Abstract
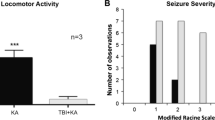
Post-traumatic epilepsy (PTE) caused by mild TBI (mild traumatic brain injury, mTBI) has a high incidence and poor prognosis, but its mechanisms are unclear. Herein, we investigated the role of reduced levels of neuronal autophagy during the latency period in the increased susceptibility to PTE. In the study, a gentle whole-body mechanical trauma rat model was prepared using Noble-Collip drums, and the extent of injury was observed by cranial CT and HE staining of hippocampal tissue. The incidence of epilepsy and its seizure form were observed 7–90 days after mTBI, and electroencephalography (EEG) was recorded during seizures in rats. Subcortical injection of non-epileptogenic dose of ferrous chloride (FeCl2) was used to observe the changes of PTE incidence after mTBI. Western blot and Real-time PCR were used to detect the level of autophagy in hippocampal cells at different time points during the latency period of PTE, and its incidence was observed after up-regulation of autophagy after administration of autophagy agonist—rapamycin. The results showed that mTBI was prepared by Noble-Collip drum, which could better simulate the clinical mTBI process. There was no intracerebral hemorrhage and necrosis in rats, no early-onset seizures, and the incidence of PTE after mTBI was 26.7%. The incidence of PTE was 56.7% in rats injected cortically with FeCl2 at a dose lower than the epileptogenic dose 48 h after mTBI, and the difference was significant compared with no FeCl2 injection, suggesting an increased susceptibility to PTE after mTBI. Further study of neuronal autophagy during PTE latency revealed that autophagy levels were reduced, and the incidence of PTE was significantly reduced after administration of rapamycin to upregulate autophagy. Taken together, the decreased level of neuronal autophagy during the latency period may be a possible mechanism for the increased susceptibility to PTE after mTBI.









Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and will be made available on reasonable request.
References
Scheffer IE, Berkovic S, Capovilla G et al (2017) ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:512–521. https://doi.org/10.1111/epi.13709
Maas AIR, Menon DK, Adelson PD et al (2017) Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16:987–1048. https://doi.org/10.1016/S1474-4422(17)30371-x
Dixon C, Lyeth B, Povlishock J et al (1987) A fluid percussion model of experimental brain injury in the rat. J Neurosurg 67:110–119. https://doi.org/10.3171/jns.1987.67.1.0110
Marmarou A, Foda M, van den Brink W et al (1994) A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg 80:291–300. https://doi.org/10.3171/jns.1994.80.2.0291
Dixon CE, Clifton GL, Lighthall JW et al (1991) A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39:253–262. https://doi.org/10.1016/0165-0270(91)90104-8
Efendioglu M, Basaran R, Akca M et al (2020) Combination therapy of gabapentin and N-acetylcysteine against posttraumatic epilepsy in rats. Neurochem Res 45:1802–1812. https://doi.org/10.1007/s11064-020-03042-x
Wang J, Lu K, Liang F et al (2013) Decreased autophagy contributes to myocardial dysfunction in rats subjected to nonlethal mechanical trauma. PLoS ONE 8:e71400. https://doi.org/10.1371/journal.pone.0071400
Tao L, Liu HR, Gao F et al (2005) Mechanical traumatic injury without circulatory shock causes cardiomyocyte apoptosis: role of reactive nitrogen and reactive oxygen species. Am J Physiol Heart Circ Physiol 288:H2811-2818. https://doi.org/10.1152/ajpheart.01252.2004
Hunt RF, Boychuk JA, Smith BN (2013) Neural circuit mechanisms of post-traumatic epilepsy. Front Cell Neurosci 7:89. https://doi.org/10.3389/fncel.2013.00089
Barranco C (2015) Activate autophagy to prevent cartilage degeneration? Nat Rev Rheumatol 11:127. https://doi.org/10.1038/nrrheum.2015.12
Chen Y, Klionsky DJ (2011) The regulation of autophagy—unanswered questions. J Cell Sci 124(Pt 2):161–170. https://doi.org/10.1242/jcs.064576
Carloni S, Buonocore G, Balduini W (2008) Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis 32(3):329–339. https://doi.org/10.1016/j.nbd.2008.07.022
Jiang P, Mizushima N (2014) Autophagy and human diseases. Cell Res 24(1):69–79. https://doi.org/10.1038/cr.2013.161
Wang D, Tian M, Qi Y et al (2015) Jinlida granule inhibits palmitic acid induced-intracellular lipid accumulation and enhances autophagy in NIT-1 pancreatic β cells through AMPK activation. J Ethnopharmacol 161:99–107. https://doi.org/10.1016/j.jep.2014.12.005
Kim JH, Hong SK, Wu PK et al (2014) Raf/MEK/ERK can regulate cellular levels of LC3B and SQSTM1/p62 at expression levels. Exp Cell Res 327(2):340–352. https://doi.org/10.1016/j.yexcr.2014.08.001
Sahani MH, Itakura E, Mizushima N (2014) Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending ontranscriptional upregulation and autophagy-derived amino acids. Autophagy 10(3):431–441. https://doi.org/10.4161/auto.27344
Clark RS, Bayir H, Chu CT et al (2008) Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy 4:88–90. https://doi.org/10.4161/auto.5173
Wong M (2013) Cleaning up epilepsy and neurodegeneration: the role of autophagy in epileptogenesis. Epilepsy curr 13:177–178. https://doi.org/10.5698/1535-7597-13.4.177
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32(3):281–294. https://doi.org/10.1016/0013-4694(72)90177-0
Ferguson PL, Smith GM, Wannamaker BB et al (2010) A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia 51:891–898. https://doi.org/10.1111/j.1528-1167.2009.02384.x
Gómez PA, de-la-Cruz J, Lora D et al (2014) Validation of a prognostic score for early mortality in severe head injury cases. J Neurosurg 121:1314–1322. https://doi.org/10.3171/2014.7.JNS131874
Register-Mihalik JK, Sarmiento K, Vander Vegt CB et al (2019) Considerations for athletic trainers: a review of guidance on mild traumatic brain injury among children from the centers for disease control and prevention and the national athletic trainers’ association. J Athl Train 54:12–20. https://doi.org/10.4085/1062-6050-451-18
Eakin K, Miller JP (2012) Mild traumatic brain injury is associated with impaired hippocampal spatiotemporal representation in the absence of histological changes. J Neurotrauma 29:1180–1187. https://doi.org/10.1089/neu.2011.2192
Jeter CB, Hergenroeder GW, Hylin MJ et al (2013) Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J Neurotrauma 30:657–670. https://doi.org/10.1089/neu.2012.2439
Jordan BD (2013) The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol 9:222–230. https://doi.org/10.1038/nrneurol.2013.33
Piccenna L, Shears G, O’Brien TJ (2017) Management of post-traumatic epilepsy: an evidence review over the last 5 years and future directions. Epilepsia Open 2:123–144. https://doi.org/10.1002/epi4.12049
Chen Z, Venkat P, Seyfried D et al (2017) Brain-heart interaction: cardiac complications after stroke. Circ Res 121:451–468. https://doi.org/10.1161/CIRCRESAHA.117.311170
Roux A, Muller L, Jackson SN et al (2016) Mass spectrometry imaging of rat brain lipid profile changes over time following traumatic brain injury. J Neurosci Methods 272:19–32. https://doi.org/10.1016/j.jneumeth.2016.02.004
Raymont V, Salazar AM, Lipsky R et al (2010) Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology 75:224–229. https://doi.org/10.1212/WNL.0b013e3181e8e6d0
Gao Y, Luo CL, Li LL et al (2017) IL-33 provides neuroprotection through suppressing apoptotic, autophagic and NF-kappaB-mediated iinflammatory pathways in a rat model of recurrent neonatal seizure. Front Mol Neurosci 10:423. https://doi.org/10.3389/fnmol.2017.00423
Li Q, Han Y, Du J et al (2018) Recombinant human erythropoietin protects against brain injury through blunting the mTORC1 pathway in the developing brains of rats with seizures. Life Sci 194:15–25. https://doi.org/10.1016/j.lfs.2017.12.014
Yuen AW, Sander JW (2014) Rationale for using intermittent calorie restriction as a dietary treatment for drug resistant epilepsy. Epilepsy Behav 33:110–114. https://doi.org/10.1016/j.yebeh.2014.02.026
Anovadiya AP, Sanmukhani JJ, Tripathi CB (2012) Epilepsy: novel therapeutic targets. J Pharmacol Pharmacother 3:112–117. https://doi.org/10.4103/0976-500X.95505
Funding
National Natural Science Foundation of China (No. 81501132).
Author information
Authors and Affiliations
Contributions
JW: topic selection, animal experiments, thesis writing; XC: animal experiments, thesis writing; FZ: data collection and processing; YL: data processing and analysis; HS: data collection and processing; KL: thesis revision, experimental guidance.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical Approval
Ethic certification was approved by ethics Committee of Shanxi Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Chai, X., Zhang, F. et al. The Role of Decreased Levels of Neuronal Autophagy in Increased Susceptibility to Post-traumatic Epilepsy. Neurochem Res 48, 909–919 (2023). https://doi.org/10.1007/s11064-022-03814-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03814-7