Abstract
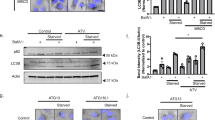
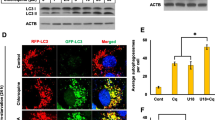
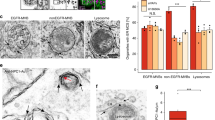
Lysosomes are known to influence cholesterol trafficking into endoplasmic reticulum (ER) membranes. Though intracellular cholesterol levels are known to influence the lipid biosynthetic responses in ER, the specific effects of lysosomal modulation on these outcomes is not known. To demonstrate this, C2C12 cells were treated with chloroquine, a lysosomotropic agent, and its effects on cellular biosynthetic capacity, structural and functional status of ER was determined. In addition to its known effects on autophagy reduction, chloroquine treatment induced accumulation of total cellular lipid and ER-specific cholesterol content. It was also observed that chloroquine caused an increase in smooth-ER content with defects in overall protein turnover. Further, since ER and mitochondria function in close association through ER membrane contact sites, it is likely that lysosomal modulation also brings about associated changes in mitochondria. In this regard, we found that chloroquine reduces mitochondrial membrane potential and mitochondrial dynamics. Collectively, the differential biosynthetic response of rise in lipid content, but not protein content, cannot be accounted by merely considering that chloroquine induced suppression of autophagy causes defects in organelle function. In this defective autophagy scenario, both biosynthetic responses such as lipid and protein synthesis are expected to be reduced rather than only the latter, as observed with chloroquine. These findings suggest that cholesterol trafficking/distribution within cellular organelles could act as an intracellular mediator of differential biosynthetic remodelling in interconnected organelles.




Similar content being viewed by others
References
Meng, Y., Heybrock, S., Neculai, D., & Saftig, P. (2020). Cholesterol handling in Lysosomes and beyond. Trends in Cell Biology, 30, 452–466. https://doi.org/10.1016/j.tcb.2020.02.007.
Goldstein, J. L., & Brown, M. S. (2015). A century of cholesterol and coronaries: from plaques to genes to statins. Cell, 161, 161–17. https://doi.org/10.1016/j.cell.2015.01.036.
Lange, Y., Ye, J., & Chin, J. (1997). The fate of cholesterol exiting lysosomes. Journal of Biological Chemistry, 272, 17018–17022. https://doi.org/10.1074/jbc.272.27.17018.
Trinh, M. N., Brown, M. S., Goldstein, J. L., Han, J., Vale, G., McDonald, J. G., Seemann, J., Mendell, J. T., & Lu, F. (2020). Last step in the path of LDL cholesterol from lysosome to plasma membrane to ER is governed by phosphatidylserine. Proceedings of the National Academy of Sciences of the United States of America, 117, 18521–18529. https://doi.org/10.1073/pnas.2010682117.
Ridsdale, A., Denis, M., Gougeon, P. Y., Ngsee, J. K., Presley, J. F., & Zha, X. (2006). Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins. Molecular Biology of the Cell, 17, 1593–1605. https://doi.org/10.1091/mbc.e05-02-0100.
Röhrl, C., & Stangl, H. (2018). Cholesterol metabolism-physiological regulation and pathophysiological deregulation by the endoplasmic reticulum. Wiener Medizinische Wochenschrift, 168, 280–285.
Thelen, A. M., & Zoncu, R. (2017). Emerging roles for the lysosome in lipid metabolism. Trends in Cell Biology, 27, 833–850. https://doi.org/10.1016/j.tcb.2017.07.006.
Essaka, D. C. (2007). Reversed-Phase HPLC Determination of Cholesterol in Food Items. Electronic Theses andDissertations. Paper 2034. https://dc.etsu.edu/etd/2034.
Kraus, N. A., Ehebauer, F., Zapp, B., Rudolphi, B., Kraus, B. J., & Kraus, D. (2016). Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte, 5, 351–358. https://doi.org/10.1080/21623945.2016.1240137.
Derendorf, H. (2020). Excessive lysosomal ion-trapping of hydroxychloroquine and azithromycin. International Journal of Antimicrobial Agents, 55, 106007 https://doi.org/10.1016/j.ijantimicag.2020.106007.
Liu, Y., & Ding, J. (2014). The combined effect of azithromycin and insulin-like growth factor-1 on cultured human meibomian gland epithelial cells. Investigative Ophthalmology and Visual Science, 55, 5596–5601. https://doi.org/10.1167/iovs.14-14782.
Van Bambeke, F., Montenez, J. P., Piret, J., Tulkens, P. M., Courtoy, P. J., & Mingeot-Leclercq, M. P. (1996). Interaction of the macrolide azithromycin with phospholipids. I. Inhibition of lysosomal phospholipase A1 activity. European Journal of Pharmacology, 314, 203–14. https://doi.org/10.1016/S0014-2999(96)00552-3.
Fiorillo, M., Tóth, F., Sotgia, F. & Lisanti, M. P. (2019). Doxycycline, Azithromycin and Vitamin C (DAV): A potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs. Aging (Albany NY), 11, 2202–2216. https://doi.org/10.18632/aging.101905.
Mizushima, N., Yoshimori, T., & Levine, B. (2010). Methods in mammalian autophagy research. Cell, 140, 313–326. https://doi.org/10.1016/j.cell.2010.01.028.
Rabanal-Ruiz, Y., & Korolchuk, V. I. (2018). mTORC1 and nutrient homeostasis: the central role of the lysosome. International Journal of Molecular Sciences, 19, 818 https://doi.org/10.3390/ijms19030818.
Mauthe, M., Orhon, I., Rocchi, C., Zhou, X., Luhr, M., Hijlkema, K. J., Coppes, R. P., Engedal, N., Mari, M., & Reggiori, F. (2018). Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy, 14, 1435–1455. https://doi.org/10.1080/15548627.2018.1474314.
DeBose-Boyd, R. A., Ou, J., Goldstein, J. L., & Brown, M. S. (2001). Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proceedings of the National Academy of Sciences of the United States of America, 98, 1477–1482. https://doi.org/10.1073/pnas.98.4.1477.
Jacquemyn, J., Cascalho, A., & Goodchild, R. E. (2017). The ins and outs of endoplasmic reticulum‐controlled lipid biosynthesis. EMBO Reports, 18, 1905–1921. https://doi.org/10.15252/embr.201643426.
Baird, T. D., & Wek, R. C. (2012). Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Advances in Nutrition, 3, 307–321. https://doi.org/10.3945/an.112.002113.
Flis, V. V., & Daum, G. (2013). Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harbor Perspectives in Biology, 5, a013235.
Fujimoto, M., Hayashi, T., & Su, T. (2012). The role of cholesterol in the association of endoplasmic reticulum membranes with mitochondria. Biochemical and Biophysical Research Communications, 417, 635–639. https://doi.org/10.1016/j.bbrc.2011.12.022.
Gerhold, J. M., Cansiz-Arda, Ş., Lõhmus, M., Engberg, O., Reyes, A., Rennes, H., Sanz, A., Holt, I. J., Cooper, H. M., & Spelbrink, J. N. (2015). Human mitochondrial DNA-protein complexes attach to a cholesterol-rich membrane structure. Scientific Reports, 5, 15292 https://doi.org/10.1038/srep15292.
Rizzuto, R., Pinton, P., Carrington, W., Fay, F. S., Fogarty, K. E., Lifshitz, L. M., Tuft, R. A., & Pozzan, T. (1998). Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science, 280, 1763–1766.
Ashrafi, G., & Schwarz, T. L. (2013). The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death & Differentiation, 20, 31–42. https://doi.org/10.1038/cdd.2012.81.
Betin, V. M., MacVicar, T. D., Parsons, S. F., Anstee, D. J., & Lane, J. D. (2012). A cryptic mitochondrial targeting motif in Atg4D links caspase cleavage with mitochondrial import and oxidative stress. Autophagy, 8, 664–676. https://doi.org/10.4161/auto.19227.
Sheng, Z., Otani, H., Brown, M. S., & Goldstein, J. L. (1995). Independent regulation of sterol regulatory element-binding proteins 1 and 2 in hamster liver. Proceedings of the National Academy of Sciences of the United States of America, 92, 935–938. https://doi.org/10.1073/pnas.92.4.935.
Matsuda, M., Korn, B. S., Hammer, R. E., Moon, Y. A., Komuro, R., Horton, J. D., Goldstein, J. L., Brown, M. S., & Shimomura, I. (2001). SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes & Development, 15, 1206–1216.
Sandri, M. (2013). Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. The International Journal of Biochemistry & Cell Biology, 45, 2121–2129. https://doi.org/10.1016/j.biocel.2013.04.023.
Fan, J., Kou, X., Jia, S., Yang, X., Yang, Y., & Chen, N. (2016). Autophagy as a potential target for sarcopenia. Journal of Cellular Physiology, 231, 1450–1459. https://doi.org/10.1002/jcp.25260.
Lee, J. H., Jeon, J. H., & Lee, M. J. (2020). Docosahexaenoic Acid, a Potential Treatment for Sarcopenia, Modulates the Ubiquitin–Proteasome and the Autophagy–Lysosome Systems. Nutrients, 12, 2597 https://doi.org/10.3390/nu12092597.
Rasmussen, B. B., Fujita, S., Wolfe, R. R., Mittendorfer, B., Roy, M., Rowe, V. L., & Volpi, E. (2006). Insulin resistance of muscle protein metabolism in aging. The FASEB Journal, 20, 768–9. https://doi.org/10.1096/fj.05-4607fje.
Chen, G. L., Sutrina, S. L., Frayer, K. L., & Chen, W. W. (1986). Effects of lysosomotropic agents on lipogenesis. Archives of Biochemistry and Biophysics, 245, 66–75. https://doi.org/10.1016/0003-9861(86)90190-6.
Tyteca, D., Van Der Smissen, P., Van Bambeke, F., Leys, K., Tulkens, P. M., Courtoy, P. J., & Mingeot-Leclercq, M. P. (2001). Azithromycin, a lysosomotropic antibiotic, impairs fluid-phase pinocytosis in cultured fibroblasts. European Journal of Cell Biology, 80, 466–78. https://doi.org/10.1078/0171-9335-00180.
Geisslinger, F., Müller, M., Vollmar, A. M., & Bartel, K. (2020). Targeting Lysosomes in Cancer as Promising Strategy to Overcome Chemoresistance-A Mini Review. Frontiers in Oncology, 10, 1156 https://doi.org/10.3389/fonc.2020.01156.
King, M. A., Ganley, I. G., & Flemington, V. (2016). Inhibition of cholesterol metabolism underlies synergy between mTOR pathway inhibition and chloroquine in bladder cancer cells. Oncogene, 35, 4518–28. https://doi.org/10.1038/onc.2015.511.
Istvan, E. (2003). Statin inhibition of HMG-CoA reductase: a 3-dimensional view. Atherosclerosis Supplements, 4, 3–8. https://doi.org/10.1016/S1567-5688(03)00003-5.
Collins, R., Reith, C., Emberson, J., Armitage, J., Baigent, C., Blackwell, L., Blumenthal, R., Danesh, J., Smith, G. D., DeMets, D., & Evans, S., et al. (2016). Interpretation of the evidence for the efficacy and safety of statin therapy. The Lancet, 388, 2532–2561. https://doi.org/10.1016/S0140-6736(16)31357-5.
Demontis, F., Piccirillo, R., Goldberg, A. L., & Perrimon, N. (2013). Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Disease Models & Mechanisms, 6, 1339–1352. https://doi.org/10.1242/dmm.012559.
Hütter, E., Skovbro, M., Lener, B., Prats, C., Rabøl, R., Dela, F., & Jansen‐Dürr, P. (2007). Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell, 6, 245–256. https://doi.org/10.1111/j.1474-9726.2007.00282.x.
Beregi, E., Regius, O., Hüttl, T., & Göbl, Z. (1988). Age-related changes in the skeletal muscle cells. Zeitschrift fur Gerontologie, 21, 83–86.
Navratil, M., Terman, A., & Arriaga, E. A. (2008). Giant mitochondria do not fuse and exchange their contents with normal mitochondria. Experimental Cell Research, 314, 164–72. https://doi.org/10.1016/j.yexcr.2007.09.013.
Jakubke, C., Roussou, R., Maiser, A., Schug, C., Thoma, F., Bunk, D., Hörl, D., Leonhardt, H., Walter, P., Klecker, T., & Osman, C. (2021). Cristae-dependent quality control of the mitochondrial genome. Science Advances, 7, eabi8886.
Peralta, S., Goffart, S., Williams, S. L., Diaz, F., Garcia, S., Nissanka, N., Area-Gomez, E., Pohjoismäki, J., & Moraes, C. T. (2018). ATAD3 controls mitochondrial cristae structure in mouse muscle, influencing mtDNA replication and cholesterol levels. Journal of Cell Science, 131, jcs217075 https://doi.org/10.1242/jcs.217075.
Terman, A., & Brunk, U. T. (1998). Ceroid/lipofuscin formation in cultured human fibroblasts: the role of oxidative stress and lysosomal proteolysis. Mechanisms of Ageing Development, 104, 277–91. https://doi.org/10.1016/S0047-6374(98)00073-6.
Sato, T., & Tauchi, H. (1975). The formation of enlarged and giant mitochondriain the aging process of human hepatic cells. Pathology International, 25(4), 403–412. https://doi.org/10.1111/j.1440-1827.1975.tb00862.x.
Terman, A., Dalen, H., Eaton, J. W., Neuzil, J., & Brunk, U. T. (2004). Aging of cardiac myocytes in culture: oxidative stress, lipofuscin accumulation, and mitochondrial turnover. Annals of New York Academy of Sciences, 1019, 70–7. https://doi.org/10.1196/annals.1297.015.
Kuhla, A., Blei, T., Jaster, R., & Vollmar, B. (2011). Aging is associated with a shift of fatty metabolism toward lipogenesis. The Journals of gerontology. Series A, Biological Sciences and Medical Sciences, 66, 1192–200. https://doi.org/10.1093/gerona/glr124.
Fussell, J., Son, J., Wagner, B. A., Sarsour, E. H., Buettner, G. R., & Goswami, P. C. (2016). A lipogenic-switch shifts metabolism from glycolysis to mitochondrial respiration during aging. Free Radical Biology and Medicine, 100, S83 https://doi.org/10.1016/j.freeradbiomed.2016.10.207.
Acknowledgements
This work was funded by Science and Engineering Research Board (CRG/2020/003399), India. We thank Indian Council of Medical Research (File No. 2017-3862/CMB-BMS) and Council of Scientific and Industrial Research (File No. 09/468(0489)/2015-EMR-I) for awarding Senior Research Fellowship to S.R. We thank Department of Science and Technology, India- Promotion of University Research and Scientific Excellence (PURSE), Fund for Improvement of S&T Infrastructure in Universities and Higher Educational Institutions (FIST) and Department of Biotechnology, India- Boost to University Interdisciplinary Life science Departments for Education and Research (BUILDER) (BT/PR12153/INF/22/200/2014) for chemiluminiscence, confocal microscope and HPLC instrumentation respectively.
Author information
Authors and Affiliations
Contributions
S.R. was involved in Data collection, Data analysis and draft paper preparation, A.R.S. contributed to Data analysis and interpretation, S.R. was involved in Data analysis, S.V. was involved in Data analysis, T.J. was involved in study conception and design, data analysis and paper preparation.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajasekaran, S., Ramaian Santhaseela, A., Ragunathan, S. et al. Altered Lysosomal Function Manipulates Cellular Biosynthetic Capacity By Remodeling Intracellular Cholesterol Distribution. Cell Biochem Biophys 81, 29–38 (2023). https://doi.org/10.1007/s12013-022-01123-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-022-01123-y