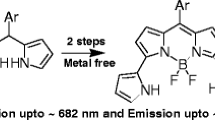
Abstract
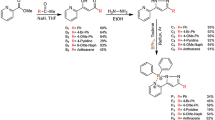
Selenium containing tetraphenyl substituted BODIPY probe was successfully synthesized from respective selenium aldehyde and tetraphenyl pyrrole using Knoevenagel-type condensation. The product was characterized using various spectroscopic techniques (1 H, 13 C, 77Se, 11B, and 19 F) and mass spectrometry. The probe was found to be selective and sensitive towards detection of superoxide over other ROS with a “turn-off” (quenched) fluorescence response. The detection limit of the probe was found to be 4.87 µM. The probe reacted with superoxide in less than a sec with a stoke shift of 35 nm.







Similar content being viewed by others
Availability of data and material
Authors are declare that the data supporting the findings of this research are available within the article.
Code Availability
Not applicable.
References
de Silva AP, Gunaratne HQN, Gunnlaugsson T et al (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566. https://doi.org/10.1021/cr960386p
Gunnlaugsson T, Glynn M, Tocci (née Hussey) GM, et al (2006) Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord Chem Rev 250:3094–3117. https://doi.org/10.1016/j.ccr.2006.08.017
Que EL, Domaille DW, Chang CJ (2008) Metals in Neurobiology: probing their Chemistry and Biology with Molecular Imaging. Chem Rev 108:1517–1549. https://doi.org/10.1021/cr800447y
Wang N, Wang H, Zhang J et al (2022) Endogenous peroxynitrite activated fluorescent probe for revealing anti-tuberculosis drug induced hepatotoxicity. Chin Chem Lett 33:1584–1588. https://doi.org/10.1016/j.cclet.2021.09.046
Yang X, Lu X, Wang J et al (2022) Near-Infrared fluorescent probe with a large Stokes Shift for detection of Hydrogen Sulfide in Food Spoilage, living cells, and zebrafish. J Agric Food Chem 70:3047–3055. https://doi.org/10.1021/acs.jafc.2c00087
Fan G, Wang N, Zhang J et al (2022) BODIPY-based near-infrared fluorescent probe for diagnosis drug-induced liver injury via imaging of HClO in cells and in vivo. Dyes Pigm 199:110073. https://doi.org/10.1016/j.dyepig.2021.110073
Chaudhury S, Sarkar PK (1983) Stimulation of tubulin synthesis by thyroid hormone in the developing rat brain. Biochim Biophys Acta - Mol Cell Res 763:93–98. https://doi.org/10.1016/0167-4889(83)90030-7
Kozumbo WJ, Trush MA, Kensler T (1985) Are free radicals involved in tumor promotion? Chem Biol Interact 54:199–207. https://doi.org/10.1016/S0009-2797(85)80163-0
Bulic B, Pickhardt M, Khlistunova I et al (2007) Rhodanine-based tau aggregation inhibitors in cell models of Tauopathy. Angew Chemie Int Ed 46:9215–9219. https://doi.org/10.1002/anie.200704051
Kepp KP (2012) Bioinorganic Chemistry of Alzheimer’s Disease. Chem Rev 112:5193–5239. https://doi.org/10.1021/cr300009x
Gorman A, Killoran J, O’Shea C et al (2004) In Vitro demonstration of the heavy-atom effect for photodynamic therapy. J Am Chem Soc 126:10619–10631. https://doi.org/10.1021/ja047649e
Hattori S, Ohkubo K, Urano Y et al (2005) Charge separation in a nonfluorescent donor – acceptor Dyad Derived from Boron Dipyrromethene Dye, leading to Photocurrent Generation. J Phys Chem B 109:15368–15375. https://doi.org/10.1021/jp050952x
Qin W, Baruah M, Van Der Auweraer M et al (2005) Photophysical properties of borondipyrromethene analogues in solution. J Phys Chem A 109:7371–7384. https://doi.org/10.1021/jp052626n
Depauw A, Kumar N, Ha-Thi M-H, Leray I (2015) Calixarene-based fluorescent sensors for Cesium cations containing BODIPY fluorophore. J Phys Chem A 119:6065–6073. https://doi.org/10.1021/jp5120288
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41:1130–1172. https://doi.org/10.1039/C1CS15132K
Shahrokhian S (2001) Lead phthalocyanine as a selective carrier for Preparation of a cysteine-selective electrode. Anal Chem 73:5972–5978. https://doi.org/10.1021/ac010541m
Vu TT, Badré S, Dumas-Verdes C et al (2009) New Hindered BODIPY derivatives: solution and amorphous state fluorescence Properties. J Phys Chem C 113:11844–11855. https://doi.org/10.1021/jp9019602
Manjare ST, Kim Y, Churchill DG (2014) Selenium- and Tellurium-Containing fluorescent Molecular Probes for the detection of biologically important Analytes. Acc Chem Res 47:2985–2998. https://doi.org/10.1021/ar500187v
Lou Z, Li P, Han K (2015) Redox-responsive fluorescent probes with different design strategies. Acc Chem Res 48:1358–1368. https://doi.org/10.1021/acs.accounts.5b00009
Nogueira CW, Zeni G, Rocha JBT (2004) Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem Rev 104:6255–6285. https://doi.org/10.1021/cr0406559
Mukherjee AJ, Zade SS, Singh HB, Sunoj RB (2010) Organoselenium Chemistry: role of intramolecular interactions. Chem Rev 110:4357–4416. https://doi.org/10.1021/cr900352j
Mugesh G, du Mont W-W, Sies H (2001) Chemistry of biologically important synthetic organoselenium compounds. Chem Rev 101:2125–2179. https://doi.org/10.1021/cr000426w
Shelar DS, Dhavan PP, Singh PR et al (2021) Synthesis and study of organoselenium compound: DNA/Protein interactions, in vitro antibacterial, antioxidant, anti-inflammatory activities and anticancer activity against carcinoma cells. J Mol Struct 1244:130914. https://doi.org/10.1016/j.molstruc.2021.130914
Poirel A, De Nicola A, Ziessel R (2012) Oligothienyl-BODIPYs: Red and Near-Infrared Emitters. Org Lett 14:5696–5699. https://doi.org/10.1021/ol302710z
Sedgwick AC, Wu L, Han H-H et al (2018) Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem Soc Rev 47:8842–8880. https://doi.org/10.1039/C8CS00185E
Wu D, Chen L, Kwon N, Yoon J (2016) Fluorescent probes containing selenium as a guest or host. Chem 1:674–698. https://doi.org/10.1016/j.chempr.2016.10.005
Balkrishna SJ, Hodage AS, Kumar S et al (2014) Sensitive and regenerable organochalcogen probes for the colorimetric detection of thiols. RSC Adv 4:11535–11538. https://doi.org/10.1039/C4RA00381K
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: Syntheses and Spectroscopic Properties. Chem Rev 107:4891–4932. https://doi.org/10.1021/cr078381n
Yang J, Liu X, Wang H et al (2018) A turn-on near-infrared fluorescence probe with aggregation-induced emission based on dibenzo[ a, c ]phenazine for detection of superoxide anions and its application in cell imaging. Analyst 143:1242–1249. https://doi.org/10.1039/C7AN01860F
Venkatesan P, Wu S-P (2015) A turn-on fluorescent probe for hypochlorous acid based on the oxidation of diphenyl telluride. Analyst 140:1349–1355. https://doi.org/10.1039/C4AN02116A
Zhang W, Liu W, Li P et al (2015) Reversible two-photon fluorescent probe for imaging of hypochlorous acid in live cells and in vivo. Chem Commun 51:10150–10153. https://doi.org/10.1039/C5CC02537K
Mulay SV, Choi M, Jang YJ et al (2016) Enhanced fluorescence turn-on imaging of Hypochlorous Acid in living Immune and Cancer cells. Chem - A Eur J 22:9642–9648. https://doi.org/10.1002/chem.201601270
Panda S, Panda A, Zade SS (2015) Organoselenium compounds as fluorescent probes. Coord Chem Rev 300:86–100. https://doi.org/10.1016/j.ccr.2015.04.006
Mulay SV, Yudhistira T, Choi M et al (2016) Substituent Effects in BODIPY in Live Cell Imaging. Chem - An Asian J 11:3598–3605. https://doi.org/10.1002/asia.201601400
Deshmukh PP, Navalkar A, Maji SK, Manjare ST (2019) Phenylselenyl containing turn-on dibodipy probe for selective detection of superoxide in mammalian breast cancer cell line. Sens Actuators B Chem 281:8–13. https://doi.org/10.1016/j.snb.2018.10.072
Madibone KS, Deshmukh PP, Navalkar A et al (2020) Cyclic Organoselenide BODIPY-Based probe: Targeting Superoxide in MCF – 7 Cancer cells. ACS Omega 5:14186–14193. https://doi.org/10.1021/acsomega.0c02074
Malankar GS, Sakunthala A, Navalkar A et al (2021) Organoselenium-based BOPHY as a sensor for detection of hypochlorous acid in mammalian cells. Anal Chim Acta 1150:338205. https://doi.org/10.1016/j.aca.2021.338205
Shelar DS, Malankar GS, Manikandan M et al (2022) Selective detection of hypochlorous acid in living cervical cancer cells with an organoselenium-based BOPPY probe. New J Chem 46:17610–17618. https://doi.org/10.1039/D2NJ02956A
Dubey R, Lee H, Nam D-H, Lim D (2011) Mild generation of selenolate nucleophiles by thiol reduction of diselenides: convenient syntheses of selenyl-substituted aryl aldehydes. Tetrahedron Lett 52:6839–6842. https://doi.org/10.1016/j.tetlet.2011.10.075
Adib M, Ayashi N, Heidari F, Mirzaei P (2016) Reaction between 4-Nitro-1,3-diarylbutan-1-ones and ammonium acetate in the Presence of Morpholine and Sulfur: an efficient synthesis of 2,4-Diarylpyrroles. Synlett 27:1738–1742. https://doi.org/10.1055/s-0035-1561852
Manjare ST, Kim S, Heo W, Do, Churchill DG (2014) Selective and sensitive superoxide detection with a New Diselenide -Based molecular probe in living breast Cancer cells. Org Lett 16:410–412. https://doi.org/10.1021/ol4033013
Ahmed MG, Romman U, Ahmed SM et al (2007) Synthesis and correlation of Spectral Properties of some substituted 1,3-Diphenyl-2-Propen-1-Ones. Bangladesh J Sci Ind Res 42:45–52. https://doi.org/10.3329/bjsir.v42i1.354
Zhang G, Zhu C, Liu D et al (2017) Solvent-free enantioselective conjugate addition and bioactivities of nitromethane to Chalcone containing pyridine. Tetrahedron 73:129–136. https://doi.org/10.1016/j.tet.2016.11.063
Liu S-R, Wu S-P (2013) Hypochlorous Acid turn-on fluorescent probe based on oxidation of Diphenyl Selenide. Org Lett 15:878–881. https://doi.org/10.1021/ol400011u
Lou Z, Li P, Pan Q, Han K (2013) A reversible fluorescent probe for detecting hypochloric acid in living cells and animals: utilizing a novel strategy for effectively modulating the fluorescence of selenide and selenoxide. Chem Commun 49:2445–2447. https://doi.org/10.1039/c3cc39269d
Acknowledgements
S. T. M. acknowledges the Science and Engineering Research Board (SERB), Govt. of India, New Delhi, for financial support (YSS/2014/000726). We acknowledge SAIF, IIT Bombay for core instrumentation facility. S. T. S acknowledges principal (Dr.) C. V. Murumkar, T. C. College Baramati, Maharashtra for their constant help and support.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Shrikrishna T. Salunke: Designed, Methodology, Investigation, and characterization of compounds, spectrophotometric investigations, and preparation of manuscript. Divyesh S. Shelar: Methodology, Investigation, characterization of compounds, photophysical study, preparation of manuscript and formal analysis. Sudesh T. Manjare: Designed, characterization of compounds, supervision, writing, reviewing and editing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salunke, S.T., Shelar, D.S. & Manjare, S.T. Synthesis and Photophysical Study of Tetraphenyl Substituted BODIPY Based Phenyl-Monoselenide Probe for Selective Detection of Superoxide. J Fluoresc 33, 437–444 (2023). https://doi.org/10.1007/s10895-022-03096-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03096-w