Abstract
The Schmidt rearrangement, a reaction that enables C-C or C-H σ bond cleavage and nitrogen insertion across an aldehyde or ketone substrate, is one of the most important and widely used synthetic tools for the installation of amides and nitriles. However, such a reaction frequently requires volatile, potentially explosive, and highly toxic azide reagents as the nitrogen donor, thus limiting its application to some extent. Here, we show a Schmidt-type reaction where aryldiazonium salts act as the nitrogen precursor and in-situ-generated cyclopenta-1,4-dien-1-yl acetates serve as pronucleophiles from gold-catalyzed Nazarov cyclization of 1,3-enyne acetates. Noteworthy is that cycloketone-derived 1,3-enyne acetates enabled ring-expansion relay to access a series of 2-pyridone-containing fused heterocycles, in which nonsymmetric cycloketone-derived counterparts demonstrated high regioselectivity. Aside from investigating the scope of this Schmidt-type reaction, mechanistic details of this transformation are provided by performing systematic theoretical calculations.
Similar content being viewed by others
Introduction
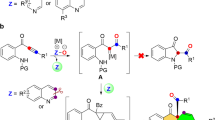
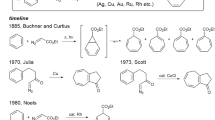
Nitrogen-based heterocycles constitute the basic structural scaffold of substantial bioactive natural products and are present in approximately half of marketed drugs, with nonplanar heterocycles featuring five- and/or six-membered ring frameworks being of great interest to the fields of pharmaceutical discovery, chemical biology, and medical chemistry1,2,3,4,5. Especially abundant among these heterocycles are those incorporating a 2-pyridinone framework, a privileged structure that is composed of the core of many alkaloids exhibiting potent biological activities (Fig. 1a)6,7,8,9,10,11. Consequently, the development of innovative strategies for the assembly of 2-pyridone-containing heterocycles has been a hot research topic in the organic community12,13,14. The Schmidt reaction represents a long-standing popular nitrogenation approach to furnish these aza-heterocycles from cyclic ketones with HN3 or alkyl azides15,16,17,18,19. However, its dependence on volatile, highly toxic, and potentially explosive azide reagents offers great possibilities for further exploration20,21,22 (Fig. 1b). Despite the astonishingly persistent efforts on hydrazoic acid replacements, the use of azide still prevails in this field23,24,25. The Beckmann rearrangement starting from cyclic oximes has been recognized as a well-known alternative pathway to approach cyclic amides26,27. However, typically catalytic Beckmann rearrangements often require some specialized active cyclic oximes, strong Brønsted acids, or preactivation of the hydroxyl (e.g., by tosylation)28,29,30,31, thus limiting the potential application to some extent. Ideally, if one easily available and widespread chemical could be activated by a suitable catalytic system while endowed with new reactivity32,33,34,35,36,37, it would advance innovative chemical technology that influences broad fields of academia and industry.
a Natural products and bioactive molecules containing 2-pyridinone structures. b Traditional Schmidt reaction with azides as the limiting reagent. c Reactivity patterns of aryldiazonium salts. d Our study on 2-pyridinone synthesis via the sequence involving Nazarov cyclization, nucleophilic addition, and Schmidt reaction.
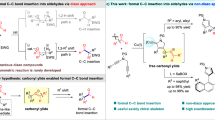
Because of their highly electrophilic character and moderate oxidizability, aryldiazonium salts, easily prepared from the corresponding anilines, demonstrate broad-spectrum reactivities and are often used as both aryl precursors and dinitrogen proelectrophiles in numerous transformations, such as the Meerwein arylation38,39, cross-coupling reactions40,41,42, [3 + 2] annulation43,44, and Japp-Klingemann reaction45,46,47. In the well-known Japp-Klingemann procedure, the addition of cyclic ketones into aryldiazonium salts produces rather unstable azo compounds, which are converted into hydrazones via 1,3-hydrogen transfer (Fig. 1c)45,46,47. In contrast, the reactivity of aryldiazonium salts as a nitrogen donor for the Schmidt reaction remains unknown. We hypothesized that the key to realizing the Schmidt reaction is to suppress the 1,3-hydrogen transfer process in the Japp-Klingemann procedure. Therefore, the removal of the proton at the α-position of azoarenes for migration while exploiting the intrinsic nucleophilicity of azoarenes may provide a feasible scheme for the Schmidt reaction.
Homogeneous gold catalysis has been acknowledged as an effective strategy for constructing functional molecules in synthetic chemistry benefited from the impressive characteristics of its high catalytic capabilities, high levels of regioselectivity, and mild reaction conditions as well as good functional group tolerance48,49,50,51,52,53. The gold-catalyzed Nazarov cyclization of nucleophilic 1,n-enyne acetates toward cyclopenta-1,4-dien-1-yl acetates is among the more exploited reactions54,55,56,57,58,59,60,61. However, to date, there are only a few reports that deal with the inherent nucleophilicity of in-situ generated cyclopenta-1,4-dien-1-yl acetates toward intermolecular nucleophilic substitution reactions55,58. In sharp contrast, the nucleophilic addition of this intermediate remains elusive. Along this line, we believe that highly electrophilic aryldiazonium salts could be trapped by in-situ-generated nucleophilic cyclopenta-1,4-dien-1-yl acetates to access the azo intermediate, which facilitates acyl transfer and the subsequent rearrangement process for the Schmidt-type reaction (Fig. 1d). Thus, this cascade activation strategy would demonstrate simple and available aryldiazonium salts as a cyclic nitrogenated partner akin to azides, but with remarkable elimination of associated hazards.
In this work, we report that gold-catalyzed transformation of 1,3-enyne acetates with aryldiazonium salts enables merging Nazarov cyclization with a Schmidt-type reaction for the general synthesis of valuable 2-pyridinone-based heterocyclic systems as well as synthetically appealing azasteroid chemistry (Fig. 1d). Of note is that the gold complex could be compatible with aryldiazonium salts without observation of their redox transformation with each other. Intrigued by the ring-expansion relay and high regioselectivity observed in nonsymmetric cycloketone-derived 1,3-enyne acetates, we carefully performed systematic theoretical calculations to elucidate the catalytic cycle for σ-bond migration, the azoarene activation mode and the influence on the regioselectivity (see the mechanism section and Supplementary Information).
Results
Reaction optimization
To test our initial hypothesis, we began by studying the gold-catalyzed reaction of 1,3-enyne acetates and aryldiazonium salts as test substrates to develop straightforward access to highly functionalized cyclopenta[c]pyridin-1-ones, which are classically synthesized via catalytic cyclization reactions that exhibit a lack of flexibility62,63,64. Diverse gold catalysts and solvents were evaluated at room temperature for 6 h, as shown in Table 1. To our delight, by using JohnPhosAu(MeCN)SbF6 (1.0 mol %) as a catalyst, the reaction of strained cyclobutanone-derived 1,3-enyne acetate 1a with 2a in 1,2-dichloroethane (DCE) afforded cyclopenta[c]pyridin-1-one product 3 in 65% yield through a Schmidt-type reaction (entry 1). Several other gold catalysts widely used in catalytic transformations, such as Ph3PAuCl, XPhosAuCl, SPhosAuCl, IPrAuNTf2, AuCl3, and AuCl, were then examined (entries 2–7). The results revealed that all these catalysts could drive the transformation to the desired product 3 except for SPhosAuCl, and the former two led to moderate yields of product 3 (entries 2–3); in contrast, the latter three demonstrated very poor catalytic capabilities and provided lower yields (<29%, entries 5–7). Taking JohnPhosAu(MeCN)SbF6 as the catalyst, we then investigated the effect of the solvent by exploiting toluene, tetrahydrofuran (THF), acetone, and DCM (entries 8–11). However, the use of the former two was almost ineffective, as only a trace amount of product 3 was detected (entries 8–9). The reaction independently proceeded in the latter two solvents, but both gave unsatisfactory results associated with the reaction yields in comparison to DCE (entries 10–11 vs entry 1). This reaction could run at higher temperatures, but gave a slightly complex system and a decrease in yields was detected (entries 12–13).
Evaluation of the substrate scope
With these acceptable reaction conditions (Table 1, entry 1), we set out to systematically investigate the scope of this transformation by examining the behaviors of 1,3-enyne acetates and aryldiazonium salts. The results are summarized in Fig. 2. First, aryldiazonium salts associated with various substituents successfully participated in this gold-catalyzed ring-expansion relay with 1a, orienting regiospecific access to the desired cyclopenta[c]pyridin-1-ones 4–18 in good yields (Fig. 2). Both electron-donating (EDG) (e.g., methyl, 2b−2d) and electron-withdrawing (EWG) (e.g., fluoro, 2e; chloro, 2f−2h; bromo, 2i; trifluoromethyl, 2j; cyano, 2k, and nitro, 2l) groups at different positions (ortho, meta, or para) demonstrated good compatibility of this transformation. Of these functional groups, both o-tolyl (2b) and o-chlorophenyl (2f) analogs with strong steric congestion were well-tolerated with this catalytic system, illustrating that the increased steric hindrance had little influence on the reactivity. In addition, three strong electron-withdrawing groups such as CF3, CN, and NO2 at the para-position remained highly reactive in this catalysis, delivering the targets 12–14 in 67–75% yields. Furthermore, the protocol was also compatible with naphthalen-2-yl counterpart 2m. Notably, three derivatives of naturally occurring chiral alcohol-based esters (L(-)-borneol (2n), L-menthol (2o), and 1-adamantanol (2p)) were also applicable, producing corresponding products 16–18 in good yields through two carbon-carbon bond cleavages. In addition to the cyclobutanone-derived substrate, less strained cyclopentanone-derived counterpart 1b worked readily, enabling a similar catalytic ring-expansion/nitrogen insertion process to deliver functionalized tetrahydroisoquinolin-1(2H)-ones 19–34 in synthetically useful yields (Fig. 2). Next, the potential variations in the structure of 1,3-enyne acetates were further examined, with p-chlorophenyl substrate 2h as the other partner. 1,3-Enyne acetate 1c, which bears a phenyl group at the C3 position of the cyclobutene ring, afforded desired product 35 in good yield. Importantly, this ring-expansion reaction proceeded with complete regioselectivity when aryl substituents were installed into the C2 position of the cyclobutene ring, in which a less steric methylene group is more prone to migrate than methine functionality with a substituent under gold-catalyzed conditions. As exemplified by 1,3-enyne substrates 1d–1f, the expected products 36–38 as single regioisomers were isolated, albeit with moderate yields. A similar phenomenon was discovered in the transformations of both bicyclo[3.2.0]hept-2-en-6-one-derived (1g) and C2-alkylated cyclopentanone-derived (1i and 1j) substrates, and regioisomeric products 39, 41 and 42 were generated with good yields. Moreover, internal alkynes 1h and 1k that bear a cyclohexenyl substituent were still tolerated, providing tricyclic products 40 and 43 in acceptable yields (Fig. 2).
After the success of the catalytic ring-expansion relay reactions of formal cycloketone-derived 1,3-enyne acetates and aryldiazonium salts, we proceeded to examine other types of naturally occurring cycloketone derivatives, and therefore offer a general ring-expansion strategy to build up other N-heterocycles incorporating two medicinally relevant privileged core structures. Azasteroids, normally derived from chemical modifications of steroids, are recognized as a privileged pharmacophore prevalent in pharmaceutical agents65,66, but relatively few approaches to their syntheses have been documented in recent years67,68,69. We questioned whether steroid-derived 1,3-enyne acetates could be included in our catalytic cycle as pro-nucleophilic partners to react with aryldiazonium salts 2. If successful, this reaction would demonstrate an efficient and regiospecific approach to form pentacyclic azasteroid motifs. Following this idea, we tested the reaction of estrone-derived 1,3-enyne acetate 1l with aryldiazonium salt 2d under the optimized conditions (Table 1, entry 1). To our delight, the desired pentacyclic azasteroid 44 was obtained in 59% yield (Fig. 3). Furthermore, an array of electron-withdrawing groups, such as halo, trifluoromethyl, cyano, and nitro substituents, at the para-position of the benzene ring of aryldiazonium salts proved to be compatible with this protocol (Fig. 3). In addition, epiandrosterone-derived substrate 1m (tetrahydropyran protection) could be utilized to react with aryldiazonium salts, furnishing diversely substituted azasteroids 50–51 in good yields (Fig. 3).
To further expand the utility of this methodology, we devoted our effort to testing aldehyde-derived 1,3-enynes as pro-nucleophiles for pyridin-2(1H)-one synthesis, because such compounds are a class of prevalent heteroaromatic structures that are frequently encountered in a broad variety of natural products, bioactive agents and approved drugs70,71,72. For this reason, reactions of aldehyde-derived 1,3-enyne acetates with aryldiazonium salts were carried out under standard conditions, and used to produce a wide range of pyridin-2(1H)-ones 52-70 with generally good yields in a regiospecific manner (Fig. 4). Aromatic aldehyde-derived 1,3-enyne acetates 1n-1w bearing both electron-donating and electron-withdrawing groups, regardless of their positions, could be readily engaged in the reaction. Various functional groups, including methyl (1o–1q), halogens (Cl, 1r–1t and F, 1 u), and trifluoromethyl (1v), were compatible with this catalytic system. The naphthalene ring (1w) was also exclusively installed into the product (61). As exemplified by substrate 1x, this protocol was also adaptable to the 1,3-enyne acetate attached to a heteroarene such as thiophene in an acceptable yield (62, 51%). Moreover, switching the aryl group at the α-position of 1,3-enyne substrates to other substituents, such as cycloalkyl (cyclopropyl 1 y and cyclohexyl 1z), benzyl (1aa), branched (1ab) and linear (1ac) alkyl, and cinnamyl (1ad), had a negligible effect on the reaction performance, orienting the complete regioselectivity to access the target pyridin-2(1H)-one products 63-68 with acceptable yields. When the methyl group (R3) on the terminal alkene moiety of 1,3-enyne acetates was replaced by phenyl and n-pentyl groups, products 69 and 70 were isolated in 68% and 73% yields, respectively. Then, we examined the generality of this catalytic annulation/nitrogen insertion cascade regarding aryldiazonium salts 2 by combining with benzaldehyde-derived substrate 1n. The detailed investigations on substituents including alkyl, halogens, trifluoromethyl, cyano, and nitro at different positions in the arene ring as well as a sterically more demanding bicyclic aromatic example, such as naphthyl group, revealed that all these attempts were applicable for the reaction (products 71–82, Fig. 4). The presence of halogen, cyano, and nitro moieties in these synthons adds greatly to the synthetic value of the cyclization products, as they can act as an additional handle for late-stage transformations. Aryldiazonium salts that bear a chiral alcohol-based ester such as L(-)-borneol (2n), L-menthol (2o), 1-adamantanol (2p) and (-)-isopulegol (2q) on the benzene ring successfully gave corresponding products 83–86 in 56-65% yields (Fig. 4).
Gram-scale synthesis and synthetic applications
To demonstrate the practicality and scalability of the present protocol, gram-scale reactions of 1a and 1o (5.0 mmol) and 2k were independently carried out with 1 mol% JohnPhosAu(MeCN)SbF6 under the optimized conditions, affording products 11 and 78 in slightly diminished yields (Fig. 5a, b). Subsequently, compound 11 reacted with methyl iodide in the presence of NaH, furnishing methyl-protected 87 in 78% yield (Fig. 5a). Treatment of 78 with Br2 gave polybrominated product 88 in 82% yield, followed by the Ir-catalyzed reaction of tetramethyldisilazane (TMDS) to access 3,5-dibromopyridin-2(1H)-one 89 in 62% yield through N-N bond cleavage (Fig. 5b). A similar transformation occurred when using 78 and TMDS in the presence of iridium catalyst (Fig. 5b).
Mechanistic studies
To gain mechanistic insights into this nitrogen insertion process, some control experiments were conducted, as shown in Fig. 6. In Zhang’s procedure, gold-catalyzed Nazarov cyclization of 1,3-enyne acetates led to two different products of cyclopentenones and unstable cyclopentadienes54. To investigate the possibility of intermediates for this reaction, a gold-catalyzed reaction of 1a was run under standard conditions for 1 h, and then 2a was placed into this reaction system. As a result, instead of the target 3, 2-methyl-3,4,5,6-tetrahydropentalen-1(2H)-one 91 was generated, along with the recovered 2a (Fig. 6a). Without a gold catalyst, the reaction between preformed 2-methyl-3,4,5,6-tetrahydropentalen-1-yl acetate 92 and 2a gave a 50% yield of 3a (Fig. 6b). Next, treatment of 92 with 2a under standard conditions provided a slightly increased yield (56%, Fig. 6c). These outcomes demonstrate that cyclopentadienes, rather than cyclopentenones, are potential reaction intermediate, and the gold catalyst is unnecessary but could promote the nitrogen insertion reaction to some extent. Let us examine in detail the roles of tetrafluoroborate anion and H2O (Fig. 6d). Without tetrafluoroborate, the reaction of 1a with benzenediazonium chloride did not proceed under the standard conditions whereas the use of 2.0 equivalents of silver tetrafluoroborate as tetrafluoroborate sources provided 3a in 58% yield. Exchanging silver tetrafluoroborate for silver acetate did not give 3a. The reaction in the absence of H2O also did not work. Evidently, when 1.0 equivalent of H2O was added to the above reaction system, product 3a was obtained in 61% yield. These results indicate that both tetrafluoroborate and H2O are crucial for this transformation (for their roles, see the mechanism section).
Possible reaction pathways
To investigate the possible mechanism of the Au(I)-catalyzed Schmidt-type reaction between cyclopentadienyl acetate and aryldiazonium salt, a theoretical study employing density functional theory (DFT) calculations was performed at M06-L/def2-TZVPP/SMDdichloroethane//M06-L/def2-SVP/SMDdichloroethane level of theory73 (Fig. 7). As a typical π-acetic metal, Au in active species catalyst could be coordinated by cyclopentadienyl acetate 1g to form complex M1 with exergonic energy of 8.4 kcal/mol. Activated by the π-acetic Au(I) cation, the coordinating C-C triple bond could be nucleophilically attacked by the intramolecular ester group to achieve stepwise acetoxy transfer via the six-membered ring transition states TS1 and TS2. The corresponding free energy barriers are 12.1 kcal/mol and 2.8 kcal/mol, respectively, indicating a rapid process. The consequence of stepwise acetoxy transfer is the formation of oxonium intermediate M3, which could be regarded as an equilibrium with intermediate M1 in thermodynamic74,75,76. Resonance structure M3’ clearly reveals the electrophilicity of its terminal methylene position, which results in an intramolecular electrophilic 1,5-annulation via transition state TS3 to irreversibly form an Au-carbene complex M4. The calculated free energy barrier for the step was detected to be 13.7 kcal/mol. The resonance structure of M4’ reveals a cationic carbon, which would cause 1,2-carbon cation rearrangement. The calculated free energy barrier for the intramolecular 1,2-shift of the unsubstituted methyl group via transition state TS4 is 10.1 kcal/mol. However, the corresponding energy barrier of the cyclopentyl shift via transition state TS5 is 17.8 kcal/mol. Therefore, fused pentalenyl acetate M5 was found to be the major product, which is fully consistent with the experimental observations.
To further analyze the regioselectivity, the Newman projections of transition states TS4 and TS5 are given in Fig. 8. In the geometry of transition state TS4, the strain of the fused five-membered ring (right part) promoted the rotation of the projecting C-C single bond, which led to the 1,2-shift of the methyl group with strain release. Meanwhile, the corresponding projecting C-C bond in TS5 is free without strain. Therefore, the lack of a driving force makes the corresponding 1,2-shift difficult via TS5.
The reaction pathway of further transformation of fused pentalenyl acetate M5 was also considered by DFT calculations. As shown in Fig. 9, the enol ester moiety of M5 could nucleophilically attack aryldiazoniom salt 2 h via transition state TS6 affording oxonium intermediate M7 with a free energy barrier of only 18.6 kcal/mol. After that, two possible pathways for the expansion of the ring could occur: acyl transfer promoted arrangement (solid black lines) or nitrogen-insertive expansion (dashed red lines). In intermediate M7, the azo moiety reveals nucleophilicity, which can attack the acyl group to achieve an intramolecular acyl transfer via transition state TS7 with a free energy barrier of only 9.7 kcal/mol. The acyldiazenium moiety in intermediate M8 leads to the cleavage of an active C-C bond via transition state TS8 with an energy barrier of only 0.8 kcal/mol. The generated carbonyl cation in intermediate M9 could undergo an intramolecular nucleophilic attack by carbonyl cation via transition state TS9, which provides an oxodihydropyridinium intermediate M10. Furthermore, a deprotonation process could be associated with a complex of water and BF4 anion via transition state TS10 with a free energy barrier of only 6.7 kcal/mol. Finally, the stepwise hydrolysis of Ac-protected product M11 could lead to the formation of the final product P-39. Moreover, resonance structure M7’ exhibits an electrophilic carbon atom, which can be nucleophilically attacked by an azo moiety via transition state TS13 to afford aziridinium intermediate M13. Although C-C bond cleavage via transition state TS14, hydrolysis of amide and deprotonation could also provide the same product P-39, the much higher free energy barrier of this process makes it an unfavorable path.
Discussion
In this work, the discovery of a Schmidt-type reaction triggered by the soft nucleophilicity of azoarenes is described. This provides a general and practical approach to highly substituted 2-pyridinones and their fused heterocyclic system from readily available 1,3-enyne acetates with aryldiazonium salts in a highly regioselective fashion. The reaction is catalyzed by a well-defined air-stable gold catalyst, involving a 3,3-rearrangement/Nazarov cyclization and nitrogen insertion pathway, under mild conditions and demonstrates wide substrate compatibility. Mechanistic studies reveal a soft nucleophilic property of azoarenes to induce C-C cleavage and nitrogen insertion. Systematic theoretical calculations led us to propose that ring strain controls the regioselectivity of C-C σ-bond migration for nonsymmetric cycloketone-derived 1,3-enyne acetates and that the nucleophilicity of azoarenes induces acyl migration to achieve Schmidt-type rearrangement, thereby giving rise to 2-pyridinone-based cyclic products with high annulation efficiency and regioselectivity from unsaturated linear substrates.
Methods
General procedure for the gold-catalyzed domino reaction of 1,3-enyne acetates and aryldiazonium tetrafluoroborates. To a 10 mL Schlenk tube under air conditions, cyclobutanone- and aldehyde-derived 1,3-enyne acetates (1, 0.2 mmol), aryldiazonium tetrafluoroborates (2, 0.4 mmol), JohnPhosAu(MeCN)SbF6 (1 mol%), and 1,2-dichloroethane (2.0 ml) were successively added. The mixture was stirred at room temperature (the reaction of cyclopentanone-derived 1,3-enyne acetates was at 50 °C) for 6 h. After the reaction was completed (indicated by TLC, petroleum ether: ethyl acetate = 2:1), the reaction mixture was concentrated by vacuum distillation and was purified by flash column chromatography to afford the desired pure products 3–86.
Data availability
The data generated in this study are provided in the Supplementary Information file. For the experimental procedures, data of NMR and HRMS analysis and computational details, see Supplementary Methods and Figures in the Supplementary Information file. The authors declare that all these data supporting the findings of this study are available within the article and Supplementary Information files and are also available from the corresponding author upon request.
Code availability
The X-ray crystallographic data generated in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number CCDC 2142493 (5), 2142494 (39), and 2142495 (55). The X-ray crystallographic data are available free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
References
Shan, Y., Su, L., Zhao, Z. & Chen, D. The construction of nitrogen-containing heterocycles from alkynyl imines. Adv. Synth. Catal. 363, 906–923 (2021).
Eicher, T., Hauptmann, S. & Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications. (Wiley-VCH, Weinheim, 2013).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Wang, Y., Zhang, W.-X. & Xi, Z.-F. Carbodiimide-based synthesis of N-heterocycles: moving from two classical reactive sites to chemical bond breaking/forming reaction. Chem. Soc. Rev. 49, 5810–5849 (2020).
Mondal, S., Yetra, S. R., Mukherjee, S. & Biju, A. T. NHC-catalyzed generation of α,β-unsaturated acylazoliums for the enantioselective synthesis of heterocycles and carbocycles. Acc. Chem. Res. 52, 425–436 (2019).
Eisenbraun, E. J., Sullins, D. W., Browne, C. E. & Shoolery, J. N. (4aS,7S,7aR)-Nepetalactam and (4aS,7S,7aR)-2-[(3R,4R,4aR,7S,7aR)-octahydro-4,7-dimethyl-1-oxocyclopenta[c]pyran-3-yl]nepetalactam. Nitrogen analogs of nepetalactone and nepetalic.psi.-anhydride. J. Org. Chem. 53, 3968–3972 (1988)..
Catozzi, N., Edwards, M. G., Raw, S. A., Wasnaire, P. & Taylor, R. J. K. Synthesis of the louisianin alkaloid family via a 1,2,4-triazine inverse-electron-demand diels−alder approach. J. Org. Chem. 74, 8343–8354 (2009).
Peukert, S., Schwahn, U., Güssregen, S., Schreuder, H. & Hofmeister, A. Poly(ADP-Ribose) polymerase-1 (PARP-1) inhibitors based on a tetrahydro-1(2H)-isoquinolinone scaffold: synthesis, biological evaluation and X-ray crystal structure. Synthesis 9, 1550–1554 (2005).
Kumar, M. R., Park, K. & Lee, S. Synthesis of amido-N-imidazolium salts and their applications as ligands in Suzuki–Miyaura reactions: coupling of hetero- aromatic halides and the synthesis of Milrinone and Irbesartan. Adv. Synth. Catal. 352, 3255–3266 (2010).
Zhang, W.-M., Dai, J., Xu, J. & Xu, H.-J. Visible-light-induced C2 alkylation of pyridine N-oxides. J. Org. Chem. 82, 2059–2066 (2017).
McClymont, K. S., Wang, F.-Y., Minakar, A. & Baran, P. S. Total synthesis of (−)-maximizcin. J. Am. Chem. Soc. 142, 8608–8613 (2020).
Su, Y., Zhao, M., Han, K.-L., Song, G.-Y. & Li, X.-W. Synthesis of 2-pyridones and iminoesters via Rh(III)-catalyzed oxidative coupling between acrylamides and alkynes. Org. Lett. 12, 5462–5465 (2010).
Tyagi, M. et al. Tandem ring opening/intramolecular [2 + 2] cycloaddition reaction for the synthesis of cyclobutane fused thiazolino−2-pyridones. J. Org. Chem. 86, 16582–16592 (2021).
Wei, G.-Z. et al. Heterologous production of unnatural flavipucine family products provides insights into flavipucines biosynthesis. Org. Lett. 23, 7708–7712 (2021).
Schmidt, K. F. Über die Einwirkung von NH auf organische verbindungen. Angew. Chem. 36, 511 (1923).
Smith, P. A. S. The Schmidt reaction: experimental conditions and mechanism. J. Am. Chem. Soc. 70, 320–323 (1948).
Aubé, J. & Milligan, G. L. Intramolecular Schmidt reaction of alkyl azides. J. Am. Chem. Soc. 113, 8965–8966 (1991).
Pearson, W. H., Walavalkar, R., Schkeryantz, J. M., Fang, W.-K. & Blickensdorf, J. D. Intramolecular Schmidt reactions of azides with carbocations: synthesis of bridged-bicyclic and fused-bicyclic tertiary amines. J. Am. Chem. Soc. 115, 10183–10194 (1993).
Szostak, M. & Aubé, J. Chemistry of bridged lactams and related heterocycles. Chem. Rev. 113, 5701–5765 (2013).
Bräse, S., Gil, C., Knepper, K. & Zimmermann, V. Organic azides: an exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 44, 5188–5240 (2005).
Hassner, A., Stern, M., Gottlieb, H. E. & Frolow, F. Synthetic methods. 33. Utility of a polymeric azide reagent in the formation of di- and triazidomethane. Their NMR spectra and the X-ray structure of derived triazoles. J. Org. Chem. 55, 2304–2306 (1990).
Marinescu, L., Thinggaard, J., Thomsen, I. B. & Bols, M. Radical azidonation of aldehydes. J. Org. Chem. 68, 9453–9455 (2003).
He, L., Wanunu, M., Byun, H.-S. & Bittman, R. Regioselective and stereospecific azidation of 1,2- and 1,3-diols by azidotrimethylsilane via a mitsunobu reaction. J. Org. Chem. 64, 6049–6055 (1999).
Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Swamy, K. C. K., Kumar, N. N. B., Balaraman, E. & Kumar, K. V. P. P. Mitsunobu and related reactions: advances and applications. Chem. Rev. 109, 2551–2651 (2009).
Gawley, R. E. The Beckmann reactions: rearrangements, elimination-additions, fragmentations, and rearrangement-cyclizations. Org. React. 35, 1–61 (1988).
Blatt, A. H. The Beckmann rearrangement. Chem. Rev. 12, 215–260 (1933).
Fujioka, H. et al. Beckmann fragmentation and successive carbon–carbon bond formation using grignard reagents via phosphonium salt intermediates. Chem. Pharm. Bull. 64, 718–722 (2016).
Alhifthi, A., Harris, B. L., Goerigk, L., White, J. M. & Williams, S. J. Beckmann fragmentation and successive carbon–carbon bond formation using grignard reagents via phosphonium salt intermediates. Org. Biomol. Chem. 15, 10105–10115 (2017).
Touchette, S. J., Dunkley, E. M., Lowder, L. L. & Wu, J. Nucleophile-intercepted Beckmann fragmentation reactions. Chem. Sci. 10, 7812–7815 (2019).
Crochet, P. & Cadierno, V. Catalytic synthesis of amides via aldoximes rearrangement. Chem. Commun. 51, 2495–2505 (2015).
Watson, A. J. A. & Williams, J. M. J. The give and take of alcohol activation. Science 329, 635–636 (2010).
Ludwig, J. R., Zimmerman, P. M., Gianino, J. B. & Schindler, C. S. Iron(III)-catalysed carbonyl–olefin metathesis. Nature 533, 374–379 (2016).
Juliá-Hernández, F., Moragas, T., Cornella, J. & Martin, R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature 545, 84–88 (2017).
Wang, L., Lear, J. M., Rafferty, S. M., Fosu, S. C. & Nagib, D. A. Ketyl radical reactivity via atom transfer catalysis. Science 362, 225–229 (2018).
Litman, Z. C., Wang, Y., Zhao, H. & Hartwig, J. F. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis. Nature 560, 355–359 (2018).
Liu, J.-Z. et al. Nitromethane as a nitrogen donor in Schmidt-type formation of amides and nitriles. Science 367, 281–285 (2020).
Ghosh, I., Marzo, L., Das, A., Shaikh, R. & Koenig, B. Visible light mediated photoredox catalytic arylation reactions. Acc. Chem. Res. 49, 1566–1577 (2016).
Hari, D. P. & Koenig, B. The photocatalyzed meerwein arylation: classic reaction of aryl diazonium salts in a new light. Angew. Chem. Int. Ed. 52, 4734–4743 (2013).
Hopkinson, M. N., Tlahuext-Aca, A. & Glorius, F. Merging visible light photoredox and gold catalysis. Acc. Chem. Res. 49, 2261–2272 (2016).
Cai, R. et al. Ligand-assisted gold-catalyzed cross-coupling with aryldiazonium salts: redox gold catalysis without an external oxidant. Angew. Chem. Int. Ed. 54, 8772–8776 (2015).
Kim, S., Rojas-Martin, J. & Toste, F. D. Visible light-mediated gold-catalysed carbon(sp2)–carbon(sp) cross-coupling. Chem. Sci. 7, 85–88 (2016).
Liu, H.-N., Cao, H.-Q., Cheung, C. W. & Ma, J.-A. Cu-mediated expeditious annulation of alkyl 3-aminoacrylates with aryldiazonium salts: access to alkyl N2-aryl 1,2,3-triazole-carboxylates for druglike molecular synthesis. Org. Lett. 22, 1396–1401 (2020).
Zhou, L.-N., Feng, F.-F., Cheung, C.-W. & Ma, J.-A. Cu-enabled [3 + 2] annulation of in situ formed nitrile ylides with aryldiazonium salts: access to 5-cyano-1,2,4-triazoles. Org. Lett. 23, 739–744 (2021).
Chen, Y.-H. et al. Utility of Japp-Klingemann reaction for the preparation of 5-carboxy-6-chloroindole via Fischer indole protocol. Tetrahedron Lett. 48, 2353–2356 (2007).
Li, J. & Corey, E. J. Name Reactions for Functional Group Transformations. (John Wiley & Sons, 2007).
Jiricek, J. & Blechert, S. Enantioselective synthesis of (-)-gilbertine via a cationic cascade cyclization. J. Am. Chem. Soc. 126, 3534–3538 (2004).
Collado, A., Nelson, D. J. & Nolan, S. P. Optimizing catalyst and reaction conditions in gold(I) catalysis-ligand development. Chem. Rev. 121, 8559–8612 (2021).
Zheng, Z.-T. et al. Homogeneous gold-catalyzed oxidation reactions. Chem. Rev. 121, 8979–9038 (2021).
Witzel, S., Hashmi, A. S. K. & Xie, J. Light in gold catalysis. Chem. Rev. 121, 8868–8925 (2021).
Lu, Z.-C., Li, T., Mudshinge, S. R., Xu, B. & Hammond, G. B. Optimization of catalysts and conditions in gold(I) catalysis-counterion and additive effects. Chem. Rev. 121, 8452–8477 (2021).
Ye, L.-W. et al. Nitrene transfer and carbene transfer in gold catalysis. Chem. Rev. 121, 9039–9112 (2021).
Qin, X.-Y. et al. Gold-catalyzed skeletal rearrangement of alkenes: regioselective synthesis of skeletally diverse tricyclic heterocycles and mechanistic investigations. ACS Catal. 11, 6951–6959 (2021).
Zhang, L.-M. & Wang, S.-Z. Efficient synthesis of cyclopentenones from enynyl acetates via tandem Au(I)-catalyzed 3,3-rearrangement and the nazarov reaction. J. Am. Chem. Soc. 128, 1442–1443 (2006).
Chen, X. et al. Gold(I)-catalyzed tandem cycloisomerization and fluorination of 1,3(4)-enyne esters with NFSI: one-pot assembly of 5-fluoro- cyclopentenones. Adv. Synth. Catal. 360, 3700–3708 (2018).
Zi, W., Wu, H.-M. & Toste, F. D. Gold(I)-catalyzed dearomative rautenstrauch rearrangement: enantioselective access to cyclopenta[b]indoles. J. Am. Chem. Soc. 137, 3225–3228 (2015).
Meng, F.-T. et al. Gold self-relay catalysis for accessing functionalized cyclopentenones bearing an all-carbon quaternary stereocenter. Org. Chem. Front. 9, 140–146 (2022).
Meng, F.-T. et al. Gold self-relay catalysis enabling [3,3]-sigmatropic rearrangement/nazarov cyclization and allylic alkylation cascade for constructing all-carbon quaternary stereocenters. Chin. J. Chem. 40, 687–692 (2022).
Rautenstrauch, V. 2-Cyclopentenones from 1-ethynyl-2-propenyl acetates. J. Org. Chem. 49, 950–952 (1984).
Shi, X.-D., Gorin, D. J. & Toste, F. D. Synthesis of 2-cyclopentenones by gold(I)-catalyzed rautenstrauch rearrangement. J. Am. Chem. Soc. 127, 5802–5803 (2005).
Bürki, C., Whyte, A., Arndt, S., Hashmi, A. S. K. & Lautens, M. Expanding the scope of the gold(I)-catalyzed rautenstrauch rearrangement: protic additives. Org. Lett. 18, 5058–5061 (2016).
Chen, Y.-P., Li, T. & Sieburth, S. M. Pyridone annulation by 4 + 2 coupling of dienolates with nitriles and nitrile equivalents. a solution to the acetonitrile problem. J. Org. Chem. 66, 6826–6828 (2001).
Wu, J.-Q. Experimental and theoretical studies on rhodium-catalyzed coupling of benzamides with 2,2-difluorovinyl tosylate: diverse synthesis of fluorinated heterocycles. J. Am. Chem. Soc. 139, 3537–3545 (2017).
Shi, L., Yu, K. & Wang, B.-Q. Regioselective synthesis of multisubstituted isoquinolones and pyridones via Rh(iii)-catalyzed annulation reactions. Chem. Commun. 51, 17277–17280 (2015).
Frye, S. V. Discovery and clinical development of dutasteride, a potent dual 5a-reductase inhibitor. Curr. Top. Med. Chem. 6, 405–421 (2006).
Logothetis, C. J., Efstathiou, E., Manuguid, F. & Kirkpatrick, P. Abiraterone acetate. Nat. Rev. Drug Discov. 10, 573–574 (2011).
Huigens, R. W. III et al. A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products. Nat. Chem. 5, 195–202 (2013).
Švenda, J. et al. Biology-oriented synthesis of a withanolide-inspired compound collection reveals novel modulators of hedgehog signaling. Angew. Chem. Int. Ed. 54, 5596–5602 (2015).
Charaschanya, M. & Aubé, J. Reagent-controlled regiodivergent ring expansions of steroids. Nat. Commun. 9, 934 (2018).
Torres, M., Gil, S. & Parra, M. New synthetic methods to 2-pyridone rings. Curr. Org. Chem. 9, 1757–1779 (2005).
Chu, D. T. W. Recent progress in novel macrolides, quinolones, and 2-pyridones to overcome bacterial resistance. Med. Res. Rev. 19, 497 (1999).
Lagoja, I. M. Pyrimidine as constituent of natural biologically active compounds. Chem. Biodivers. 2, 1–50 (2005).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Correa, A. et al. Golden carousel in catalysis: the cationic gold/propargylic ester cycle. Angew. Chem. Int. Ed. 47, 718–721 (2008).
Jiang, J.-X. et al. Rationalization of the selectivity between 1,3- and 1,2-migration: a DFT study on gold(i)-catalyzed propargylic ester rearrangement. Org. Biomol. Chem. 14, 3558–3563 (2016).
Bernardo, O., González-Pelayo, S., Fernández, I. & López, L. A. Gold-catalyzed reaction of propargyl esters and alkynylsilanes: synthesis of vinylallene derivatives through a twofold 1,2-rearrangement. Angew. Chem. Int. Ed. 60, 25258–25262 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21971090 and 22271123, Bo Jiang).
Author information
Authors and Affiliations
Contributions
F.T.M. and Y.N.W. contributed equally to this work. F.T.M., X.Y.Q., and J.L. conducted and analyzed the experimental studies. Y.N.W., S.J.L., and Y.L. conducted the DFT computational study. B.J., S.J.T., and Y.L. discussed the reaction mechanism. All authors wrote the manuscript. B.J., W.J.H., and Y.L. conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meng, FT., Wang, YN., Qin, XY. et al. Azoarene activation for Schmidt-type reaction and mechanistic insights. Nat Commun 13, 7393 (2022). https://doi.org/10.1038/s41467-022-35141-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-35141-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.