Abstract
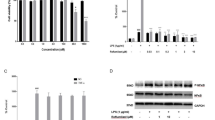
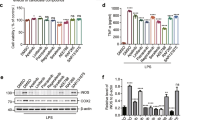
Selonsertib is a first-in-class apoptosis signal-regulating kinase 1 (ASK1) inhibitor in clinical trials for treating NASH and diabetic kidney disease due to its anti-inflammatory and anti-fibrotic activities. In the present study, we investigated the anti-neuroinflammatory effects and brain pharmacokinetic properties of selonsertib. It inhibited inflammatory cytokines and NO production by suppressing phosphorylated ASK1 in the LPS-stimulated microglial cell line, BV2 cells. Consistent with the in vitro results, selonsertib attenuated plasma and brain TNF-α levels in the LPS-induced murine neuroinflammation model. In vitro and in vivo pharmacokinetic studies of selonsertib were conducted in support of central nervous system (CNS) drug discovery. In both Caco-2 and MDR-MDCK cells, selonsertib exhibited a high efflux ratio, showing that it is a P-gp substrate. Selonsertib was rapidly and effectively absorbed into the systemic circulation after oral treatment, with a Tmax of 0.5 h and oral bioavailability of 74%. In comparison with high systemic exposure with Cmax of 16.2 µg/ml and AUC of 64 µg·h/mL following oral dosing of 10 mg/kg, the brain disposition of selonsertib was limited, with Cmax of 0.08 µg/g and Kp value of 0.004. This study demonstrates that selonsertib can be a therapeutic agent for neuroinflammatory diseases.





Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Abdel-Magid AF (2018) ASK1: a therapeutic target for the treatment of multiple diseases. ACS Publications 12–13. https://doi.org/10.1021/acsmedchemlett.8b00621
Guo X, Namekata K, Kimura A, Harada C, Harada T (2017) ASK1 in neurodegeneration. Adv Biol Regul 66:63–71. https://doi.org/10.1016/j.jbior.2017.08.003
Lanier M, Pickens J, Bigi SV, Bradshaw-Pierce EL, Chambers A, Cheruvallath ZS, Cole D, Dougan DR, Ermolieff J, Gibson T (2017) Structure-based design of ASK1 inhibitors as potential agents for heart failure. ACS Med Chem Lett 8:316–320. https://doi.org/10.1021/acsmedchemlett.6b00481
Liles JT, Corkey BK, Notte GT, Budas GR, Lansdon EB, Hinojosa-Kirschenbaum F, Badal SS, Lee M, Schultz BE, Wise S (2018) ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J Clin Investig 128:4485–4500. https://doi.org/10.1172/JCI99768
Okazaki T (2017) ASK family in infection and inflammatory disease. Adv Biol Regul 66:37–45. https://doi.org/10.1016/j.jbior.2017.10.001
Song J, Park KA, Lee WT, Lee JE (2014) Apoptosis signal regulating kinase 1 (ASK1): potential as a therapeutic target for Alzheimer’s disease. Int J Mol Sci 15:2119–2129. https://doi.org/10.3390/ijms15022119
Guo X, Harada C, Namekata K, Matsuzawa A, Camps M, Ji H, Swinnen D, Jorand-Lebrun C, Muzerelle M, Vitte PA, Ruckle T, Kimura A, Kohyama K, Matsumoto Y, Ichijo H, Harada T (2010) Regulation of the severity of neuroinflammation and demyelination by TLR-ASK1-p38 pathway. EMBO Mol Med 2:504–515. https://doi.org/10.1002/emmm.201000103
Takada E, Furuhata M, Nakae S, Ichijo H, Sudo K, Mizuguchi J (2013) Requirement of apoptosis-inducing kinase 1 for the induction of bronchial asthma following stimulation with ovalbumin. Int Arch Allergy Immunol 162:104–114. https://doi.org/10.1159/000353240
Mnich SJ, Blanner PM, Hu LG, Shaffer AF, Happa FA, O’Neil S, Ukairo O, Weiss D, Welsh E, Storer C, Mbalaviele G, Ichijo H, Monahan JB, Hardy MM, Eda H (2010) Critical role for apoptosis signal-regulating kinase 1 in the development of inflammatory K/BxN serum-induced arthritis. Int Immunopharmacol 10:1170–1176. https://doi.org/10.1016/j.intimp.2010.06.023
Fujisawa T, Takahashi M, Tsukamoto Y, Yamaguchi N, Nakoji M, Endo M, Kodaira H, Hayashi Y, Nishitoh H, Naguro I (2016) The ASK1-specific inhibitors K811 and K812 prolong survival in a mouse model of amyotrophic lateral sclerosis. Hum Mol Genet 25:245–253. https://doi.org/10.1093/hmg/ddv467
Kadowaki H, Nishitoh H, Urano F, Sadamitsu C, Matsuzawa A, Takeda K, Masutani H, Yodoi J, Urano Y, Nagano T (2005) Amyloid β induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death & Differentiation 12:19–24. https://doi.org/10.1038/sj.cdd.4401528
Mukherjee S, Zhelnin L, Sanfiz A, Pan J, Li Z, Yarde M, McCarty J, Jarai G (2019) Development and validation of an in vitro 3D model of NASH with severe fibrotic phenotype. Am J translational Res 11:1531
Ji N, Yang Y, Cai C-Y, Lei Z-N, Wang J-Q, Gupta P, Shukla S, Ambudkar SV, Kong D, Chen Z-S (2019) Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1-and ABCG2-overexpressing cancer cells. Cancer Lett 440:82–93. https://doi.org/10.1016/j.canlet.2018.10.007
Yan J, Zhang Y, Sheng G, Ni B, Xiao Y, Wang S, Wang T, Ma Y, Wang H, Wu H (2021) Selonsertib Alleviates the Progression of Rat Osteoarthritis: An in vitro and in vivo Study. Front Pharmacol 12:1787. https://doi.org/10.3389/fphar.2021.687033
Younossi ZM, Stepanova M, Lawitz E, Charlton M, Loomba R, Myers RP, Subramanian M, McHutchison JG, Goodman Z (2018) Improvement of hepatic fibrosis and patient-reported outcomes in non‐alcoholic steatohepatitis treated with selonsertib. Liver Int 38:1849–1859. https://doi.org/10.1111/liv.13706
Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP (2018) The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 67:549–559. https://doi.org/10.1002/hep.29514
Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, De Oliveira ACP (2019) Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci 20:2293. https://doi.org/10.3390/ijms20092293
Hailman E, Lichenstein HS, Wurfel MM, Miller DS, Johnson DA, Kelley M, Busse LA, Zukowski MM, Wright SD (1994) Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med 179:269–277. https://doi.org/10.1084/jem.179.1.269
Whitton P (2007) Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol 150:963–976. https://doi.org/10.1038/sj.bjp.0707167
Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B (2002) Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem 81:1285–1297. https://doi.org/10.1046/j.1471-4159.2002.00928.x
Zhao W, Xie W, Le W, Beers DR, He Y, Henkel JS, Simpson EP, Yen AA, Xiao Q, Appel SH (2004) Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J Neuropathology Experimental Neurol 63:964–977. https://doi.org/10.1093/jnen/63.9.964
Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL (1998) Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res 780:294–303. https://doi.org/10.1016/S0006-8993(97)01215-8
Gee MS, Kim S-W, Kim N, Lee SJ, Oh MS, Jin HK, Bae J-s, Inn K-S, Kim N-J, Lee JK (2018) A novel and selective p38 mitogen-activated protein kinase inhibitor attenuates LPS-induced neuroinflammation in BV2 microglia and a mouse model. Neurochem Res 43:2362–2371. https://doi.org/10.1007/s11064-018-2661-1
Himmelbauer MK, Xin Z, Jones JH, Enyedy I, King K, Marcotte DJ, Murugan P, Santoro JC, Hesson T, Spilker K, Johnson JL, Luzzio MJ, Gilfillan R, de Turiso FG (2019) Rational Design and Optimization of a Novel Class of Macrocyclic Apoptosis Signal-Regulating Kinase 1 Inhibitors. J Med Chem 62:10740–10756. https://doi.org/10.1021/acs.jmedchem.9b01206
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A (2018) Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 488. https://doi.org/10.3389/fncel.2018.00488
Gomez-Nicola D, Perry VH (2015) Microglial dynamics and role in the healthy and diseased brain: a paradigm of functional plasticity. The Neuroscientist 21:169–184. https://doi.org/10.1177/1073858414530512
Jimenez-Ferrer I, Jewett M, Tontanahal A, Romero-Ramos M, Swanberg M (2017) Allelic difference in Mhc2ta confers altered microglial activation and susceptibility to α-synuclein-induced dopaminergic neurodegeneration. Neurobiol Dis 106:279–290. https://doi.org/10.1016/j.nbd.2017.07.016
Garaschuk O, Verkhratsky A (2019) Physiology of microglia. Microglia:27–40. https://doi.org/10.1007/978-1-4939-9658-2_3
Henneman W, Sluimer J, Barnes J, Van Der Flier W, Sluimer I, Fox N, Scheltens P, Vrenken H, Barkhof F (2009) Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology 72:999–1007. https://doi.org/10.1212/01.wnl.0000344568.09360.31
Venegas C, Heneka MT (2017) Danger-associated molecular patterns in Alzheimer’s disease. J Leukoc Biol 101:87–98. https://doi.org/10.1189/jlb.3MR0416-204R
Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE (2013) Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78:631–643. https://doi.org/10.1016/j.neuron.2013.04.014
Perry VH (2012) Innate inflammation in Parkinson’s disease. Cold Spring Harbor perspectives in medicine 2:a009373. https://doi.org/10.1101/cshperspect.a009373
Boje KM, Arora PK (1992) Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res 587:250–256. https://doi.org/10.1016/0006-8993(92)91004-X
Chen S-H, Oyarzabal EA, Hong J-S (2016) Critical role of the Mac1/NOX2 pathway in mediating reactive microgliosis-generated chronic neuroinflammation and progressive neurodegeneration. Curr Opin Pharmacol 26:54–60. https://doi.org/10.1016/j.coph.2015.10.001
Mishra A, Bandopadhyay R, Singh PK, Mishra PS, Sharma N, Khurana N (2021) Neuroinflammation in neurological disorders: pharmacotherapeutic targets from bench to bedside. Metab Brain Dis 36:1591–1626. https://doi.org/10.1007/s11011-021-00806-4
Jones JH, Xin Z, Himmelbauer M, Dechantsreiter M, Enyedy I, Hedde J, Fang T, Coomaraswamy J, King KW, Murugan P (2021) Discovery of Potent, Selective, and Brain-Penetrant Apoptosis Signal-Regulating Kinase 1 (ASK1) Inhibitors that Modulate Brain Inflammation In Vivo. J Med Chem 64:15402–15419. https://doi.org/10.1021/acs.jmedchem.1c01458
Xin Z, Himmelbauer MK, Jones JH, Enyedy I, Gilfillan R, Hesson T, King K, Marcotte DJ, Murugan P, Santoro JC (2020) Discovery of CNS-penetrant apoptosis signal-regulating kinase 1 (ASK1) inhibitors. ACS Med Chem Lett 11:485–490. https://doi.org/10.1021/acsmedchemlett.9b00611
Methaneethorn J, Naosang K, Kaewworasut P, Poomsaidorn C, Lohitnavy M (2020) Development of a Physiologically-Based Pharmacokinetic Model of Delta(9)-Tetrahydrocannabinol in Mice, Rats, and Pigs. Eur J Drug Metab Pharmacokinet 45:487–494. https://doi.org/10.1007/s13318-020-00616-6
Methaneethorn J, Poomsaidorn C, Naosang K, Kaewworasut P, Lohitnavy M (2020) A Delta(9)-Tetrahydrocannabinol Physiologically-Based Pharmacokinetic Model Development in Humans. Eur J Drug Metab Pharmacokinet 45:495–511. https://doi.org/10.1007/s13318-020-00617-5
Ya KM, Methaneethorn JP, Tran QBP, Trakulsrichai SM, Wananukul WM, Lohitnavy MP (2021) Development of a Physiologically Based Pharmacokinetic Model of Mitragynine, Psychoactive Alkaloid in Kratom (Mitragyna Speciosa Korth.), In Rats and Humans. J Psychoact Drugs 53:127–139. https://doi.org/10.1080/02791072.2020.1849877
Funding
This research was supported by Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN21C1139, Republic of Korea).
Author information
Authors and Affiliations
Contributions
JHL, SHJ: Investigation, Methodology, Writing-original draft, JSL: Investigation, Methodology, SA, HY: Methodology, Formal analysis, Resources, SHK: Conceptualization, Wiriting-review, Supervision, JSS: Conceptualization, Wiriting-review, Funding acquisition, Supervision.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical Approval
All applicable national and institutional guidelines for the care and use of animals were followed. The protocols for animal experiments were approved by the Animal Ethics Committee of Korea Research Institute of Chemical Technology with the code number of 2022-7 F-02-01(approval date: 2022-02-09).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J.H., Ji, S.H., Lim, J.S. et al. Anti-neuroinflammatory Effects and Brain Pharmacokinetic Properties of Selonsertib, an Apoptosis signal-regulating Kinase 1 Inhibitor, in mice. Neurochem Res 47, 3829–3837 (2022). https://doi.org/10.1007/s11064-022-03777-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03777-9