Abstract
A novel fluorescent probe possessing anthracene with an indole unit was designed and synthesized to detect chromium(III) ions (Cr3+) with high sensitivity and selectivity. The probe was synthesized in one step by mixing two commercially available chemicals, 2-aminoanthracene and Indole-5-carboxaldehyde. The probe molecule (ANT–In) demonstrates distinct properties, for instance, “turn-on” fluorescence response, high sensitivity and selectivity in less than one minute, and low detection limit (0.2 µM) via hydrolysis of the C = N bond. Additionally, the probe ANT–In was successfully used to identify the presence of chromium(III) ions in real water samples.
Similar content being viewed by others
Introduction
Chromium(III) ions are essential for biological, chemical, and environmental systems [1]. It is a necessary trace element in the human diet for the “glucose tolerance factor” to function correctly [1]. Chromium(III) ions are essential in the metabolism of lipids, carbohydrates, proteins, and nucleic acids in living organisms because they activate some enzymes and stabilize some proteins and nucleic acids [2]. Thus, the National Research Council strongly advises ingesting 50–200 µg d− 1 of chromium(III) ion daily [3, 4]. Chromium(III) ion shortage in the diet can affect the metabolism of glucose and lipids [5] and causes an increase in risk factors for diabetes [6], cardiovascular disease [7], and nervous system diseases [8]. Nevertheless, high chromium(III) ion concentration can negatively affect enzymatic activities and DNA damage [9, 10]. Chromium(III) ions can also be harmful during industrial activities, including manufacturing steelworks chromate, tanning, and chrome pigment [11,12,13]. Therefore, chromium(III) ion detection with reliable, effective, and practical methods is in high demand.
There are several instruments for detecting trace chromium(III) ions, including atomic absorption spectroscopy (AAS), atomic emission spectroscopy (AES), and inductively coupled plasma mass spectroscopy (ICP-MS) [14,15,16,17,18,19]. On the other hand, fluorescence approaches have received much attention compared to the mentioned methods above because of their great sensitivity and selectivity, low cost, ease of operation, and real-time detection [20,21,22,23,24]. For the detection of chromium(III) ions, various fluorescent probes have been developed, such as rhodamine [25,26,27], Bodipy [28, 29], coumarine [30], and anthracene [31, 32] based fluorescent probes. However, many of them have drawbacks such as cross-sensitivity towards Al3+ and Fe3+, turn-off response, slow reaction, and high detection limit. Consequently, developing fluorescent probes for chromium(III) ions to overcome these obstacles is in high demand.
Aminoanthracene-based probes have gained significant interest because of their simple structure, high quantum yield, chemical stability, and ease of chemical modification. Therefore, many anthracene-based fluorescent probes have been reported recently [33,34,35,36,37,38,39,40,41].
Herein, we developed an anthracene and indole-based fluorescent probe (ANT–In) to detect chromium(III) ions. ANT–In demonstrates privileged properties such as operability in aqueous mediums, fast turn-on response, excellent sensitivity and selectivity, and applicability in real water samples.
Experimental Details
General Methods
All reagents were purchased from commercial suppliers (Aldrich and Merck) and used without further purification. 1 H NMR and 13 C NMR were measured on a Varian VNMRJ 600 Nuclear Magnetic Resonance Spectrometer. Mass analyses were conducted with Thermo Q Exactive Orbitrap device. Fluorescence emission spectra were obtained using the Varian Cary Eclipse Fluorescence spectrophotometer.
Preparation of UV-vis and Emission Measurement Solutions
The stock solution of probe molecule ANT–In (1 mM) was prepared in CH3CN, and stock solutions of metal ion salts (20 mM) were prepared in triple distilled deionized water. The metal ions solution was added to the probe solution (2 mL) using a micropipette during the measurements. For fluorescence measurements, samples were contained in 10.0 mm path length quartz cuvettes (2.0 mL volume). Upon excitation at 400 nm, the emission spectra were integrated over the range 410 to 700 nm (Both excitation and emission slit width 5 nm / 5 nm). All measurements were conducted in triplicate at least.
Synthesis of ANT-In
2-aminoanthracene (100.0 mg, 0.517 mmol) and Indole-5-carboxaldehyde (75 mg, 0.517 mmol) were mixed in 10 mL ethanol in the presence of catalytic amount (2–3 drops) of acetic acid (AcOH). The solution mixture was refluxed for 6 h under the nitrogen atmosphere. The obtained solid was filtered and recrystallized in an EtOH-CH2Cl2 mixture (3:1 v/v) to get the desired product of 121 mg ANT–In as a dark green solid (74%) (Scheme 1). 1 H NMR (600 MHz, DMSO-d6) δ 11.43 (s, 1 H), 8.83 (s, 1 H), 8.55 (d, J = 13.8 Hz, 2 H), 8.15–8.11 (m, 2 H), 8.07–8.04 (m, 2 H), 7.85 (d, J = 10.1 Hz, 1 H), 7.81 (s, 1 H), 7.57 (d, J = 6.9 Hz, 1 H), 7.53–7.47 (m, 3 H), 7.44 (t, J = 2.8 Hz, 1 H), 6.58 (s, 1 H). 13 C NMR (150 MHz, DMSO-d6) δ 164.9, 152.3, 141.0, 135.1, 134.7, 133.8, 132.9, 132.3, 131.2, 130.9, 130.8, 130.7, 129.8, 129.1, 128.8, 128.7, 128.3, 126.7, 125.43, 123.9, 119.8, 115.0, 105.5. HRMS (ESI): m/z: Calcd. for (C23H16N2) [M]+ : 320.13135 found, 321.13586.
Preparation of Real Water Samples
The recovery experiments were performed to determine the Cr3+ ions in drinking water, and tap water. Drinking and tap water samples were collected from the district of Gebze in Kocaeli Province in Turkey. First, different Cr3+ ions concentrations were spiked into the actual water samples and detected with ANT–In based on fluorescence measurements. Next, the Cr3+ concentrations were calculated using a linear regression equation by spiked water samples’ fluorescent response (λem = 500 nm). The experiments were repeated three times to get an average value of the detected Cr3+ concentrations. Then, the recovery percentages were calculated to evaluate the degree of deviation of the detected value compared to the amount of added Cr3+.
Results and Discussions
The synthesis route of ANT–In has been shown in Scheme 1. The probe molecule was synthesized via a facile reaction of 2-aminoanthracene and Indole-5-carboxaldehyde (Scheme 1). As specified in the Supporting Information (SI), the probe’s chemical identity confirmed by 1H NMR, 13C NMR and HRMS techniques (SI).
Firstly, we determined the ideal sensing medium for Cr3+ detection. Since ANT–In is not entirely soluble in aqueous solutions, a suitable organic co-solvent is required to raise the probe’s solubility. CH3CN: H2O (7:3 v/v) was an effective system among different solvent combinations. To rule out any pH changes, we evaluated the effect of pH variations on the fluorescence intensity of the sensing medium, demonstrating that ANT–In was pH insensitive (pH 6.0–12.0) and pH adjustment of the sensing media did not affect ANT–In’s ability to detect Cr3+ (Fig. S1). In addition, the probe can detect Cr3+ with a quite wide range of pH values from pH 6–10. Hence, HEPES buffer was used to set the pH of the sensing medium to pH = 7.0.
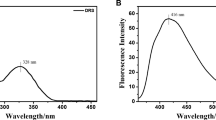
The free ANT–In was fluorescence off mode because of photoinduced electron transfer (PET), in which the lone pair electrons of the nitrogen atom are transferred to the anthracene unit. Upon adding Cr3+ to the ANT–In, a new emission peak emerged at 500 nm. The saturation point was obtained when 4 eq Cr3+ were introduced with a 26-fold enhancement (Fig. 1). Whereas the fluorescence response was rapid (1 < min), and complete saturation took 5 min (Fig. S2). We calculated the detection limit as 0,2 µM based on a signal-to-noise ratio of 3 (Fig. S3).
The sensitivity of ANT–In to other possible metal species, including Cr3+, Fe3+, Al3+, Na+, Li+, Ag+, Ca2+, Mg2+, Ba2+, Pd2+, Hg2+, Cu2+, Zn2+, Pb2+, Ni2+, Cd2+, Co2+, Ce3+, Cr6+ was investigated under same conditions. Fortunately, all other metal ions did not result in any fluorescence change except for Al3+ and Fe3+, but their intensities were lower than that indicated by Cr3+, even if their concentration (10µM) were much more than Cr3+ (2µM) concentration (Fig. 2a). Meanwhile, we investigated the interference of other metal ions for Cr3+. The interference experiments revealed that ANT–In could detect Cr3+ in mixtures of other metal ions without difficulty (Fig. 2b).
(a) Fluorescence intensities of ANT–In (10 µM) in 7:3 CH3CN/HEPES at pH = 7.0 in the presence of Cr3+ (2.0 equiv.) and other metal ions (10.0 equiv) (b) Fluorescence intensities of ANT–In (10 µM) in 7:3 CH3CN/HEPES at pH = 7.0 in the presence of Cr3+ (2.0 equiv.) and 10.0 equiv. other metal ions 1, Cr3+; 2, Fe3+; 3, Al3+; 4, Na+; 5, Li+; 6, Ag+; 7, Ca2+; 8, Mg2+; 9, Ba2+; 10, Pd2+; 11, Hg2+; 12, Cu2+; 13, Zn2+; 14, Pb2+; 15, Ni2+; 16, Cd2+; 17, Co2+; 18, Ce3+; 19, Cr6+
The HRMS technique was used to gain a better insight into the sensing mechanism. Using HRMS analysis of the probe solution (ANT–In + Cr3+), a main molecular ion peak at m/z 194.09509 indicated the exact molecular weight of 2-aminoanthracene. As depicted in Scheme 2, Cr3+ readily coordinate with the nitrogen atom of the C = N unit and the nitrogen atom in the indole part. Afterwards, nucleophilic addition of a water molecule leads to hydrolysis in forming highly emissive starting material 2-aminoanthracene.
In order to provide support for the postulated sensing mechanism, NMR analysis was performed. The NMR results revealed that initially, there was no signal at 9.87 ppm (Fig. 3a); however, after treating the ANT–In solution with Cr3+ ions, a new aldehyde proton, belonging to Indole-5-carboxaldehyde, appeared at 9.87 ppm (Fig. 3b). The conclusion that can be drawn from this finding is that a Cr3+ ion-mediated hydrolysis process occurred.
Encouraged by the probe’s high sensitivity and selectivity for Cr3+, we performed the practical application in real water samples. First, tap and drinking water samples were obtained and used in experiments without further purification. Subsequently, a specific amount of Cr3+ was spiked in the water samples (17.5 µM). To minimize the matrix effect, we developed independent calibration curves for each water sample (Fig. S4), and recovery values were calculated 101.8% and 98.4% for drinking water and tap water, respectively (Table 1). In light of obtained results, our probe ANT–In (10 µM) could determine the amount of Cr3+, and it was practicable and reliable for Cr3+ measurement in real water samples.
Conclusion
In this article, we designed and synthesized a simple fluorescence probe ANT–In for detecting Cr3+ in an aqueous medium. The ANT–In exhibited remarkable selectivity, short response time (less than 1 min), and low detection limit. In addition, the designed probe successfully detected Cr3+-spiked drinking and tap water samples.
Data Availability
All the data associated with this research has been presented in the paper.
References
Gómez V, Callao MP (2006) Chromium determination and speciation since 2000. TrAC Trends Anal Chem 25:1006–1015. https://doi.org/10.1016/j.trac.2006.06.010
Arakawa H, Ahmad R, Naoui M, Tajmir-Riahi HA (2000) A comparative study of calf thymus DNA binding to Cr(III) and Cr(VI) ions. Evidence for the guanine N-7-chromium-phosphate chelate formation. J Biol Chem 275:10150–10153. https://doi.org/10.1074/jbc.275.14.10150
Mertz W, Schwarz K (1955) Impaired intravenous glucose tolerance as an early sign of dietary necrotic liver degeneration. Arch Biochem 58:504–506
Arakawa H, Ahmad R, Naoui M, Tajmir-Riahi H-A (2000) A Comparative Study of Calf Thymus DNA Binding to Cr(III) and Cr(VI) Ions: evidence for the guanine n-7-chromium-phosphate chelate formation*. J Biol Chem 275:10150–10153. https://doi.org/10.1074/jbc.275.14.10150
Zhou Z, Li Y, Wu Y (2014) Ratiometric fluorescence probe for two-photon bioimaging of Cr3 + in living cells. Tetrahedron Lett 55:4075–4077. https://doi.org/10.1016/j.tetlet.2014.06.014
Zhou Y, Zhang J, Zhang L et al (2013) A rhodamine-based fluorescent enhancement chemosensor for the detection of Cr3+ in aqueous media. Dye Pigment 97:148–154. https://doi.org/10.1016/j.dyepig.2012.12.006
Liu H, Wan X, Liu T et al (2014) Cascade sensitive and selective fluorescence OFF–ON–OFF sensor for Cr3+ cation and F– anion. Sens Actuators B Chem 200:191–197. https://doi.org/10.1016/j.snb.2014.04.027
Wu Y-S, Li C-Y, Li Y-F et al (2014) A ratiometric fluorescent chemosensor for Cr3+ based on monomer–excimer conversion of a pyrene compound. Sens Actuators B Chem 203:712–718. https://doi.org/10.1016/j.snb.2014.07.046
Raspor P, Batic M, Jamnik P et al (2000) The influence of chromium compounds on yeast physiology: (A review). Acta Microbiol Immunol Hung 47:143–173. https://doi.org/10.1556/AMicr.47.2000.2-3.2
Cervantes C, Campos-García J, Devars S et al (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347. https://doi.org/10.1111/j.1574-6976.2001.tb00581.x
Marqués MJ, Salvador A, Morales-Rubio A, de la Guardia M (2000) Chromium speciation in liquid matrices: a survey of the literature. Fresenius J Anal Chem 367:601–613. https://doi.org/10.1007/s002160000422
Kumar AR, Riyazuddin P (2009) Comparative study of analytical methods for the determination of chromium in groundwater samples containing iron. Microchem J 93:236–241. https://doi.org/10.1016/j.microc.2009.07.012
Wang L-L, Wang J-Q, Zheng Z-X, Xiao P (2010) Cloud point extraction combined with high-performance liquid chromatography for speciation of chromium(III) and chromium(VI) in environmental sediment samples. J Hazard Mater 177:114–118. https://doi.org/10.1016/j.jhazmat.2009.12.003
Zhu H, Fan J, Wang B, Peng X (2015) Fluorescent, MRI, and colorimetric chemical sensors for the first-row d-block metal ions. Chem Soc Rev 44:4337–4366. https://doi.org/10.1039/C4CS00285G
Lee MH, Kim JS, Sessler JL (2015) Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev 44:4185–4191. https://doi.org/10.1039/C4CS00280F
Wu D, Sedgwick AC, Gunnlaugsson T et al (2017) Fluorescent chemosensors: the past, present and future. Chem Soc Rev 46:7105–7123. https://doi.org/10.1039/C7CS00240H
Kaur B, Kaur N, Kumar S (2018) Colorimetric metal ion sensors – A comprehensive review of the years 2011–2016. Coord Chem Rev 358:13–69. https://doi.org/10.1016/j.ccr.2017.12.002
Wu D, Chen L, Lee W et al (2018) Recent progress in the development of organic dye based near-infrared fluorescence probes for metal ions. Coord Chem Rev 354:74–97. https://doi.org/10.1016/j.ccr.2017.06.011
Park S-H, Kwon N, Lee J-H et al (2020) Synthetic ratiometric fluorescent probes for detection of ions. Chem Soc Rev 49:143–179. https://doi.org/10.1039/C9CS00243J
Karakuş E, Erdemir E, Suna G et al (2021) Fluorescein Based Three-channel Probe for the Selective and Sensitive Detection of CO32– Ions in an Aqueous Environment and Real Water Samples. J Fluoresc 31:1617–1625. https://doi.org/10.1007/s10895-021-02779-0
Erdemir E, Suna G, Gunduz S et al (2022) Rapid, ultrasensitive, highly selective detection of toxic Hg(II) ions in seabass, swordfish and water samples. Food Chem 371:131309. https://doi.org/10.1016/j.foodchem.2021.131309
Suna G, Erdemir E, Liv L et al (2022) Multi-channel detection of Au(III) ions by a novel rhodamine based probe. Sens Actuators B Chem 360:131658. https://doi.org/10.1016/j.snb.2022.131658
Erdemir E, Suna G, Gunduz S et al (2022) Tetraphenylethylene–thiosemicarbazone based ultrafast, highly sensitive detection of hypochlorite in aqueous environments and dairy products. Anal Chim Acta 1218:340029. https://doi.org/10.1016/j.aca.2022.340029
Karakuş E, Cakan-Akdogan G, Emrullahoğlu M (2015) A guanidinium modified rhodamine-based fluorescent probe for in vitro/vivo imaging of gold ions. Anal Methods 7:8004–8008. https://doi.org/10.1039/C5AY01581B
Huang K, Yang H, Zhou Z et al (2008) Multisignal Chemosensor for Cr3+ and Its Application in Bioimaging. Org Lett 10:2557–2560. https://doi.org/10.1021/ol800778a
Zhou Z, Yu M, Yang H et al (2008) FRET-based sensor for imaging chromium(III) in living cells. Chem Commun 3387–3389. https://doi.org/10.1039/B801503A
Li D, Li C-Y, Qi H-R et al (2016) Rhodamine-based chemosensor for fluorescence determination of trivalent chromium ion in living cells. Sens Actuators B Chem 223:705–712. https://doi.org/10.1016/j.snb.2015.09.126
Shiraishi Y, Sumiya S, Hirai T (2010) A coumarin–thiourea conjugate as a fluorescent probe for Hg(II) in aqueous media with a broad pH range 2–12. Org Biomol Chem 8:1310–1314. https://doi.org/10.1039/B924015B
Kursunlu AN, Şahin E, Güler E (2015) Bodipy/dipyridylamino-based “turn-on” fluorescent chemosensor for trivalent chromium cations: characterization and photophysical properties. RSC Adv 5:5951–5957. https://doi.org/10.1039/C4RA12874E
Guha S, Lohar S, Banerjee A et al (2012) Thiophene anchored coumarin derivative as a turn-on fluorescent probe for Cr3+: cell imaging and speciation studies. Talanta 91:18–25. https://doi.org/10.1016/j.talanta.2011.12.014
Guha S, Lohar S, Banerjee A et al (2012) Anthracene appended coumarin derivative as a Cr(III) selective turn-on fluorescent probe for living cell imaging: a green approach towards speciation studies. Anal Methods 4:3163–3168. https://doi.org/10.1039/C2AY25693B
Erdemir S, Kocyigit O (2016) Anthracene excimer-based “turn on” fluorescent sensor for Cr3+ and Fe3+ ions: Its application to living cells. Talanta 158:63–69. https://doi.org/10.1016/j.talanta.2016.05.017
Fabbrizzi L, Licchelli M, Pallavicini P et al (1994) An Anthracene-Based Fluorescent Sensor for Transition Metal Ions. Angew Chemie Int Ed English 33:1975–1977. https://doi.org/10.1002/anie.199419751
Bohne C, Ihmels H, Waidelich M, Yihwa C (2005) N-Acylureido Functionality as Acceptor Substituent in Solvatochromic Fluorescence Probes: Detection of Carboxylic Acids, Alcohols, and Fluoride Ions. J Am Chem Soc 127:17158–17159. https://doi.org/10.1021/ja052262c
Ihmels H, Meiswinkel A, Mohrschladt CJ et al (2005) Anthryl-Substituted Heterocycles as Acid-Sensitive Fluorescence Probes. J Org Chem 70:3929–3938. https://doi.org/10.1021/jo047841z
Lohani CR, Kim J-M, Lee K-H (2009) Facile synthesis of anthracene-appended amino acids as highly selective and sensitive fluorescent Fe3+ ion sensors. Bioorg Med Chem Lett 19:6069–6073. https://doi.org/10.1016/j.bmcl.2009.09.036
Erdemir S, Malkondu S (2015) Novel “turn on” fluorescent sensors based on anthracene and carbazone units for Cu (II) ion in CH3CN–H2O. J Lumin 158:86–90. https://doi.org/10.1016/j.jlumin.2014.09.038
Chinna ayya swamy PC, Shanmugapriya J, Singaravadivel S et al (2018) Anthracene-based highly selective and sensitive fluorescent “Turn-on.” Chemodosimeter for Hg2+ ACS Omega 3:12341–12348. https://doi.org/10.1021/acsomega.8b01142
Singh R, Mitra K, Singh S et al (2019) Highly selective fluorescence ‘turn off’ sensing of picric acid and efficient cell labelling by water-soluble luminescent anthracene-bridged poly(N-vinyl pyrrolidone). Analyst 144:3620–3634. https://doi.org/10.1039/C8AN02417K
Karakuş E, Gunduz S, Liv L, Ozturk T (2020) Fluorescent and electrochemical detection of Cu (II) ions in aqueous environment by a novel, simple and readily available AIE probe. J Photochem Photobiol A Chem 400:112702. https://doi.org/10.1016/j.jphotochem.2020.112702
Karakuş E (2020) An anthracene based fluorescent probe for the selective and sensitive detection of Chromium (III) ions in an aqueous medium and its practical application. Turkish J Chem 44:941–949. https://doi.org/10.3906/kim-2003-41
Acknowledgements
The authors gratefully acknowledge TUBITAK UME for financial support, Muhiddin ÇERGEL for NMR analysis and special thanks to Erman KARAKUŞ for his supervision.
Author information
Authors and Affiliations
Contributions
Garen SUNA: Formal analysis, Investigation, Data curation, Writing – original draft. Simay GUNDUZ: Conceptualization, Methodology, Investigation, Data curation, Supervision, Writing – original draft, Writing – review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Declaration Statement
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suna, G., Gunduz, S. An Anthracene and Indole-based Fluorescent Probe for the Detection of Chromium(III) Ions in Real Water Samples. J Fluoresc 33, 185–190 (2023). https://doi.org/10.1007/s10895-022-03041-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03041-x