419:349–361. https://doi.org/10.1007/s11104-017-3348-0
Ma M, Walck JL, Ma Z, Wang L, Du G (2018) Grazing disturbance increases transient but decreases persistent soil seed bank. Ecol Appl 28:1020–1031. https://doi.org/10.1002/eap.1706
Ma MJ, Zhou XH, Du GZ (2010) Role of soil seed bank along a disturbance gradient in an alpine meadow on the Tibet plateau. Flora 205:128–134. https://doi.org/10.1016/j.flora.2009.02.006
Ma MJ, Zhou XH, Du GZ (2013) Effects of disturbance intensity on seasonal dynamics of alpine meadow soil seed banks on the Tibetan Plateau. Plant Soil 369:283–295. https://doi.org/10.1007/s11104-012-1560-5
Matus G, Verhagen R, Bekker RM, Grootjans AP (2003) Restoration of the Cirsio dissecti- Molinietum in The Netherlands: can we rely on soil seed banks? Appl Veg Sci 6:73–84. https://doi.org/10.1111/j.1654-109X.2003.tb00566.x
Miehe G, Schleuss PM, Seeber E et al (2019) The Kobresia pygmaea ecosystem of the Tibetan highlands-origin, functioning and degradation of the world’s largest pastoral alpine ecosystem. Sci Total Environ 648:754–771. https://doi.org/10.1016/j.scitotenv.2018.08.164
Miller GR, Cummins RP (2003) Soil seed banks of woodland, heathland, grassland, mire and montane communities, Cairngorm Mountains, Scotland. Plant Ecol 168:255–266. https://doi.org/10.1023/A:1024464028195
Mohammed SA, Denboba MA (2020) Study of soil seed banks in ex-closures for restoration of degraded lands in the central Rift Valley of Ethiopia. Sci Rep 10:956. https://doi.org/10.1038/s41598-020-57651-1
Moreno-de las Heras M, Turnbull L, Wainwright J, (2016) Seed-bank structure and plant-recruitment conditions regulate the dynamics of a grassland-shrubland Chihuahuan ecotone. Ecology 97(9):2303–2318. https://doi.org/10.1002/ecy.1446
Müller F, Bergmann M, Dannowski R et al (2016) Assessing resilience in long-term ecological data sets. Ecol Indic 65:10–43. https://doi.org/10.1016/j.ecolind.2015.10.066
Nabiollahi K, Golmohamadi F, Taghizadeh-Mehrjardi R, Kerry R, Davari M (2018) Assessing the effects of slope gradient and land use change on soil quality degradation through digital mapping of soil quality indices and soil loss rate. Geoderma 318:16–28. https://doi.org/10.1016/j.geoderma.2017.12.024
Nakajima T, Lal R, Jiang S (2015) Soil quality index of a crosby silt loam in central Ohio. Soil till Res 146:323–328. https://doi.org/10.1016/j.still.2014.10.001
Nie ZN, Zollinger RP, Behrendt R (2015) Impact of deferred grazing and fertilizer on herbage production, soil seed reserve and nutritive value of native pastures in steep hill country of southern Australia. Grass Forage Sci 70:394–405. https://doi.org/10.1111/gfs.12136
Pan T, Zou X, Liu Y, Wu S, He G (2017) Contributions of climatic and non-climatic drivers to grassland variations on the Tibetan plateau. Ecol Eng 108:307–317. https://doi.org/10.1016/j.ecoleng.2017.07.039
Philipson CD, Cutler MEJ, Brodrick PG et al (2020) Active restoration accelerates the carbon recovery of human modified tropical forests. Science 369:838. https://doi.org/10.1126/science.aay4490
Plue J, Calster HV, Auestad I et al (2021) Buffering effects of soil seed banks on plant community composition in response to land use and climate. Global Ecol Biogeogr 30:128–139. https://doi.org/10.1111/geb.13201
Sabatier R, Joly F, Hubert B (2017) Assessing both ecological and engineering resilience of a steppe agroecosystem using the viability theory. Agr Syst 157:146–156. https://doi.org/10.1016/j.agsy.2017.07.009
Shang ZH, Deng B, Ding LM et al (2013) The effects of three years of fencing enclosure on soil seed banks and the relationship with above-ground vegetation of degraded alpine grasslands of the Tibetan plateau. Plant Soil 364:229–244. https://doi.org/10.1007/s11104-012-1362-9
Shang ZH, Yang SH, Wang YL, Shi JJ, Ding LM, Long RJ (2016) Soil seed bank and its relation with above-ground vegetation along the degraded gradients of alpine meadow. Ecol Eng 90:268–277. https://doi.org/10.1016/j.aquabot.2013.05.004
Soil Survey Staff (2014) Keys to Soil Taxonomy, 12th ed. In: United States Department of Agriculture and Natural Resources Conservation Service, Washington, DC
Thompson K, Bakker JP, Bekker RM (1997) The soil banks of North West Europe: methodology, density and longevity. Cambridge University Press, Cambridge
Thompson K, Grime JP (1979) Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. J Ecol 67(3):893-921. http://www.jstor.org/stable/2259220
Yang XJ, Baskin CC, Baskin JM et al (2021) Global patterns of potential future plant diversity hidden in soil seed banks. Nat Commun 12:7023. https://doi.org/10.1038/s41467-021-27379-1
Valko O, Deak B, Torok P et al. (2020) Vegetation and seed bank dynamics highlight the importance of post-restoration management in sown grasslands. bioRxiv 1-16. https://doi.org/10.1101/2020.01.20.913426
Van Moorsel SJ, Hahl T, Petchey OL, Ebeling A, Eisenhauer N, Schmid B, Wagg C (2021) Co-occurrence history increases ecosystem stability and resilience in experimental plant communities. Ecology 102(1):e03205. https://doi.org/10.1002/ecy.3205
Wu GL, Liu ZH, Zhang L, Chen JM, Hu TM (2010) Long-term fencing improved soil properties and soil organic carbon storage in an alpine swamp meadow of western China. Plant Soil 332:331–337. https://doi.org/10.1007/s11104-010-0299-0
Zhang R, Bai Y, Zhang T et al (2019) Driving factors that reduce soil carbon, sugar, and microbial biomass in degraded alpine grasslands. Rangeland Ecol Manag 72:396–404. https://doi.org/10.1016/j.rama.2018.10.001
Acknowledgments
We thank two anonymous reviewers for their very helpful suggestions.
Funding
This work was supported by the National Natural Science Foundation of China (U21A20183; 31870433; 31961143012; 42041005), the Second Tibetan plateau Scientific Expedition and Research (STEP) Program (Grant No. 2019QZKK0302), the Fundamental Research Funds for the Central Universities (lzujbky-2021-ct10), and the “111” Program 2.0 (BP0719040).
Author information
Authors and Affiliations
Contributions
Z. S. and C. S. designed the research; C. S., M. H., R. Z., L. M., Y. B., T. Z., W. W., J. N., S. L., R. J., Z. S., did the research; N. G., A. A. D., Z. S. analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Guo, N., Sang, C., Huang, M. et al. Long-term active restoration of degraded grasslands enhances vegetation resilience by altering the soil seed bank. Agron. Sustain. Dev. 43, 6 (2023). https://doi.org/10.1007/s13593-022-00862-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-022-00862-9