Abstract
The recent literature on causation has seen the introduction of several distinctions within causation, which are thought to be important for understanding the widespread scientific practice of focusing causal explanations on a subset of the factors that are causally relevant for a phenomenon. Concepts used to draw such distinctions include, among others, stability, specificity, proportionality, or actual-difference making. In this contribution, I propose a new distinction that picks out an explanatorily salient class of causes in biological systems. Some select causes in complex biological systems, I argue, have the property of enabling coherent causal control of these systems. Examples of such control variables include hormones and other signaling molecules, e.g., TOR (target of rapamycin), morphogens or the products of homeotic selector genes in embryonic pattern formation. I propose an analysis of this notion based on concepts borrowed from causal graph theory.
Similar content being viewed by others
1 Introduction
While the traditional literature on causation has focused mainly on the question of how to distinguish causal from non-causal relations, a new debate is currently going on that attempts to draw significant distinctions within causation, using concepts such as stability, specificity or proportionality (Woodward, 2010). Such distinctions are thought to be important in order to make sense of the scientific practice of causal selection. This term designates the process of singling out, from the totality of conditions that jointly cause a phenomenon, such causal variables or levels that have explanatory relevance (e.g., Waters, 2007; Woodward, 2010; Ross, 2018, forthcoming; Baxter, 2019; Lean, 2020; Gebharter & Eronen, 2021; Weber, 2022). The basic premise of this debate is that all causal relations are not alike. Some relations have features which others lack completely or have to a lesser degree and those features make them significant for explanatory or for otherFootnote 1 purposes.
The most widely discussed distinctions in the literature include stability, proportionality, causal specificity (all three discussed in Woodward’s seminal 2010 paper), and actual- versus potential-difference making cause (Waters, 2007). A more recent addition is Ross’s and Woodward’s distinction between irreversible or one-hit and reversible or sustainable causation (Ross & Woodward, 2022). Here is just a rough idea of these notions before going into details:
-
(1)
Stability has to do with the range of conditions under which a causal dependency holds.
-
(2)
Proportionality concerns the choice of causal variables in such a way that they contain all and only relevant information about the effect.
-
(3)
Causal specificity designates whether a causal variable supports fine-grained control or merely acts like a switch.
-
(4)
Actual-difference making causes are causes that vary in an actual population and fully or partially explain some actual variation in this population.
-
(5)
Irreversible versus reversible causation has to do with the question of whether an effect can be undone by reversing the cause variable to its initial state.
While I accept that all these distinctions are relevant to the practice of the biological sciences, I want to introduce here a new feature of some biologically particularly significant causes than cannot be reduced to any of the distinctions (1) – (5), namely the coherence of their effects. The basic idea is that many biological systems contain a specific type of control variable that has a coordinating effect on a large number of downstream variables. Examples of such variables includes hormones and other signaling molecules, morphogens, selector genes, or so-called “master regulators”. I want to show here that these causal factors share a causal property that cannot be captured by any of the existing distinctions and that may not be fully formalizable.
In Section 2, I will first provide an intuitive account of this property, which I call coherent causal control.Footnote 2 In Section 3, I will suggest a definition that uses notions borrowed from causal graph theory. Section 4 will present some further biological examples in order to demonstrate the biological significance of this idea. In Section 5, I show that my notion of coherent causal control cannot be captured in terms of the now standard distinctions within causation that I have just reviewed. Section 6 compares the concept to some cognate notions that have been discussed in recent philosophy of biology as well as in the philosophy of mind, namely the notions of control mechanism, control variable and domain coverage. Finally, in Section 7 I discuss the implications for the whole project of using distinctions within causation as a causal selection criterion.
2 An intuitive account of coherent causal control
The basic phenomenon I am interested in is this: Biologists have discovered molecules that can be manipulated such that they change the values of a large number (i.e., several dozen or more) of downstream variables in such a way that these target variables together produce a coherent response in a biological system. By ‘coherent’ I mean that these downstream variables take on a distribution of values (or time functions) that allow the system in question to perform a specific biological activity at some defined rate, e.g., an increased or decreased regular heart beat as opposed to cardiac arrhythmia or cardiac arrest, or to assume a specific developmental pathway such as eye or limb development as opposed to disorganized growth or developmental arrest. The control variable typically also selects from a range (two or more) of alternative options, e.g., increased, constant or decreased heart rate or alternative pathways such as head versus thorax development. For this to work, the values that the causal descendant variables take in response to the value of the control variable must somehow be tuned to each other such as to perform some biological activity (at some defined rate). It is this kind of tuning that my present analysis tries to capture.
I shall first try to give an intuitive account of the phenomenon I am after by using the example of the hormone insulin. When insulin is released by pancreatic cells, it has numerous physiological effects. For example, it stimulates glucose uptake by various organs, it activates glycogen synthesis in liver and muscle cells (glycogen is a storage form of glucose), it stimulates fatty acid synthesis, it inhibits fatty acid degradation, etc. Some of these effects have the function of removing glucose from the blood stream in order to keep blood sugar concentration within range. More generally, insulin can be viewed as a signal that puts the metabolism into “storage” mode when it is in a state where high-energy compounds are abundantly available (e.g., after a meal). Intuitively, we are inclined to say that the sum total of the effects of insulin “make sense biologically”. It would not make sense if insulin stimulated fatty acid synthesis and degradation at the same time. That would seem incoherent. By contrast, the normal response of various metabolic functions to the insulin signal is coherent in the sense that it allows certain high-level metabolic functions to operate, in particular energy storage and blood glucose regulation. For this to work, the various effects of insulin must be tuned to each other. It is this tuning or the “making biological sense” aspect of the causal effects of certain biological factors that I wish to capture with my notion of coherent causal control.
Other biological examples of the phenomenon I have in mind include (1) hormones in general, (2) signal-transducing molecules like protein kinases and phosphatases and the “second messengers” such as cyclic AMP, inositol phosphate (IP3) or calcium ions that control them, (3) gradient-forming morphogens and the products of homeotic selector genes in embryonic pattern formation, or (4) so-called “master regulators” of growth and metabolism such as TOR (target of rapamycin). This list is far from complete; it is supposed to provide just a few typical examples. From now on, I will refer to such variables as coherent control variables (CCVs). The cases of homeotic selector genes and TOR will be examined in more detail in Sect. 4. In the following Section I will try to go beyond the intuitive understanding presented here and provide a more formal definition.
3 Definition of coherent control variables (CCVs)
I propose a definition of the concept of CCV by using concepts from causal graph theory (Pearl, 2009). This definition comes in two steps. The first defines the concept of coherent control variable (CCV) in terms of causally coherent value distributions of the relevant descendant variables in a causal graph. The notion of coherent distribution is then defined in the second step.
My definition assumes that a biological system S that performs some activities or functions F1 … Fn or undergoes processes P1 … Pm is representableFootnote 3 by a causal graph containing at least one variable X that controls the values of a set D of relevant descendant variables. The members of D are somehow causally relevant to the performance of the functions or processes (such as blood glucose regulation in my insulin example). Now X is a coherent control variable (CCV) iff:
-
(1)
X has a set D of relevant descendant variables in system S such that, under appropriate conditions, an ideal exogeneous intervention on X that were to change its value would change the values of the variables in D from their initial distribution to a coherent distribution.
-
(2)
A coherent distribution of the variables in D is a distribution that would cause system S to perform activities F1 … Fn (at rates f1 … fn) or undergo processes P1 … Pm (at rates p1 … pn)
CCVs clearly require a certain structure of the underlying causal network, with a lot of direct causal descendants. They can be switch-like, which means that they select just between two value assignments, or they can be causally specific, i.e., admitting of more than two values that map bijectively (or nearly so) to a range of different value combinations of the effect variables. But in addition, their values somehow constrain the distribution of values taken by their descendants such that the latter can perform a functionally significant activity (or several such activities) at some specific rate or undergo a certain developmental pathway or several. More precisely, a change in a CCV causes a change in the distribution of values of the downstream variables from some initial state to a coherent state that supports some coordinated biological activity. The initial state may or may not itself be coherent in the sense of (2) – due to previous action of the same CCV or other factors – or it may be a default. Which states of a complex system are selected as “initial” as well as the choice of an end point at which coherence is assessed depends on the investigative context.
It is this coordinating action on the values taken by downstream variables in response to a change in the control variable that characterizes many control variables in biological systems.Footnote 4 Some combinations of values “make biological sense” because they allow some activities or processes that contribute to an organism’s biological functionsFootnote 5 (e.g., blood glucose regulation) to be performed, or to be performed at the appropriate rate. Often, the functionally most appropriate value combinations depend on the organism’s physiological state or developmental stage. Coherent control variables make sure that the right value combinations with respect to biological functioning are selected under the given circumstances.
My use of the term “cause” in the second part of the definition requires some attention. Basically, I mean it in an interventionist sense (Woodward, 2003). However, since interventionist causation can be rather weak, it is necessary that the variables in D have a large effect on the relevant activities or processes, like insulin has a large effect on blood glucose level. To have a large effect doesn’t mean that the effect variable is completely determined or even that some change is made highly probable by the cause; there is always a certain amount of noise in biological systems. It just means that there are possible changes in the cause variable that change the value of the effect variables by a large amount, for instance from 0 to 1 (for a binary variable) or, say, from < 10% to > 90% (for a continuous variable).
In theory, there are different ways in which coherent control could be achieved.Footnote 6 The CCV may (I) act as a trigger that sets the downstream variables to some values but then lets them find a coherent distribution themselves, via downstream interactions such as mutual feedback. Alternatively, (II) the CCV may directly set the downstream variables to a coherent distribution without any coherence-enhancing downstream interactions and keep the values fixed for a certain amount of time. Finally, (III) the CCV may continue to constrain the downstream variables after having set them to some value distribution. This constraining may involve the CCV participating in the downstream interactions, e.g., by responding to feedback.
Types I and III of causal control could work in two different ways: (a) The CCV could either cause value combinations of the downstream variables that are far from a coherent distribution, leaving it entirely to the downstream interactions (which involve the CCV itself in type III but not in Type I) to produce the coherent state. Or (b) the CCV could set the values of the downstream variables such that they are close to a coherent state and then let dynamic interactions fine-tune it. Obviously, “far” and “close” are vague and the difference between Ia and Ib and IIIa and IIIb, respectively, is thus a matter of degree. For this reason, I prefer to group all these kinds of causal control under the new category of coherent causal control, even though in particular Type Ia may look more like self-coordination than control by a single factor.
All of these kinds of control may occur in biological systems. However, dynamical interactions are frequent in biological systems and the CCVs in many cases receive feedback from downstream variables,Footnote 7 therefore I am inclined to think that most cases of coherent causal control are going to lie somewhere in the spectrum between Types IIIa and IIIb. It is beyond the scope of the present paper to systematically review and analyze the different kinds of causal control that occur in biological systems, as the sheer number and complexity of mechanisms that exhibit this kind of phenomenon is staggering. What is more, my analysis is intended to be conceptual and not empirical. My main point is that, irrespectively of the exact mechanisms that bring about the coordination of numerous causal factors in biological systems, there is a significant category of causal control that is characterized by the fact that the variables that are being controlled are somehow coordinated with each other and that some variable takes on a central role in this coordination, no matter by what exact mechanism(s). Furthermore, I contend that this kind of control represents a distinction within causation that is not captured by any of the existing distinctions that have been proposed by philosophers to date.
In all cases, what makes a given causal variable a CCV is the structure of the network with causes that have a lot of relevant downstream effects plus a certain way of constraining the values of the downstream variables in a way that makes biological sense. This effect of coherent control is realized by the specific effects that the CCV has on various other biological factors, for example, by specifically activating or inhibiting a set of enzymes or by binding to different types of receptors.
Before I turn to an examination of some further examples, I will point out what the kind of causal control I have in mind is not.
It could be suggested that what characterizes the control variables in which I am interested here is the high connectivity or their place as hubs in complex networks. Indeed, the structure of networks of protein–protein interactions or of gene co-expression patterns have generated much interest in systems biology (e.g., Bork et al., 2004). It was shown that such networks often have so-called “small world”-properties, which means that they contain nodes with higher connectivity – the hubs – and less connected regions such that many elements of these networks are only connected through the nearest hubs (kind of like your small-town local airport). However, while coherent control variables in my sense may also be network hubs, the property I am after is not merely defined by a high connectivity. This is evident in the fact that connectivity is a structural relation between nodes or variables in a network, while coherent causal control is both a structural property and a relation between the possible values that the variables can take dependent in the value of the cause variable.
In the following section, I would like to apply my analysis to some more candidate examples.
4 Some candidates
I have used the example of insulin as a paradigmatic case of coherent control. In this section, I wish to examine a few additional examples in order to demonstrate the biological significance of this phenomenon.
Morphogens
This term designates a class of substances, most of them proteins, that function in the patterning of embryonic axes during early development. Morphogens form gradients and act in a concentration-dependent manner to determine the subsequent developmental fate of the cells as a function of their position in the embryo (Rogers & Schier, 2011). The processes of embryonic pattern formation in which morphogens function exhibit a remarkable precision and robustness, generating the same pattern irrespectively of the size of the embryo and even when the developmental process is externally disturbed. According to current thinking in developmental biology it is the self-regulating nature of these gradients that arises from complex dynamic interactions between numerous factors that is responsible for the robustness of pattern formation (Weber, 2022). In any case, it is clear that morphogens have an effect on the gene expression patterns of embryonic cells. It is these gene expression patterns that differentiate the cells into different developmental pathways, for example, a pathway towards neural development. In order to do so, the morphogens have to turn some genes on and others off. Obviously, not all gene expression patterns will lead to an orderly development in accordance with position. If we consider the gene expression level of genes as causal variables, only some very specific value combinations of these variables will permit developmental processes to generate the species-typical form of the organism. This is why morphogens must satisfy the requirements that I try to capture here with the notion coherent causal control.
I would like to emphasize that coherent causal control need not be static; morphogens and similar substances exert coherent control in a dynamic fashion (Jaeger et al., 2004) and thus may represent one of the self-coordinating types of coherent causal control, probably Type IIIa according to my little taxonomy from the previous section, as morphogens exhibit dynamic feedback.
Homeotic selector genes
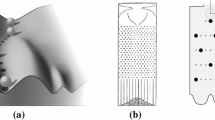
These are genes that play a role in generating repeated structures such as vertebrae, digits or segments with region-specific morphology. In the fruit fly Drosophila, mutations in these genes can cause bizarre modifications of the body plan, some of which have already been discovered as early as 1915 in T.H. Morgan’s famous fly lab (Gehring, 1998). For example, homeotic fly mutants may sport a second pair of wings or legs on their head. Different segments of the insect body express different combinations of such selector genes, which gives each region its unique identity. These combinations are responsive to the concentration of morphogens, and according to current thinking the selector genes form the basis of some sort of a cellular memory retaining a long-lasting record of the positional information initially provided by the morphogens (Alberts et al., 2015, 1164). Like the morphogens, the homeotic selector genes control the expression of gene combinations that will cause cells to follow the specific fate corresponding to its earlier position in a morphogen gradient (or a system of gradients). For example, the homeotic selector gene Abdominal-B was shown to control the activity of several classes of target genes in the fruit fly, in particular genes that encode cell adhesion proteins, cell polarity genes (encoding proteins differentiating the cell membrane into a side facing the surface and one facing the interior of the tissue), and proteins regulating cytoskeleton formation. These genes are expressed specifically in the fly larva’s abdomen, where they form, among other structures, the larva’s respiratory organ known as the “spiracle” (see Fig. 1).
Regulatory targets of the homeotic selector gene Abdominal-B. Image reproduced with permission from (Lohmann, 2006)
In addition to the homeotic selector genes, there are also region-specific selector genes such as eyeless (homologous to Pax6 in mammals) that will activate precisely those genes needed to make an eye (Halder et al., 1995). In all these examples, the coherent control property makes sure that the gene combinations (and also the timing of gene expression) are matched to each other such that a particular developmental pathway can go forward. Homeotic selector genes have long been known to autoregulate by dynamic feedback, which may explain the stability of the cell differentiation states that they determine (Kuziora & McGinnis, 1988). Thus, they also seem to represent type III causal control.
Master regulators
Another example is a very loose class of biomolecules that are known as “master regulators”.Footnote 8 Typically proteins, such regulators control the rate of various biological processes via numerous downstream effects. To give an example, the proteins TOR and mTOR that were initially discovered as the targets of the immune suppressant drug rapamycin regulate and integrate numerous metabolic processes, protein synthesis and cell growth. The TOR and mTOR proteins (“m” designates the mammalian version) are protein kinases, which means that they attach phosphate groups to other proteins at specific sites and thus activate or deactivate them. When we look at the enormously complex interaction network of mTOR (see Fig. 2), the question arises why the TORs are drawn in a central position and thus picked out as “master regulators”. I suggest that it is a combination of their high connectivity within the network and the fact that they are CCVs. For there are molecules in this network that are as highly or even more connected, e.g., the tuberous sclerosis proteins TSC1 and TSC2 (see Fig. 2) as well as proteins that are also CCVs (e.g., insulin or the signal transducing protein Wnt, see Fig. 2), but only mTOR combines the causal role of a CCV with a highly connected, hub-like position in cell metabolism and growth control.
Interaction network of mTOR, by Charles Betz, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=20984537. mTOR appears twice in this network because it forms a complex with two different binding partners (raptor and rictor), which play distinct regulatory roles
The mTOR network, too, exhibits complex dynamics due to the occurrence of feedback, including feedback on various forms of mTOR (Varusai & Nguyen, 2018) and thus probably also represents one of the types of causal control with dynamic feedback.
In Fig. 2, we also see that especially the mTOR-raptor complex is highly connected to numerous other proteins (including hormones, other enzymes and transcription factors) both as a recipient and a source of regulatory influence. The immediate function of the mTOR/raptor complex is the phosphorylation (attachment of a phosphate) group to specific sites at other proteins and thereby to activate or inhibit the activity of these other proteins. Surely, the various regulatory effects of the mTOR-raptor kinase on other proteins must be fitted with each other such as to coordinate cell metabolism in a growth-dependent way. This means that only some activity patterns of mTOR/raptor downstream regulatory targets will be permitted. For instance, ribosome synthesis activity must be appropriate for the cell’s protein synthesis rate; it wouldn’t make sense to stop ribosome synthesis while the cell needs a lot of proteins because it’s growing. The causal property of coherent control makes sure that only those combinations of activity values that allow metabolism and cell growth to be coordinated are selected by the different values that mTOR/raptor protein kinase activity can take. This is why mTOR is also a CCV in the sense I am trying to work out here.
In all these cases, I suggest, there is more than a particular structural relation such as a central place or hub position in a network. It is true that hormones, morphogens, the protein products of homeotic selector genes and so-called “master regulators” are characterized by a high connectivity. However, many biological entities are somehow causally connected to a lot of other entities. My proposal is that some biological entities are also characterized by playing a specific causal role, which I have characterized as coherent causal control. I will show now that this type of causation cannot be captured in terms of the standard distinctions within causation.
5 Causally coherent control and the standard distinctions within causation
I will show here that coherent causal control is orthogonal to the classic distinctions within causation such as stability, proportionality and causal specificity and their likes.
Stability
Some causal relations are more stable than others, which means that they hold across a broader range of background conditions (Woodward, 2010). The paradigm of highly stable causal relations are physical laws. At the other end of the stability spectrum, we find relations such as the one between certain human genetic loci and dyslexia. Too unstable to constitute “genes for reading”, these causal links only manifest themselves in a narrow range of genetic and environmental backgrounds. What about CCVs? For starters, it should be noted that my definition given in Sect. 3 contains the dummy proviso “under appropriate conditions”. A proviso is necessary because it should be clear that the response elicited by a change in the control variable will depend on numerous conditions, for example, the availability of metabolic energy, temperature, water content, ionic strength, etc. The coherent response may only be realized in an organism that is alive and healthy. Thus, the causal link between a CCV and its coherent responses in the descendant variables will normally exhibit the typical fragility of causal generalizations in biology. However, fragility alone is hardly sufficient for CCVs. There are numerous fragile causal relations that do not involve CCVs known in biology, e.g., the effect of CO2 concentration on plant growth.
Proportionality
Proportional causal attributions are pitched at the maximally informative level of generality. To use an example due to Stephen Yablo, if trained pigeons peck at all red blotches, to assert that they peck at scarlet blotches is true, but as a causal generalization it is not maximally informative. This concept of proportionality has been used in attempts to account for the indispensability of higher-level causes vis-à-vis their lower-level realizers or more generally as a tool for choosing the right level of explanation (Woodward, 2010). If this works, then many biological causes should have this feature, including the CCVs. However, nothing in the concept of proportionality entails that the effects of a proportional variable are coherent in my sense. Hence, the coherence requirement also goes beyond proportionality.
Causal specificity
Now for the feature that has been discussed the most in the philosophy of biology. While biologists mean by “specificity” usually the feature that many biological interactions like those between enzymes and their substrates or between receptors and their ligands are limited to one or just a few partners, philosophers of causation have come to use the term in several different ways. The biologists’ kind of specificity is also among them, and some refer to it as “one–one specificity” (Lean, 2020; Woodward, 2010). This kind of specificity characterizes a relation between causal variables. Another kind of variable specificity is effect specificity, which is present when a cause has only one effect and absent when a cause has many effects (Ross, 2021). But the term is also used to characterize a relation between the values of causal variables (Bourrat, 2019; Ross, 2021). One such notion is value-specificity of a cause for its effect, which is present in a causal relation, according to Gebharter and Eronen (2021), when each value of the effect is caused by a different value of the cause, thus enabling fine-grained control of the effect by the cause variable.
Another kind of value-specificity that has particularly interested philosophers of biology is known simply as “causal specificity” in the philosophical literature. This term designates causal relations in which the causally linked variables admit more than two values (unlike on–off switches) and the possible values of the cause and effect variables map onto each other bijectively, or nearly so. This kind of relation is also distinguished by enabling fine-grained control of an effect variable by a cause variable, like in a light dimmer. While Woodward (2010) treats this feature as one that causal relations do or do not have, there are also quantitative versions of causal specificity (Griffiths et al., 2015). An example of a highly specific causal relation is the one between DNA sequences and the RNA and protein sequences that they encode, but there are more.
Many but not all CCVs are causally specific in this sense. Even though the values that the control variable can take will often be continuous, there is sometimes a range of values that will elicit the same response. If we take the example of the morphogens, their concentration forms a continuous gradient, however, they normally just control the choice between a single-digit number of distinct developmental fates. In the case of hormones, there are typically also threshold values and thus a limited number of response states. Thus, the link between coherent control variables and the different states they control will be somewhat causally specific, but not highly so. There are even CCVs that are simple switches, for example, certain inducing signals in vertebrate development (Weber, 2022, Chapter 4). In any case, causal specificity sensu Woodward is not a distinguishing feature of CCVs. It should also be noted that what Ross (2021) calls variable non-specificity of the effects is necessary but insufficient for being a CCV.
Because causal specificity has been much discussed as a feature that potentially distinguishes DNA and genes from other factors involved in development, I wish to flag here that I do not consider whole genes or whole genomic DNAs to be CCVs. Perhaps there are genomic sequences that have that role, but I suspect that most CCVs are gene products, i.e., RNAs or proteins.
Actual- versus potential-difference making cause
Another idea is to distinguish between actual- versus potential-difference making causes (Waters, 2007). The former designates a cause that actually varies, for example, between the individuals in a population and that fully or partially accounts for variation in the effect variable. Some of the examples discussed here may satisfy these requirements, in particular the morphogens if their action is construed in accordance with idealized models (Weber, 2022). However, some standard examples of actual-difference making causes are not CCVs. A case in point is the Drosophila white gene, which is the actual-difference maker for red vs. white eyes in some populations of flies, but neither the gene itself nor its product (an enzyme needed to transport precursors for the eye pigment) have the CCV property.
Irreversible (one-hit) versus reversible (sustainable) causes
A more recent distinction is the one between reversible and irreversible causes. A rock that shatters a bottle or the injection of a lethal dose of a substance into a living organism are irreversible causes, i.e., returning the cause variable to its initial state will not case the effect variable to assume its initial state. By contrast, turning off a light switch and turning it back on again will return the lights to their initial state (Ross & Woodward, 2022). Typical CCVs belong to the broader class of reversible causes, however, it cannot be excluded that there are also irreversible cases, e.g., in the realm of epigenetics but also in developmental biology. In any case, the distinction is orthogonal to what I am trying to capture with the idea of causal coherence.
Thus, the causal role of the examples of control variables considered here cannot be captured in terms of the standard distinctions within causation. This should also be evident in the fact that coherent causal control refers to both the structure of the causal graph and to the relationship between the values that the variables can take. The existing distinctions refer to either the structure of the causal graph (one–one specificity, variable specificity of effects), or the modal strength of the regularity (stability), or the choice of causal variables (proportionality), or the exact mapping of the values sets (causal specificity), or to the population level (actual-difference making).
I suggest therefore the contrast between control variables with coherent effect and causal variables lacking this property as a new and independent distinction within causation.
6 Comparison to some cognate notions
In this section I would like to compare my idea of coherent causal control to some other causal notions that have been discussed in the context of biology.Footnote 9
Control mechanisms and biological regulation
The notion of control mechanism is widespread in biology, but the question what distinguishes them from ordinary mechanisms has not been discussed much. A notable exception is Bich and Bechtel (2022). They suggest that a distinguishing feature of such mechanisms is that they measure one or several variables and adjust the activity of other mechanisms according to value of the measured variable(s). While the notion of “measurement” might need some unpacking, I think it is clear that this kind of control mechanism is distinguished by its regulatory function.Footnote 10 Now, while some CCVs also have a regulatory function of the type discussed by Bich and Bechtel, e.g., the case of insulin, the CCV architecture is not necessary to perform such a function. Thus, the concept of CCV is not identical to the concept of control mechanism.
Campbell’s notion of control variable
Campbell (2010) has sketched a concept of control variable in an attempt to specify what it means to describe the causal functioning of a complex system at the right level. On his account, control variables are such causal variables interventions on which are systematically correlated with outcomes that are of interest to us. His main example are psychological variables, thus vindicating the mental level – as opposed to the physical level – as the right one for explaining human behavior.
The goals of Campbell’s account are different from mine, as I am not trying to solve a levels problem.Footnote 11 Nonetheless, some of my examples probably qualify as control variables in Campbell’s sense, in particular the case of hormones. But Campbell’s conception is broader and will pick out things that are not characterized by the coherence of their effect but anything that can be intervened upon in such a way as to generate interesting correlations to the variable that is being manipulated, for example, ionizing radiation or heavy metal poisoning (anything that satisfies a dose–response relationship, as Campbell notes).
Stegmann’s notion of domain coverage
Stegmann (unpublished) has proposed an analysis for the notion of master regulator. An example he discusses is the mechanism that regulates the biosynthetic pathway for the amino acid arginine in bacteria. The expression of the eight enzymes needed by the bacteria to make arginine is controlled by the arginine repressor (ArgR), a transcription factor that binds to the operator region of the arginine operon (a string of genes controlled by a common regulatory region, like the famous lac operon) when arginine is present. In Stegmann’s account, the regulatory targets of ArgR are assigned to a domain in virtue of being part of the same process, namely arginine biosynthesis. Then, what characterizes the causality of the master regulator ArgR is its high domain coverage. This means that the same causal variable regulates all the effects in its domain. Stegmann notices that this property is distinct from specificity; a master regulator in this sense could be highly specific in the sense of not regulating any other processes, or it could be entirely non-specific, i.e., have a large number of other effects as well.
Stegmann suggests that high domain coverage is necessary (but not sufficient) for what biologists call a “master regulator”, a claim that I do not wish to evaluate here. What matters here is that the notion of coherent causal control is distinct. One difference is that high domain coverage is a purely causal feature without any functional connotations. Furthermore, high domain coverage is not necessary for what I call coherent causal control. I am after causes that are distinguished by playing some coordinating role in the organism, a role that ensures that various causal factors assume values that allow some biological function or process to succeed. For this, it is not necessary that they control all or most of the relevant effects in a domain. It is enough if they control the variables that have a particularly strong impact, for example, because they affect a rate-limiting step.
Thus, the notion of coherent causal control is distinct from any other causal concept that has been considered in the recent literature on causation and explanation in the biological sciences.
7 Conclusions with respect to the general project of drawing distinctions within causation
It is my contention that in the contemporary biological sciences CCVs take center stage in the quest for understanding complex systems, without necessarily having any of the other properties that are thought to distinguish causal relations from others (stability, proportionality, specificity, actual-difference making). In this section, I would like to discuss some implications for the whole project of drawing distinctions within causation.
The known distinctions between causation target either properties having to do with the scope of causal regularities (in the case of stability), with the choice of causal variables (in the case of proportionality), with population-level properties (actual-difference making), with the structure of the causal graph (one–one specificity) or with the mapping between the sets of values that the cause and effect variables can take (causal specificity sensu Woodward). Causally coherent control is different from all the previous distinctions within causation in that it is defined both on terms of causal graph structure and a relation between the sets of values. By contrast to causal specificity, the mapping of the value set is not just a dyadic, bijective relation between two sets. Instead, it is an n-adic (n > 2) relation between the value set of control variable and the respective value sets of the downstream target variables. The coherence of this set is grounded not in some mathematical property but in the mutual fitting together of the values with respect to the processes in which they are involved. This coherence is not something that could be defined in abstract causal terms; in the definition that I have given, a reference to biological functioning, processes or pathways seems ineliminable.Footnote 12
In a way, thus, my notion of causally coherent control exposes the limit of the project of drawing distinctions within causation that refer only to abstract properties such as the mathematical form of the value maps. It should be noted that my concern here is not the extent in which biological causes can or cannot be represented by a formal causal framework. A framework such as Pearl’s (2009) may provide a good way of distinguishing between causal and non-causal relations in most areas of biology. But my issue here is not how to distinguish between causal and non-causal but how to draw sensible distinctions within causation. Previous attempts to do so rely mainly on formal properties such as causal specificity in its different senses. My main point here is that there exists an important class of biological causes that – even though they are representable as causes in a formal framework – is not distinguished from other causes just by having many causal descendants nor by the form of the mapping from cause to effect values but by having a coordinating effect on the downstream variables. What it means to have a coordinating effect can only be understood by reference to biological functions and processes.Footnote 13
While there are cases in which the standard distinctions within causation guide causal selection practices, there appears to be an important class of biological causes that is not distinguished by any of the properties that underly these distinctions. Many of these causes are given the honorific title of “signal”, others are referred to – even more gloriously – as “master regulators”, “instructive causes” or “selectors”. I have argued here that what distinguishes these causes is that they exert a kind of control over many causal descendants that is characterized by the coherence of their response. Coherence means that the values of the downstream variables take on combinations of values that enable certain functions or activities to be performed or developmental or other pathways to proceed in an orderly fashion. Of course, it is a truism that some biological causes have specific functional roles, but it seems that the project of drawing distinctions within causation has harbored the hope that all causal relations that have explanatory relevance in biology could be singled out irrespectively of functional considerations, by attending only to abstract properties of the causal relation itself. If the notion of coherent causal control does indeed pick out a relevant class of causal factors in biological systems, as I have argued here, this assumption falters.
Notes
Other purposes may include practical ones such as medical intervention (Ross forthcoming) or heuristic utility for further research (Weber, 2022).
I have published a version of this intuitive account elsewhere (Weber, 2022). The purpose of the present paper is to provide a more formal definition and to examine the relation to other distinctions within causation and to some cognate notions that have been proposed. Furthermore, I present an additional example here.
Such a representation may be idealized in various ways. As an anonymous reviewer points out, representing biological phenomena in terms of variables that take on defined values is a considerable simplification.
Of course, technological artifacts often have similar control variables. For example, luxury cars (so I hear) have different drive modes adjusting various parameters related to how gently (or not) the user wants to drive.
I don’t want to commit to a specific account of biological function, but perhaps a goal-contribution account (Weber, 2017) would work best here.
I am indebted to an anonymous reviewer for suggesting the threefold distinction.
This notion should not be confused with that of master volume controls in electronics. Sound engineers, to my knowledge, mean by this term a control that allows them to regulate the gain or volume of all available tracks or channels at once and by the same amount. I am not sure there is a good biological analogue for master controls in this sense; maybe regulatory mechanisms for whole metabolic pathways (such as Stegmann’s example of the arginine biosynthesis pathway to be discussed in Section 5).
The notion of coherence is also used in a recent account of mechanistic explanation due to Colombo et al. (2015). However, their goals as well as the concept of coherence used are quite different. They work with a probabilistic conception of epistemic coherence, while mine is an ontological notion. Their objective is to offer a general account of mechanistic explanation while my account targets a very specific kind of causal role (which may be part of one or of several mechanism.
The biochemist Hans Krebs has aptly defined regulation as “the adjustment of activities with reference to a purpose” (Krebs, 1959). For “purpose”, we can simply substitute “function”.
I owe this insight to Ulrich Stegmann.
I am indebted to an anonymous reviewer for nudging me to be clear about this point.
References
Alberts, B., Johnson, A., Lewis, J., Morgan, D., Raff, M., Roberts, K., & Walter, P. (2015). Molecular biology of the cell. 6th Revised edition. Revised. Norton & Company.
Anderson, W. (2020). The compatibility of differential equations and causal models reconsidered. Erkenntnis, 85(2), 317–332. https://doi.org/10.1007/s10670-018-0029-1
Baxter, J. (2019). How biological technology should inform the causal selection debate. Philosophy, Theory, and Practice in Biology, 11.https://doi.org/10.3998/ptpbio.16039257.0011.002
Bich, L., & Bechtel, W. (2022). Control mechanisms: Explaining the integration and versatility of biological organisms. Adaptive Behavior, 30(5), 389–407. https://doi.org/10.1177/10597123221074429
Bork, P., Jensen, L. J., von Mering, C., Ramani, A. K., Lee, I., & Marcotte, E. M. (2004). Protein interaction networks from yeast to human. Current Opinion in Structural Biology, 14(3), 292–299. https://doi.org/10.1016/j.sbi.2004.05.003
Bourrat, P. (2019). Variation of information as a measure of one-to-one causal specificity. European Journal for Philosophy of Science, 9(1), 11. https://doi.org/10.1007/s13194-018-0224-6
Campbell, J. (2010). Control variables and mental causation. Proceedings of the Aristotelian Society, 110, 15–30. https://www.jstor.org/stable/41061508. Accessed 14 June 2022.
Colombo, M., Hartmann, S., & van Iersel, R. (2015). Models, mechanisms, and coherence. The British Journal for the Philosophy of Science, 66(1), 181–212. https://doi.org/10.1093/bjps/axt043
Craver, C. (2007). Explaining the brain: Mechanisms and the mosaic unity of neuroscience. Oxford University Press.
Friend, T. (2021). Intervening on time derivatives. Studies in History and Philosophy of Science, 89(October), 74–83. https://doi.org/10.1016/j.shpsa.2021.07.005
Gebharter, A., & Eronen, M. I. (2021). Quantifying proportionality and the limits of higher-level causation and explanation. The British Journal for the Philosophy of Science. https://doi.org/10.1086/714818
Gehring, W. J. (1998). Master control genes in development and evolution: The homeobox story. Yale University Press.
Griffiths, P. E., Pocheville, A., Calcott, B., Stotz, K., Kim, H., & Knight, R. (2015). Measuring causal specificity. Philosophy of Science, 82, 529–555.
Halder, G., Callaerts, P., & Gehring, W. J. (1995). Induction of ectopic eyes by targeted expression of the eyeless gene in drosophila. Science, 267(5205), 1788–1792.
Jaeger, J., Surkova, S., Blagov, M., Janssens, H., Kosman, D., Kozlov, K. N., et al. (2004). Dynamic control of positional information in the early Drosophila embryo. Nature, 430(6997), 368–371. https://doi.org/10.1038/nature02678
Krebs, H. (1959). Chairman’s introductory address: Rate-limiting factors in cell respiration. In Ciba foundation symposium — regulation of cell metabolism (pp. 1–16). John Wiley & Sons, Ltd. https://doi.org/10.1002/9780470719145.ch1
Kuziora, M. A., & McGinnis, W. (1988). Autoregulation of a Drosophila homeotic selector gene. Cell, 55(3), 477–485. https://doi.org/10.1016/0092-8674(88)90034-7
Lean, O. (2020). Binding specificity and causal selection in drug design. Philosophy of Science, 87(1), 70–90. https://doi.org/10.1086/706093
Lohmann, I. (2006). Hox genes: Realising the importance of realisators. Current Biology: CB, 16(23), R988-989. https://doi.org/10.1016/j.cub.2006.10.044
Pearl, J. (2009). Causality. Cambridge University Press. https://doi.org/10.1017/CBO9780511803161
Rogers, K. W., & Schier, A. F. (2011). Morphogen gradients: From generation to interpretation. Annual Review of Cell and Developmental Biology, 27(1), 377–407. https://doi.org/10.1146/annurev-cellbio-092910-154148
Ross, L. N. (2018). Causal selection and the pathway concept. Philosophy of Science, 85(4), 551–572. https://doi.org/10.1086/699022
Ross, L. N. (2021). Causes with material continuity. Biology & Philosophy, 36(6), 52. https://doi.org/10.1007/s10539-021-09826-x
Ross, L. N. (forthcoming). Causal control: A rationale for causal selection. In B. Hanley and C. K. Waters (Eds.), Philosophical perspectives on causal reasoning in biology (Vol. XIX). Minnesota Studies in Philosophy of Science. University of Minnesota Press.
Ross, L. N., & Woodward, J. F. (2022). Irreversible (one-hit) and reversible (sustaining) causation. Philosophy of Science, 1–10.https://doi.org/10.1017/psa.2022.70
Stegmann, U. (Unpublished). Nonspecific causes and master regulators. Manuscript received on 18 January 2022.
Varusai, T. M., & Nguyen, L. K. (2018). Dynamic modelling of the mTOR signalling network reveals complex emergent behaviours conferred by DEPTOR. Scientific Reports, 8(1), 643. https://doi.org/10.1038/s41598-017-18400-z
Waters, C. K. (2007). Causes that make a difference. The Journal of Philosophy, 104(11), 551–579.
Weber, M. (2016). On the incompatibility of dynamical biological mechanisms and causal graphs. Philosophy of Science, 83(5), 959–971. https://doi.org/10.1086/687878
Weber, M. (2017). How objective are biological functions? Synthese, 194(12), 4741–4755. https://doi.org/10.1007/s11229-017-1483-z
Weber, M. (2022). Philosophy of developmental biology. Cambridge University Press.
Wimsatt, W. C. (2007). Re-engineering Philosophy for Limited Beings: Piecewise Approximations to Reality. Harvard University Press.
Woodward, J. (2003). Making things happen: A theory of causal explanation. Oxford University Press.
Woodward, J. (2010). Causation in biology: Stability, specificity, and the choice of levels of explanation. Biology and Philosophy, 25, 287–318.
Acknowledgements
I wish to thank Lauren Ross for organizing the PSA 2020/21 symposium on distinctions within causation and for helpful comments. The paper also benefited from valuable suggestions from the audience at EPSA 2021, the Lake Geneva Biological Interest Group and from Lorenzo Casini, Ulrich Stegmann and two anonymous reviewers.
Funding
Open access funding provided by University of Geneva
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
N/A.
Informed consent
N/A
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: EPSA21: Selected papers from the biennial conference in Turin
Guest Editors: A.C. Love, C. Marchionni, M. Redei, J. Williamson
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weber, M. Coherent causal control: a new distinction within causation. Euro Jnl Phil Sci 12, 69 (2022). https://doi.org/10.1007/s13194-022-00499-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13194-022-00499-1