Abstract
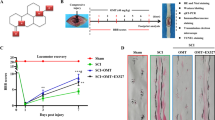
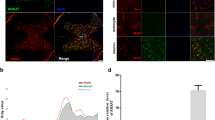
Spinal cord injury (SCI), resulting in damage of the normal structure and function of the spinal cord, would do great harm to patients, physically and psychologically. The mechanism of SCI is very complex. At present, lots of studies have reported that autophagy was involved in the secondary injury process of SCI, and several researchers also found that calcium ions (Ca2+) played an important role in SCI by regulating necrosis, autophagy, or apoptosis. However, to our best of knowledge, no studies have linked the spinal cord mechanical injury, intracellular Ca2+, and autophagy in series. In this study, we have established an in vitro model of SCI using neural cells from fetal rats to explore the relationship among them, and found that mechanical injury could promote the intracellular Ca2+ concentration, and the increased Ca2+ level activated autophagy through the CaMKKβ/AMPK/mTOR pathway. Additionally, we found that apoptosis was also involved in this pathway. Thus, our study provides new insights into the specific mechanisms of SCI and may open up new avenues for the treatment of SCI.




Similar content being viewed by others
References
Ramer L, Ramer M, Bradbury E (2014) Restoring function after spinal cord injury: towards clinical translation of experimental strategies The Lancet Neurology. Lancet Neurol 13(12):1241–1256. https://doi.org/10.1016/s1474-4422(14)70144-9
Ahuja C, Wilson J, Nori S, Kotter M, Druschel C, Curt A, Fehlings M (2017) Traumatic spinal cord injury Nature reviews Disease primers. Nat reviews Disease primers 3:17018. https://doi.org/10.1038/nrdp.2017.18
Selvarajah S, Hammond E, Schneider E (2015) Trends in Traumatic Spinal Cord Injury. JAMA JAMA 314(15):1643. https://doi.org/10.1001/jama.2015.11194
Furlan J, Craven B, Fehlings M, -Utility (2016)Analysis Neurosurgery. Neurosurgery, 79(3):418–425. https://doi.org/10.1227/neu.0000000000001314
Kwon B, Tetzlaff W, Grauer J, Beiner J, Vaccaro A (2004) Pathophysiology and pharmacologic treatment of acute spinal cord injury The spine journal: official journal of the North American Spine Society. The spine journal: official. J North Am Spine Soc 4(4):451–464. https://doi.org/10.1016/j.spinee.2003.07.007
Zhou K, Sansur C, Xu H, Jia X (2017) The Temporal Pattern, Flux, and Function of Autophagy. Spinal Cord Injury International journal of molecular sciences International journal of molecular sciences 18(2). https://doi.org/10.3390/ijms18020466
Romeo-Guitart D, Marmolejo-Martínez-Artesero S, Casas C (2020) Is it the time of autophagy fine-tuners for neuroprotection? Autophagy Autophagy 16(11):2108–2109. https://doi.org/10.1080/15548627.2020.1794355
Stavoe A, Holzbaur E (2019) Autophagy in Neurons Annual review of cell and developmental biology. Annual. Rev cell Dev biology 35:477–500. https://doi.org/10.1146/annurev-cellbio-100818-125242
Tran A, Warren P, Silver J (2020) Regulation of autophagy by inhibitory CSPG interactions with receptor PTPσ and its impact on plasticity and regeneration after spinal cord injury Experimental neurology. Exp Neurol 328:113276. https://doi.org/10.1016/j.expneurol.2020.113276
Liao H, Wang Z, Ran R, Zhou K, Ma C, Zhang H (2021) Biological Functions and Therapeutic Potential of Autophagy in Spinal Cord Injury Frontiers in cell and developmental biology. Frontiers. in cell and developmental biology 9:761273. https://doi.org/10.3389/fcell.2021.761273
Zhou K, Chen H, Xu H, Jia X (2021) Trehalose Augments Neuron Survival and Improves Recovery from Spinal Cord Injury via mTOR-Independent Activation of Autophagy Oxidative medicine and cellular longevity. Oxidative medicine and cellular longevity, 2021:8898996. https://doi.org/10.1155/2021/8898996
Li Y, Zhang J, Zhou K, Xie L, Xiang G, Fang M, Han W, Wang X, Xiao J (2021) Elevating sestrin2 attenuates endoplasmic reticulum stress and improves functional recovery through autophagy activation after spinal cord injury Cell biology and toxicology. Cell Biol Toxicol 37(3):401–419. https://doi.org/10.1007/s10565-020-09550-4
Doherty J, Baehrecke E (2018) Life, death and autophagy Nature cell biology. Nat Cell Biol 20(10):1110–1117. https://doi.org/10.1038/s41556-018-0201-5
New J, Thomas S (2019) Autophagy-dependent secretion: mechanism, factors secreted, and disease implications Autophagy. Autophagy 15(10):1682–1693. https://doi.org/10.1080/15548627.2019.1596479
Sun D, Deshpande L, Sombati S, Baranova A, Wilson M, Hamm R, DeLorenzo R (2008) Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2 + homeostatic mechanisms in hippocampal neurons surviving brain injury The European journal of neuroscience. Eur J Neurosci 27(7):1659–1672. https://doi.org/10.1111/j.1460-9568.2008.06156.x
Maneshi M, Sachs F, Hua S (2015) A Threshold Shear Force for Calcium Influx in an Astrocyte Model of Traumatic Brain Injury Journal of neurotrauma. J Neurotrauma 32(13):1020–1029. https://doi.org/10.1089/neu.2014.3677
Decuypere J, Bultynck G, Parys J (2011) A dual role for Ca(2+) in autophagy regulation Cell calcium. Cell Calcium 50(3):242–250. https://doi.org/10.1016/j.ceca.2011.04.001
Cárdenas C, Miller R, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung K, Yang J, Parker I et al (2010) Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2 + transfer to mitochondria Cell. Cell 142(2):270–283. https://doi.org/10.1016/j.cell.2010.06.007
Rimessi A, Bonora M, Marchi S, Patergnani S, Marobbio C, Lasorsa F, Pinton P (2013) Perturbed mitochondrial Ca2 + signals as causes or consequences of mitophagy induction Autophagy. Autophagy 9(11):1677–1686. https://doi.org/10.4161/auto.24795
Orem B, Rajaee A, Stirling D (2020) IPR-mediated intra-axonal Ca release contributes to secondary axonal degeneration following contusive spinal cord injury Neurobiology of disease. Neurobiology of disease. 146:105123. https://doi.org/10.1016/j.nbd.2020.105123
Yang J, Yu J, Li D, Yu S, Ke J, Wang L, Wang Y, Qiu Y, Gao X, Zhang J et al (2017) Store-operated calcium entry-activated autophagy protects EPC proliferation via the CAMKK2-MTOR pathway in ox-LDL. exposure Autophagy Autophagy 13(1):82–98. https://doi.org/10.1080/15548627.2016.1245261
Que H, Liu Y, Jia Y, Liu S (2011) Establishment and assessment of a simple and easily reproducible incision model of spinal cord neuron cells in vitro In vitro cellular & developmental biology Animal. In vitro cellular &. Dev biology Anim 47(8):558–564. https://doi.org/10.1007/s11626-011-9443-2
Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R et al (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2 Molecular cell. Molecular cell. 25:193–205. https://doi.org/10.1016/j.molcel.2006.12.009. 2
Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon J, Janle E, Lobo J, Ferruzzi M, Davies P et al (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism The Journal of biological chemistry. J Biol Chem 285(12):9100–9113. https://doi.org/10.1074/jbc.M109.060061
Gordon P, Holen I, Fosse M, Røtnes J, Seglen P (1993) Dependence of hepatocytic autophagy on intracellularly sequestered calcium The Journal of biological chemistry. J Biol Chem 268(35):26107–26112
Williams A, Sarkar S, Cuddon P, Ttofi E, Saiki S, Siddiqi F, Jahreiss L, Fleming A, Pask D, Goldsmith P et al (2008) Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem biology Nat Chem biology 4(5):295–305. https://doi.org/10.1038/nchembio.79
Khan M, Joseph S (2010) Role of inositol trisphosphate receptors in autophagy in DT40 cells The Journal of biological chemistry. J Biol Chem 285(22):16912–16920. https://doi.org/10.1074/jbc.M110.114207
Law B, Wang M, Ma D, Al-Mousa F, Michelangeli F, Cheng S, Ng M, To K, Mok A, Ko R et al (2010) Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis Molecular cancer therapeutics. Molecular cancer therapeutics. 9:718–730. https://doi.org/10.1158/1535-7163.Mct-09-0700. 3
Pan J, Cai R, Chen Y, Li Y, Lin W, Wu J, Wang X (2018) Analysis the effect of hyperbaric oxygen preconditioning on neuronal apoptosis, Ca2 + concentration and caspases expression after spinal cord injury in rats European review for medical and pharmacological sciences. European review for medical and pharmacological sciences. 22:3467–3473. https://doi.org/10.26355/eurrev_201806_15172. 11
Huang Y, Lin J, Chen X, Lin J (2021) Pannexin-1 Contributes to the Apoptosis of Spinal Neurocytes in Spinal Cord Injury Frontiers in physiology. Frontiers in physiology. 12:656647. https://doi.org/10.3389/fphys.2021.656647
Kanno H, Ozawa H, Sekiguchi A, Itoi E (2009) Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death Neurobiology of disease. Neurobiology of disease. 33:143–148. https://doi.org/10.1016/j.nbd.2008.09.009. 2
Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E (2011) Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine Spine 36(22):E1427–1434. https://doi.org/10.1097/BRS.0b013e3182028c3a
Muñoz-Galdeano T, Reigada D, Del Águila Á, Velez I, Caballero-López M, Maza R, Nieto-Díaz M (2018) Cell Specific Changes of Autophagy in a Mouse Model of Contusive Spinal Cord Injury Frontiers in cellular neuroscience. Frontiers in cellular neuroscience. 12:164. https://doi.org/10.3389/fncel.2018.00164
Wang P, Xie Z, Xie C, Lin C, Wang J, Xuan L, Zhang C, Wang Y, Huang Z, Teng H (2018) AMP-activated protein kinase-dependent induction of autophagy by erythropoietin protects against spinal cord injury in rats CNS neuroscience & therapeutics. CNS neuroscience & therapeutics. 24:1185–1195. https://doi.org/10.1111/cns.12856. 12
Li X, Du J, Lu Y, Lin X (2019) Neuroprotective effects of rapamycin on spinal cord injury in rats by increasing autophagy and Akt signaling Neural regeneration research. Neural regeneration research. 14:721–727. https://doi.org/10.4103/1673-5374.247476. 4
Fang B, Li X, Bao N, Tan W, Chen F, Pi X, Zhang Y, Ma H (2016) Role of autophagy in the bimodal stage after spinal cord ischemia reperfusion injury in rats. Neurosci Neurosci 328:107–116. https://doi.org/10.1016/j.neuroscience.2016.04.019
Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E (2012) Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J neurotrauma J neurotrauma 29(5):946–956. https://doi.org/10.1089/neu.2011.1919
Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, He C, Du H, Zhang L (2014) Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats Molecular neurobiology. Molecular neurobiology. 49:276–287. https://doi.org/10.1007/s12035-013-8518-3. 1
Guo Y, Liu S, Zhang X, Wang L, Gao J, Han A, Hao A (2015) G-CSF promotes autophagy and reduces neural tissue damage after spinal cord injury in mice Laboratory investigation; a journal of technical methods and pathology. Laboratory investigation; a journal of technical methods and pathology. 95:1439–1449. https://doi.org/10.1038/labinvest.2015.120. 12
Zhang H, Wang Z, Wu F, Kong X, Yang J, Lin B, Zhu S, Lin L, Gan C, Fu X et al (2013) Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury Molecular neurobiology. Molecular neurobiology. 48:452–464. https://doi.org/10.1007/s12035-013-8432-8. 3
Wei X, Zhou Z, Li L, Gu J, Wang C, Xu F, Dong Q, Zhou X (2016) Intrathecal Injection of 3-Methyladenine Reduces Neuronal Damage and Promotes Functional Recovery via Autophagy Attenuation after Spinal Cord Ischemia/Reperfusion Injury in Rats Biological & pharmaceutical bulletin. Biological & pharmaceutical bulletin. 39:665–673. https://doi.org/10.1248/bpb.b15-00610. 5
Ren X, Wan C, Niu Y (2019) Overexpression of lncRNA TCTN2 protects neurons from apoptosis by enhancing cell autophagy in spinal cord injury FEBS open bio. FEBS open bio. 9:1223–1231. https://doi.org/10.1002/2211-5463.12651. 7
Lin S, Tian H, Lin J, Xu C, Yuan Y, Gao S, Song C, Lv P, Mei X (2020) Zinc promotes autophagy and inhibits apoptosis through AMPK/mTOR signaling pathway after spinal cord injury Neuroscience letters. Neurosci Lett 736:135263. https://doi.org/10.1016/j.neulet.2020.135263
Rong Y, Liu W, Lv C, Wang J, Luo Y, Jiang D, Li L, Zhou Z, Zhou W, Li Q et al (2019) Neural stem cell small extracellular vesicle-based delivery of 14-3-3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin-1. Aging Aging 11(18):7723–7745. https://doi.org/10.18632/aging.102283
Gao K, Niu J, Dang X (2020) Neuroprotection of melatonin on spinal cord injury by activating autophagy and inhibiting apoptosis via SIRT1/AMPK signaling pathway Biotechnology letters. Biotechnol Lett 42(10):2059–2069. https://doi.org/10.1007/s10529-020-02939-5
Wang P, Lin C, Wu S, Huang K, Wang Y, Bao X, Zhang F, Huang Z, Teng H (2018) Inhibition of Autophagy is Involved in the Protective Effects of Ginsenoside Rb1 on Spinal Cord Injury Cellular and molecular neurobiology. Cell Mol Neurobiol 38(3):679–690. https://doi.org/10.1007/s10571-017-0527-8
Wang Z, Zhou L, Zheng X, Liu W (2018) Effects of dexamethasone on autophagy and apoptosis in acute spinal cord injury. Neuroreport Neuroreport 29(13):1084–1091. https://doi.org/10.1097/wnr.0000000000001076
Feng Z, Min L, Chen H, Deng W, Tan M, Liu H, Hou J (2021) Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury Redox biology. Redox biology. 43:101984. https://doi.org/10.1016/j.redox.2021.101984
Xia M, Zhang Q, Zhang Y, Li R, Zhao T, Chen L, Liu Q, Zheng S, Li H, Qian Z et al (2022) Growth Differentiation Factor 15 Regulates Oxidative Stress-Dependent Ferroptosis Post Spinal Cord Injury by Stabilizing the p62-Keap1-Nrf2 Signaling Pathway Frontiers in aging neuroscience. Front Aging Neurosci 14:905115. https://doi.org/10.3389/fnagi.2022.905115
Sugaya T, Kanno H, Matsuda M, Handa K, Tateda S, Murakami T, Ozawa H, Itoi E (2019) B-RAF Inhibitor Dabrafenib Attenuates RIPK3-Mediated Necroptosis and Promotes Functional Recovery after Spinal. Cord Injury Cells Cells 8(12). https://doi.org/10.3390/cells8121582
Hongna Y, Hongzhao T, Quan L, Delin F, Guijun L, Xiaolin L, Fulin G, Zhongren S (2020) Jia-Ji Electro-Acupuncture Improves Locomotor Function With Spinal Cord Injury by Regulation of Autophagy Flux and Inhibition of Necroptosis Frontiers in neuroscience. Frontiers in neuroscience. 14:616864. https://doi.org/10.3389/fnins.2020.616864
Wang J, Zhang F, Xu H, Yang H, Shao M, Xu S, Lyu F (2022) TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury Clinical and translational medicine. Clinical and translational medicine. 12:e894. https://doi.org/10.1002/ctm2.894. 6
Jiang W, He F, Ding G, Wu J (2022) Topotecan Reduces Neuron Death after Spinal Cord Injury by Suppressing Caspase-1-Dependent Pyroptosis Molecular neurobiology. Molecular neurobiology. https://doi.org/10.1007/s12035-022-02960-x
Yang D, Shu T, Zhao H, Sun Y, Xu W, Tu G (2020) Knockdown of macrophage migration inhibitory factor (MIF), a novel target to protect neurons from parthanatos induced by simulated post-spinal cord injury oxidative stress Biochemical and biophysical research communications. Biochemical and biophysical research communications. 523:719–725. https://doi.org/10.1016/j.bbrc.2019.12.115. 3
Kuzhandaivel A, Nistri A, Mladinic M (2010) Kainate-mediated excitotoxicity induces neuronal death in the rat spinal cord in vitro via a PARP-1 dependent cell death pathway (Parthanatos) Cellular and molecular neurobiology. Cell Mol Neurobiol 30(7):1001–1012. https://doi.org/10.1007/s10571-010-9531-y
Acknowledgements
No.
Funding
This work was supported by the National Natural Science Foundation of China Youth Science Fund Project (81701228 to FBL), the National Natural Science Foundation of China (81871821 to JL) and the Fundamental Research Funds for the Central Universities of Central South University (160171025 to FSL).
Author information
Authors and Affiliations
Contributions
All authors participated in data acquisition. FBL, GHL, JL and BW contributed to the conception and design of the study. FSL, CJ, ZL and XBW did the data analysis and interpretation. FSL and FBL contributed to the drafting and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Research Ethics Committee of The Second Xiangya Hospital, Central South University, Hunan, P.R. China.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, FS., Jiang, C., Li, Z. et al. Ca2+ Regulates Autophagy Through CaMKKβ/AMPK/mTOR Signaling Pathway in Mechanical Spinal cord Injury: An in vitro Study. Neurochem Res 48, 447–457 (2023). https://doi.org/10.1007/s11064-022-03768-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03768-w