Abstract
The new mineral khurayyimite Ca7Zn4(Si2O7)2(OH)10·4H2O occurs in colorless spherulitic aggregates in small cavities of altered spurrite marbles located in the northern part of the Siwaqa pyrometamorphic rock area, Central Jordan. It is a low-temperature, hydrothermal mineral and is formed at a temperature lower than 100 °C. Synchrotron single-crystal X-ray diffraction experiments have revealed that khurayyimite crystallizes in space group P21/c, with unit cell parameters a = 11.2171(8), b = 9.0897(5), c = 14.0451(10) Å, β = 113.297(8)º, V = 1315.28(17) Å3 and Z = 2. The crystal structure of khurayyimite exhibits tetrahedral chains of periodicity 6. The sequence of SiO4 and ZnO2(OH)2-tetrahedra along the chain is Si–Si-Zn. The neighboring SiO4-tetrahedra of the corrugated chains are bridged by additional ZnO2(OH)2-tetrahedra to form 3-connected dreier rings. The chains can be addressed as loop-branched sechser single chains {lB, 11∞}[6Zn4Si4O21]. The chains are linked by clusters of five CaO6 and two CaO7 polyhedra with additional OH groups and H2O molecules in the coordination environment. Based on the connectedness and one-dimensional polymerisations of tetrahedra (TO4)n−, chains of khurayyimite belong to the same group as vlasovite Na2ZrSi4O11, since they can be described with geometrical repeat unit cTr = 2T4 3T4 and topological repeat unit cVr = 2V2 3V2.
Similar content being viewed by others
Introduction
Spurrite marbles of the Siwaqa area, a pyrometamorphic complex in Central Jordan, have anomalously high contents of Zn (Khoury et al. 2016; Sokol et al. 2017; Vapnik et al. 2019). Most common Zn-bearing minerals are sulphides, but Zn can be found in selenides and oxides, too. In the northern part of the Siwaqa region, the mineral tululite Ca14(Fe3+, Al)(Al, Zn, Fe3+, Si, P, Mn, Mg)15O36, was found in medium-temperature (800 – 850 °C) combustion metamorphic (CM) rocks i.e. Zn-rich marbles with high Ca:Al ratio (Khoury et al. 2016). A natural equivalent of CaZn2(OH)6·2H2O (Stahl and Jacobs 1997), named qatranaite (Vapnik et al. 2019), was recently discovered in the same area. Qatranaite was found in a single outcrop within cuspidine veins cutting spurrite marbles. This mineral is a product of low-temperature (< 70 °C) alteration of pyrometamorphic rocks by hyper-alkaline solutions (Vapnik et al. 2019). Furthermore, the mineral clinohedrite CaZn(SiO4)·H2O was reported to replace sphalerite in the bleaching zones cutting through dark spurrite marbles from the same type locality (Khoury et al. 2016).
In the same area, we have found the new low-temperature hydrothermal mineral khurayyimite (IMA 2018–140), with ideal chemical formula Ca7Zn4(Si2O7)2(OH)10·4H2O. To the best of our knowledge, no synthetic analogue is known and therefore, it is a new compound in the system CaO-ZnO-SiO2-H2O. The name khurayyimite is given after Mount Khurayyim (Jabal al Khurayyim), Siwaqa pyrometamorphic rock area, central Jordan. Khurayyimite was found in the immediate vicinity of this mountain. Type material was deposited in the mineralogical collection of the Fersman Mineralogical Museum, Leninskiy pr., 18/k2, 115162 Moscow, Russia, catalogue number: 5298/1.
Occurrence and genesis
The mineral khurayyimite, Ca7Zn4(Si2O7)2(OH)10·4H2O, occurs in small cavities and veins in altered spurrite marbles together with calcite, ettringite-thaumasite series minerals, Ca-hydrosilicates like jennite, Ca9(Si3O9)2(OH)6·8H2O and foshagite, Ca4(SiO3)3(OH)2 along with minerals of the tobermorite group. The type locality (N31°24.23′; E36°15.06′) is in Central Jordan, in the northern part of the Siwaqa pyrometamorphic rock area, circa 80 km south of Amman. Daba-Siwaqa is the largest area of the Hatrurim Complex (Mottled Zone) within the Dead Sea rift region, which exhibits twenty fields of pyrometamorphic rocks (Geller et al. 2012; Novikov et al. 2013).
Rock-forming minerals of unaltered dark spurrite marbles are spurrite Ca5(SiO4)2(CO3), calcite CaCO3, fluorapatite Ca5(PO4)3F and cuspidine Ca4Si2O7(F,OH)2. Accessory minerals spinel MgAl2O4—magnesioferrite MgFe23+O4, franklinite ZnFe3+2O4, fluormayenite Ca12Al14O32[☐4F2] —fluorkyuygenite Ca12Al14O32[(H2O)4F2], sphalerite (Zn,Fe)S, pyrite FeS2, chalcocite Cu2S, hematite Fe2O3, clinohedrite CaZn(SiO4)·H2O. Furthermore, barite BaSO4, celestine SrSO4, selenides of Ni, Fe and Cu, greenockite CdS, elbrusite Ca3(Zr1.5U6+0.5)Fe3+3O12, perovskite CaTiO3 and vorlanite (CaU6+)O4 can be found (Galuskin et al. 2011a).
The formation of low-temperature zinc-bearing hydrated minerals in spurrite rock of the Hatrurim Complex was discussed in detail by Vapnik et al. (2019) in a publication on the mineral qatranaite, CaZn2(OH)6(H2O)2. The authors describe dark and fractured spurrite rocks, where cm-sized white zones are visible along the cracks. Within these zones re-crystallization of fine-grained spurrite, occurrence of metacrysts (up to 0.5 cm in size), and local enrichments in cuspidine are observed. The occurrence of qatranaite is restricted to cuspidine zones, whereas clinohedrite and khurayyimite are associated with the hydrated fragments of re-crystallized spurrite rock. Sphalerite is a widespread mineral in spurrite rock and it is considered to be a source of the zinc for the low-temperature minerals (Khoury et al. 2016). The stability of thaumasite (Jallad et al. 2003; Matschei and Glasser 2015) indicates that qatranaite, khurayyimite and clinohedrite are formed from highly alkaline solutions at ∼70 °C, after the crystallization of thaumasite and calcite veins (Vapnik et al. 2019).
Results
Physical and optical properties
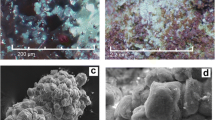
Khurayyimite forms colorless spherulitic aggregates up to 200–300 µm in size (Figs. 1, 2). Individual elongated platy crystals in the spherules are nearly 50 µm long, 20 µm wide and up to 10 µm thick. Crystals show white streak and white vitreous lustre. The measured micro-indentation hardness of khurayyimite gave Vickers Hardness VHN25 = 242 (average of 13 measurement), range 220–264 kg/mm2, which corresponds to a value of 3.5–4 on the Mohs scale. Cleavage or parting were not observed. Tenacity is brittle and fracture is splintery. Because of the small size of the crystals, the density could not be measured. Instead, we calculated the density on the basis of the empirical formula and unit cell volume, as refined from single-crystal X-ray diffraction data. The calculated density is 2.806 g·cm−3. The mineral dissolves in 10% HCl. Khurayyimite is optically negative, α = 1.603(2), β = 1.607(2), γ = 1.610(2) (at λ = 589 nm), 2Vmeas. = 50(10)º and 2Vcalc. = 40.9º. Dispersion of the optical axes is very weak; the optical orientation is: Z = b, X^c = 20(5)°, and it is non-pleochroic. Gladstone-Dale’s compatibility factor is superior (1-(KP/KC) = -0.012).
Khurayyimite and associated minerals: a holotype specimen, contact of unaltered spurrite marble (brown) and altered spurrite marble (light), mainly composed of calcite, minerals of the ettringite-thaumasite series, Ca-hydrosilicates containing cavities with khurayyimite; b, c and d BSE images of typical spherulitic aggregates of khurayyimite: Cal—calcite, Fos—foshagite, Gnk—greenockite, HSi—mixture of Ca-hydrosilicates, Khu—khurayyimite and Spu—spurrite
Spherulitic aggregates of khurayyimite, from the same area as shown in Fig. 1d, presented in: a plane-polarized transmitted light and b cross-polarized transmitted light. Associated minerals are: Afw—afwillite with finely dispersed Fe hydroxides, Cal—calcite, HSi—mixture of Ca-hydrosilicates, Khu—khurayyimite and Spu—spurrite
Chemical composition
Quantitative wavelength-dispersive electron-microprobe analyses of khurayyimite and the associated minerals were carried out using a CAMECA SX100 electron probe micro-analyser. A beam diameter of 10 µm was used. A counting time for peaks was 30 s and 15 s for the background. Diopside and sphalerite were used as reference materials for the analysis of Ca, Si and Zn (all Kα lines). The holotype crystals of khurayyimite show uniform composition. The results based on eleven analyses are summed in the Table 1. The empirical formula, calculated on the basis of 28 O with 10(OH)− and 4H2O is Ca7.070Zn3.894Si4.018O14(OH)10·4H2O. The simplified and ideal formula is: Ca7Zn4(Si2O7)2(OH)10·4H2O, which implies the following weight percentages: CaO 35.03, ZnO 29.05, SiO2 21.45, H2O 14.47. The total sum is quite low with ∼96.28 wt%, because the measurement was done with a broad beam of 10 µm. Using a narrower beam the total wt% was higher, but the ratio of (Ca + Zn)/Si was worse. Because of the small size of the khurayyimite spherulites and difficulties to select pure material, H2O and CO2 contents were not determined by chemical methods. Moreover, absence of CO32− groups and presence of H2O and hydroxyl groups in khurayyimite were confirmed by the structural investigations and Raman spectroscopy.
X-ray crystallography
Single crystal diffraction experiments at ambient conditions were performed at the X06DA beamline of the Swiss Light Source (Paul Scherrer Institute, Villigen, Switzerland). The beamline was equipped with an Aerotech one-axis goniometer and a PILATUS 2 M detector.
Data collection was carried out at ambient conditions using the DA+ acquisition software (Wojdyla et al. 2018). The radiation source was a SLS super-bending magnet (2.9 T). A wavelength of 0.70849 Å was obtained using a Bartels monochromator. The detector was placed 80 mm from the sample, resulting in a maximum resolution of 0.7 Å. A total of 1800 frames were recorded using fine-sliced (0.1°) ω-scans at 0.2 s per frame. Experimental details are given in Table 2.
Determination of lattice parameters, data reduction and absorption correction were processed with the program CrysAlisPro (Rigaku 2020). The average structure was solved using SIR2004 (Burla et al. 2005). The least-squares refinements were performed using the program Shelxl97 (Sheldrick 2008). Bond valence sum calculations were done with the BondStr program (Brown and Altermatt 1985; Rodríguez-Carvajal 2005). For the analysis of the chains in the structure of khurayyimite the Crystana software was employed (Klein and Liebau 2014). Figures of the crystal structure and deviations of the polyhedra from their ideal geometries expressed with the quadratic elongation l and the angle variance σ2 as defined by (Robinson et al. 1971) were calculated using Vesta3 (Momma and Izumi 2011). All H-sites were located by difference Fourier analysis. The resulting structure model was refined using 223 parameters and 4676 independent reflections. All of the atoms, except H, were described using anisotropic displacement parameters. Hydrogen positions were refined at a fixed value of Uiso = 0.05 Å2 for the H2O molecules and OH groups bonded to cations. OH distances were constrained to 0.90(5) Å. Refinement details are summarized in Table 2. Table 3 lists atomic coordinates. In Table 4 selected bond distances, bond valence sums, quadratic elongation and bond angle variance are given. In the Table 5 parameters for H-bonds (D–A) are listed. A CIF is available in the Supplement.
As khurayyimite occurs only in tiny amounts X-ray powder diffraction data were not collected. Instead we calculated the powder pattern with Jana2006 (Petříček et al. 2014) using the structural data obtained from the single-crystal structure refinements. The seven strongest powder X-ray diffraction lines, d in Å (I %) hkl are: 3.8333 (100%) \(\overline{2}13\); 10.3107 (81%) 100;214 2.9519 (68%) 031; 5.455 (59%)\(\overline{1}21\); 2.6607 (57%) 114; 2.9084 (55%) \(\overline{1}31\) and 3.4083 (42 %) \(\overline{2}04\).
Crystal structure
The structure of khurayyimite exhibits dimers of SiO4 tetrahedra, which are connected by ZnO2(OH2)-tetrahedra to form corrugated tetrahedral chains of periodicity six, extending along b. Each of the dimers Si2O7 of this sechser chain is bridged by another Zn1O2(OH)2-tetrahedron resulting in dreier rings of two Si- and one Zn-centered tetrahedra (Fig. 3a). According to the silicate nomenclature of Liebau (1985), these chains can be addressed as loop-branched sechser single chains {lB, 11∞}[6Zn4Si4O21](OH)8.
The structure of khurayyimite: a loop-branched sechser single chains with dreier rings b single chain linking the two clusters of CaOn polyhedra c clusters of CaOn polyhedra with OH¯ groups and H2O molecules d different orientations of clusters in the unit cell e periodicity of crooked chain f crystal structure with chains, clusters and hydrogen bonds between them
The chains are linking the clusters of seven CaOn polyhedra made of two Ca1O7 and five octahedra with Ca2, Ca3 and Ca4 atoms in the center (Fig. 3b and c). The clusters occur in two different orientations in the unit cell (Fig. 3d). The sechser chains are twisting around clusters sharing corners and edges with the CaOn polyhedra. Three oxygen atoms shared by Zn-centered tetrahedra and CaOn polyhedra are connected to additional H atoms (O8–H8, O9–H9, O10–H10 and O12–H12). Another hydrogen atom, H11 is attached to O11, an apical oxygen between Ca1-, Ca2- and Ca3-centered polyhedra (Fig. 3c). Ca2 is coordinated by one O anion, four OH¯ groups and one H2O molecule (H13A–O13w–H13B), where Ca4 is coordinated by three O anions, two OH¯ groups and one H2O molecule (H14A–O14w–H14B) (Fig. 3c, Table 5). The chains extend infinitely along b. Along the a-direction the corrugation of the chains creates ∼11 Å thick sheets (Fig. 3d, e). These sheets are interconnected by oxygen (O12) shared by Ca2O6 polyhedra and Zn1O4 tetrahedra (Fig. 4). In addition, the narrow gaps between the sheets, formed parallel to [001], are filled by strong hydrogen bonds formed between five OH¯ groups two H2O molecules attached to the chains or Ca-clusters.
The periodicity of crooked loop-branched sechser single chains {lB, 11∞}[6Zn4Si4O21](OH)8 along b is 9.0897 Å, which corresponds to the b lattice parameter of the cell. The stretching factor of the chain is rather small. Both SiO4 tetrahedra within the chain show average bond lengths of 1.6329(6) and 1.6367(7) and low measures of distortion (Table 4). Two Zn-centered tetrahedra are equally distorted (see Table 4). Still Zn1O4 has longer average bonds of 1.9642(6) Å than Zn2O4 1.9388(7). Actually, the larger Zn1O4 tetrahedron forms a loop with the Si2O7 groups. Strong repulsive forces between the tetrahedra of the dreier ring are pressing O1 atom as far as possible forming a triangle with longer Zn1O4 edge (∼3.176 Å) and two shorter SiO4 edges (2.66 and 2.68 Å). These repulsive forces are, according to Liebau (1985), a possible reason why such loops are only rarely observed.
Raman spectroscopy
The Raman spectrum of khurayyimite was recorded using a WITec alpha 300R confocal Raman microscope equipped with an air-cooled solid laser (488 nm) and a charge-coupled device detector operating at -61 °C. The laser radiation was coupled to a microscope through a single-mode optical fibre with a diameter of 3.5 μm. An air Zeiss LD EC Epiplan-Neofluan DIC-100/0.75NA objective was used. Raman scattered light was focused on a broad band single mode fibre with effective Pinhole size about 30 μm and monochromator with a 600 mm−1 grating. The power of the laser at the sample position was ∼30 mW. Integration times of 3 s with accumulation of 15 scans and a resolution 3 cm−1 were chosen. The monochromator was calibrated using the Raman scattering line of a silicon plate (Ntziouni et al. 2022). The following bands in the Raman spectra of khurayyimite, Ca7Zn4(Si2O7)2(OH)10·4H2O, were observed (cm−1, Fig. 5): 80, 112, 143, 171, 203, 246, 270, 314, 342, 378, 436, 465, 524, 586, 676, 740, 821, 911, 916, 975, 1624, 2898, 3074, 3109, 3503, 3555, 3580, 3603, 3618, 3633.
The main bands correspond to Si-O vibrations in (Si2O7) 6-groups: ν1 911 cm-1, ν4 676 cm-1, ν2 378 cm-1 (Galuskin et al. 2011b) and Zn-O tetrahedra ZnO2(OH)2: ν1 465 cm-1; ν2 + ν4 310cm1, 314 cm-1, 342 cm-1 (Lin et al. 1999; Kesic et al. 2014). The band at 586 cm-1 is related to SiO-Zn vibrations (Chandra Babu and Buddhudu 2014), and band at 524 cm-1 is related to Si-O-Si vibrations. The main bands below 203 cm-1 are due to vibrations of Ca-O in CaO4(OH)3 polyhedra and CaO(OH)4(OH2), CaO4(OH)2, CaO3(OH)2(OH2) octahedra. Bands at 2898, 3074 and 3109 cm-1 are related to O-H vibration in H2O with strong hydrogen bonds, whereas the main bands at 3503, 3580, 3603 and 3618 cm-1 correspond to vibrations of OH groups.
Discussion
The structure of this mineral comprises new and very unusual loop-branched sechser single chains that can be described with the formula {lB, 11∞}[6Zn4Si4O21](OH)8, following the classification of Liebau (1985). This formula denotes a loop-branched (lB) single chain (11∞) with a six-tetrahedra repetition unit (sechser) made of four ZnO4 and four SiO4 corner-sharing tetrahedra (Zn4Si4O21). In the chains, Si2O7 dimers and ZnO2(OH)2 tetrahedra are connected by corners building the loops i.e. dreier ring (Fig. 3a). This combination of the two SiO4 and one ZnO4 tetrahedra in a ring is very rare due to the strong repulsive forces in this formation. The volume of the ZnO4 tetrahedra with ∼3.97 Å3 is two times larger than the volume of SiO4 tetrahedra (∼ 2.05 Å3) (Abrahams and Bernstein 1969; Kato and Nukui 1976).
So far, only a few compounds are reported with the same ring formation but utterly different structures (see Fig. 6). One of them is a structure of synthetic K2ZnSi2O6 (Hogrefe and Czank 1995), where two layers with three-membered rings (2 × SiO4, 1 × ZnO4) are forming the loop-branched zweier framework {lB,3∞}[2(Zn1Si2)O6]. In the structure of K2ZnSi4O10 (Kohara and Kawahara 1990), a tectosilicate framework is built by ten-membered rings of SiO4 tetrahedra interconnected with five-member rings (4 × SiO4, 1 × ZnO4), four-membered rings (3 × SiO4, 1 × ZnO4) and three-membered rings (2 × SiO4, 1 × ZnO4).
Quite the contrary, three-membered rings comprising two ZnO4 and one SiO4 are widespread among zinc silicates, like in the minerals willemite Zn2SiO4 (Simonov et al. 1977), hemimorphite Zn4Si2O7(OH)2·H2O (Li and Bass 2020), hodgkinsonite Zn2MnSiO4(OH)2 (Rentzeperis 1963), junitoite, CaZn2Si2O7·H2O (Hamilton and Finney 1985), K2Zn2Si8O19 (Kohara and Kawahara 1990) etc.
Different combinations of chains and frameworks made of ZnO4 and SiO4 tetrahedra are known. In ZnSiO3 (Morimoto et al. 1975), with Zn atoms in six- and four-fold coordination, two pyroxene-like chains are branched with two ZnO4 tetrahedra forming four-member loops. The crystal structures of LT and HT forms BaZn2Si2O7 (Lin et al. 1999) have a disilicate group Si2O7 linked via corners with ZnO4 tetrahedra in a three-dimensional framework, which exhibits sixmember rings (2 × Si2O7, 2 × ZnO4), four member-rings (2 × SiO4, 2 × ZnO4) and three-membered rings (1 × SiO4, 2 × ZnO4).
Loop-branched sechser single chains are observed in vlasovite Na2ZrSi4O11 (Sokolova et al. 2006; Voronkov and Pyatenko 1962). However, chains of vlasovite {lB, 11∞}[6Si4O22] exhibit four-membered rings of SiO4-tetrahedra linked to form a chain (Liebau 1985). According to the structural hierarchy for chain, ribbon and tube silicates established by Day and Hawthorne (2020), khurayyimite has the same geometrical repeat unit cTr = 2T4 3T4 and topological repeat unit cVr = 2V2 3V2 as the mineral vlasovite and the three synthetic compounds HNb(H2O)[Si4O11](H2O), Cs0.66H0.33Nb(H2O)[Si4O11] and Na2H(NbO)[Si4O11](H2O)1.25 reported by Salvadó et al. (2001), and therefore they belong to the same group. This new structural hierarchy is based on the connectedness of one-dimensional polymerization of the (TO4)n− tetrahedra.
The geometrical repeat unit has ng = 6 tetrahedra. Its connectivity is denoted as cTr = 2T4 3T4; i.e. contains four ZnO4 tetrahedra with connectivity of two (2T4) and four SiO4 tetrahedra with connectivity of three (Fig. 7b). The topological repeat unit is denoted by degree of vertex (r) and the number of vertices (c) in the topological repeat unit. All the branches are moved to the one side of the chain. The topological repeat unit cVr = 2V2 3V2 in khurayyimite is half of the size of geometrical repeat unit (Fig. 7c). According to the classification of Day and Hawthorne (2020) the mineral vlasovite Na2ZrSi4O11 (Sokolova et al. 2006) with chains made of four-membered rings of SiO4 tetrahedra belongs to the same group (Fig. 7d-f). Another member of the group is synthetic compound HNb(H2O)[Si4O11](H2O) with chains made of six-membered rings.
References
Abrahams SC, Bernstein JL (1969) Remeasurement of the structure of hexagonal ZnO. Acta Crystallogr B 25:1233–1236
Brown ID, Altermatt D (1985) Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr B 41:244–247
Burla MC, Caliandro R, Camalli M, Carrozzini B, Cascarano GL, De Caro L, Giacovazzo C, Polidori G, Spagna R (2005) SIR2004: an improved tool for crystal structure determination and refinement. J Appl Crystallogr 38:381–388
Chandra Babu B, Buddhudu S (2014) Analysis of structural and electrical properties of Ni2+:Zn2SiO4 ceramic powders by sol–gel method. J Sol-Gel Sci Technol 70:405–415
Day MC, Hawthorne FC (2020) A structure hierarchy for silicate minerals: chain, ribbon, and tube silicates. Mineral Mag 84:165–244
Galuskin EV, Armbruster T, Galuskina IO, Lazic B, Winiarski A, Gazeev VM, Dzierżanowski P, Zadov AE, Pertsev NN, Wrzalik R, Gurbanov AG, Janeczek J (2011) Vorlanite (CaU6+)O4 - A new mineral from the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus. Russia Am Mineral 96(1):188–196
Galuskin EV, Galuskina IO, Lazic B, Armbruster T, Zadov AE, Krzykawski T, Banasik K, Gazeev VM, Pertsev NN (2011) Rusinovite, Ca10(Si2O7)3Cl2: a new skarn mineral from the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus, Russia. Eur J Mineral 23:837–844
Geller YI, Burg A, Halicz L, Kolodny Y (2012) System closure during the combustion metamorphic “Mottled Zone” event. Israel Chem Geol 334:25–36
Hamilton RD, Finney JJ (1985) The structure of junitoite, CaZn2Si2O2·H2O. Mineral Mag 49:91–95
Hogrefe AR, Czank M (1995) Synthetic Dipotassium Zinc Disilicate. Acta Crystallogr C51:1728–1730
Jallad KN, Santhanam M, Cohen MD (2003) Stability and reactivity of thaumasite at different pH levels. Cem Concr Res 33(3):433–437
Kato K, Nukui A (1976) Die Kristallstruktur des monoklinen Tief-Tridymits. Acta Crystallogr B 32:2486–2491
Kesic Z, Lukić I, Zdujić M, Jovalekic C, Liu H, Skala D (2014) Mechanochemical synthesis of CaO·ZnO·K2CO3 catalyst: Characterization and activity for methanolysis of sunflower oil. Chem Ind Chem Eng Q21:41–41
Khoury HN, Sokol EV, Kokh SN, Seryotkin YV, Nigmatulina EN, Goryainov SV, Belogub EV, Clark ID (2016) Tululite, Ca14(Fe3+, Al)(Al, Zn, Fe3+, Si, P, Mn, Mg)15 O36: a new Ca zincate-aluminate from combustion metamorphic marbles, central Jordan. Mineral Petrol 110:125–140
Klein H-J, Liebau F (2014) Computerized crystal-chemical classification of silicates and related materials with CRYSTANA and formula notation for classified structures. J Solid State Chem. https://doi.org/10.1016/j.jssc.2008.05.042
Kohara S, Kawahara A (1990) Structure of synthetic dipotassium zinc tetrasilicate. Acta Crystallogr C 46:1373–1376
Li Y, Bass JD (2020) Single Crystal Elastic Properties of Hemimorphite, a Novel Hydrous Silicate. Minerals 10(5):425–434
Liebau F (1985) Structural Chemistry of Silicates: Structure, Bonding, and Classification. Springer-Verlag, Berlin Heidelberg. https://doi.org/10.1007/978-3-642-50076-3
Lin JH, Lu GX, Du J, Su MZ (1999) Phase transition and crystal structures of BaZn2Si2O7. J Phys Chem Solids 60(7):975–983
Matschei T, Glasser FP (2015) Thermal stability of thaumasite. Mater Struct 48:2277–2289
Momma K, Izumi F (2011) VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276
Morimoto N, Nakajima Y, Syono S, Akimoto S, Matsui Y (1975) Crystal structure of pyroxene-type ZnSiO3 and ZnMgSi2O6. Acta Cryst B31:1041–1049
Novikov I, Vapnik Y, Safonova I (2013) Mud volcano origin of the Mottled Zone, South Levant. Geosci Front 4:597–619
Ntziouni A, Thomson J, Xiarchos I, Li X, Bañares MA, Charitidis C, Portela R, Diz EL (2022) Review of Existing Standards, Guides, and Practices for Raman Spectroscopy. Appl Spectrosc 76(7):747–772
Petříček V, Dušek M, Palatinus L (2014) Crystallographic Computing System JANA2006: General features. Z Für Krist - Cryst Mater 229:345–352
Rentzeperis PJ (1963) The crystal structure of hodgkinsonite Zn2Mn[(OH)2SiO4]. Z Für Krist -Cryst Mater 119:117–138
Rigaku (2020) CrysAlisProSoftware System, Version 171.40.53, Rigaku Oxford Diffraction Ltd, Yarnton, Oxfordshire, England
Robinson K, Gibbs GV, Ribbe PH (1971) Quadratic Elongation: A Quantitative Measure of Distortion in Coordination Polyhedra. Science 172:567–570
Rodríguez-Carvajal J (2005) The program Bond_Str and its GUI GBond_Str. Available at: http://mill2.chem.ucl.ac.uk/ccp/web-mirrors/plotr/BondStr/Bond_Str.htm. Last access 14 Nov 2022
Salvadó MA, Pertierra P, García-Granda S, Khainakov SA, García JR, Bortun AI, Clearfield A (2001) Novel Silicate Anion: Si8O2212-. Hydrothermal Synthesis and X-ray Powder Structure of Three New Niobium Silicates. Inorg Chem 40:4368–4373
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Simonov MA, Sandomirskii PA, Egorov T, Belov NV (1977) The crystal structure of willemite Zn2(SiO4). Dokl Akad Nauk SSSR 237:581–588
Sokol EV, Kozmenko OA, Khoury HN, Kokh SN, Novikova SA, Nefedov AA, Sokol IA, Zaikin P (2017) Calcareous sediments of the Muwaqqar Chalk Marl Formation, Jordan: Mineralogical and geochemical evidences for Zn and Cd enrichment. Gondwana Res 46:204–226
Sokolova E, Hawthorne FC, Ball NA, Mitchell RH, Ventura GD (2006) Vlasovite, Na2Zr(Si4O11), from the Kipawa alkaline Complex, Quebec, Canada: Crystal-Structure refinement and infrared Spectroscopy. Can Mineral 44:1349–1356
Stahl R, Jacobs H (1997) Zur Kristallstruktur von CaZn2(OH)6·2H2O. Z Für Anorg Allg Chem 623:1287–1289
Vapnik Y, Galuskin EV, Galuskina IO, Kusz J, Stasiak M, Krzykawski T, Dulski M (2019) Qatranaite, CaZn2(OH)6∙2H2O: a new mineral from altered pyrometamorphic rocks of the Hatrurim Complex, Daba-Siwaqa. Jordan Eur J Mineral 31(3):575–584
Voronkov AA, Pyatenko YA (1962) The crystal structure of vlasovite. Soviet Physics -Crystallography 6:755–760
Wojdyla JA, Kaminski JW, Panepucci E, Ebner S, Wang X, Gabadinho J, Wang M (2018) DA+ data acquisition and analysis software at the Swiss Light Source macromolecular crystallography beamlines. J Synchrotron Radiat 25:293–303
Acknowledgements
BK acknowledges help from Hannes Krüger, Anuschka Pauluhn and Valerie Goettgens during the synchrotron experiments. The research leading to these results has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 730872, project CALIPSOplus. The field works and microprobe investigations were supported by the National Science Centre (NCN) of Poland, grant 2021/41/B/ST10/00130.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Contributions
EVG, IOG, YV and MM collected the samples in Israel, EVG and IOG performed petrological investigations, microprobe analysis, Raman spectroscopy and optical measurements. BK performed SC XRD investigation at the synchrotron. BK solved and analyzed the structure. BK and IOG wrote the paper with help from all coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Editorial handling: G. Giester
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krüger, B., Galuskina, I.O., Galuskin, E.V. et al. Khurayyimite Ca7Zn4(Si2O7)2(OH)10·4H2O: a mineral with unusual loop-branched sechser single chains. Miner Petrol 117, 191–200 (2023). https://doi.org/10.1007/s00710-022-00804-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-022-00804-z