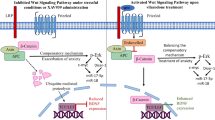
Abstract
Evidence shows that inflammatory responses may encompass the onset of severe depressive illness. Traditionally used licorice contains 18β-glycyrrhetinic acid (18βGA), which has been demonstrated to reduce inflammation and oxidative stress. This study investigates the antidepressant effects of 18βGA and the underlying mechanism in rats exposed to chronic unpredictable mild stress (CUMS). Wistar rats were exposed to CUMS for 36 consecutive days to establish depression. 18βGA (10, 20, and 50 mg/kg) or fluoxetine was given once daily (from day 30 to day 36). Thereafter, behavior parameters (sucrose preference test, forced-swimming test, open-field test, body weight), pro-inflammatory cytokines, neurotransmitters, adrenocorticotropic hormone (ACTH), corticosterone (CORT), and liver biomarkers were studied. Immunohistochemistry and western blot analyses were conducted to investigate the protein’s expression. 18βGA (20 and 50 mg/kg) treatment increased sucrose intake, locomotion in the open-field test, decreased immobility time in the forced swim test, and improved body weight in CUMS-exposed rats. The therapy of 18βGA dramatically declined cytokines, ACTH and CORT and improved 5HT and norepinephrine in CUMS rats. Furthermore, BDNF and TrkB proteins were down-regulated in CUMS group, which was increased to varying degrees by 18βGA at doses of 20 and 50 mg/kg. Therefore, 18βGA ameliorates depressive-like behavior persuaded by chronic unpredictable mild stress, decreases neuroinflammation, liver biomarkers, stress hormones, and improves body weight, brain neurotransmitter concentration via activating on BDNF/TrkB signaling pathway in both PFC and hippocampus in rats.
Graphical Abstract

Similar content being viewed by others
Introduction
World Health Organization indicated that depression is a severely devastating and life-threatening mental condition that affects an estimated 300 million individuals of all ages worldwide [1]. The outbreak of the COVID-19 pandemic has also increased many factors that contribute to poor mental health [2]. By 2030, depression is expected to be the world’s second most crippling burden [3, 4]. Anhedonia, helplessness sentiments, limited physical activity, unpleasant moods, loss of weight, low mood, and cognitive issues are pathological phases of depression [5, 6]. Despite significant advancements in the etiology of depression, most antidepressants now used in clinical practice have modest effectiveness, uncertain safety, low response rates, and severe adverse effects [7, 8]. Consequently, the discovery of better, safer, and more effective pharmaceuticals is continually required across the globe. Antidepressants based on the monoaminergic insufficiency theory, in particular, play an important role in medical practice, but they are also linked to treatment resistance in 33% of cases [9].
In addition, the research implies that oxidative stress and inflammatory illnesses are associated with depression [10, 11]. Several research studies have linked the production of pro-inflammatory mediators and depressive symptoms [6, 12]. Furthermore, it has been shown that the cytokines interleukin-1beta (IL-1beta), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha) increase in animals experiencing depression [6, 13]. Despite the fact that the pathophysiology of depression development has been thoroughly studied, further research is required to comprehend the important molecular mechanism. Furthermore, most studies on depression concentrate on alterations in the prefrontal cortex (PFC) and the hippocampal tissues [14, 15].
Moreover, natural herbal substances with strong biological activity have recently received a lot of attention. Licorice’s main ingredient, 18β-Glycyrrhetinic acid (Molecular Weight: 470.68; PubChem Substance ID: 24895033), a pentacyclic triterpene, has sparked a lot of attention due to its pharmacological properties. Enoxolone, glycyrrhetin, 3β-hydroxy-11oxo-18β, 20β-olean12-en-29oic acid, uralenic acid, or sub-glycyrrhelinic acid are all names for 18β-glycyrrhetinic acid. 18β-glycyrrhetinic acid have been described to reduce airway inflammation [16, 17], alpha-naphthylisothiocyanate-induced cholestasis [18], D-GalN/LPS-induced acute liver injury [19], cyclophosphamide-induced hepatotoxicity [20], prostate tumor [21], lung cancer [22], gastric tumor [23], streptozotocin-induced diabetes [24], pulmonary arterial hypertension [25], visceral leishmaniasis [26], rotavirus replication [27], bacterial load [28, 29], antipsychotic-induced hyperprolactinemia [30], myocardial infarction [31], hyperoxia exposure effects in neonatal rats [32], rheumatoid arthritis [33], fructose-induced nephropathy [34], radiation-induced skin damage [35], cisplatin-induced oxidative intestinal damage [36].
Moreover, 18β-glycyrrhetinic acid has also been known for inhibition of microglia activation and stimulation of remyelination to decrease experimental autoimmune encephalomyelitis [37], neuroprotection in ischemic stroke through HMGB1 inhibition [38], reduces demyelination by altering the M1/M2 phenotype of the microglia [39], improvement in overall cerebral ischemia/reperfusion [40], neuroprotective effects through JAK1/STAT1 signaling pathway [41]. In addition, adjunct therapy with Glycyrrhiza Glabra extract (Licorice) in patients rapidly improved outcomes in depression [42] and immobility time in the mouse model [43].
Since 18β-Glycyrrhetinic acid (18βGA) has been revealed for antiinflammatory and antioxidant capabilities. This research intended to observe the antidepressant-like influence of 18βGA and the underlying mechanism in chronic unpredictable mild stress (CUMS) rats.
CUMS is a well-known, established paradigm in animal research that has been extensively used to study depressive disorders and medicines [44]. The CUMS paradigm, which is significant, accurately reproduces various behavioral and neuroendocrine changes in depressed individuals. Many behavioral changes, including loss of enjoyment and apathy, are carried on by CUMS in the vast majority of animals. These behavioral modifications and changes in a few endocrine and neurological factors are comparable to those in depressed people. CUMS provides predictive, construct, and face validity. By increasing the production of pro-inflammatory mediators such as IL-1β, TNF-α, and IL-6, CUMS causes an inflammation associated with depressive-like behavior [12].
Additionally, there is growing evidence that CUMS may alter the HPA axis and raise blood corticosterone levels (CORT) and ACTH levels. Additionally, defective negative feedback in the HPA may cause the CUMS-induced rise in serum corticosterone levels [45]. To investigate the relationship between 18βGA and stress level, its effects on CORT and ACTH levels were investigated in CUMS animals. Increasing evidence also suggests that stress may harm the liver in addition to causing mental changes, with oxidative stress and inflammation possibly playing a role [46]. To comprehend the connection between chronic stress, neuroinflammation, and altered liver indicators, we also examined how CUMS altered liver enzymes and how 18βGA impacted the altered liver markers.
Further, the hippocampus, prefrontal neurogenesis, and the BDNF-TrkB signaling pathway may offer novel observations into the discovery of antidepressants [47, 48]. Through its receptor, tropomyosin receptor kinase B (TrkB), brain-derived neurotrophic factor (BDNF) functions as an antidepressant. Moreover, TrkB, as a receptor for BDNF, is linked to neurotrophic and neuroprotective functions [11, 49]. The aforementioned data demonstrates that the BDNF/TrkB pathway, via its neurogenesis and antioxidant events, is expected to play a crucial role in developing and preventing depression. Meanwhile, because 18βGA has the neuroprotective and antioxidant capacity [32, 50, 51], we hypothesized that 18βGA could activate the BDNF/TrkB signaling pathway to promote neurogenesis and protection of neurons, and antioxidant functions, resulting in an antidepressant. To test this hypothesis, researchers used a rat depression model subjected to CUMS, which may better mimic the fundamental symptoms of human sadness [52, 53]. On this premise, the antidepressant effects of 18βGA were evaluated using regularly utilized depressive-behavior assays. Molecular techniques such as IHC, western blot, and multiple kits were utilized to understand the pathway’s protein levels, neuroinflammation markers, stress hormone levels, and liver markers. The first-ever detailed analysis of 18βGA’s antidepressant mechanisms was then conducted to determine if the BDNF/TrkB signaling pathway was in charge of regulating the neurotrophic and antioxidant defenses.
Materials and Methods
Drugs and Compounds
18βGA (97 percent purity, Sigma-Aldrich, USA, Molecular Weight: 470.68; PubChem Substance ID: 24895033) and FXT (Cadila Pharmaceuticals Ltd, India) were obtained from the appropriate suppliers. ERBA Diagnostics, Mannheim, provided the ALP, ALT, and AST kits. Krishgen Biosystems, India, provided kits for cytokine, corticosterone, and ACTH ELISA kit. Abcam provided all of the antibodies (Cambridge, United Kingdom). Antibodies to BDNF, TrkB, and β-actin were procured from Santa Cruz Biotechnology, Inc., USA, and goat anti-Rabbit from Abcam, USA. Commercial ELISA kits for IL-1beta, IL-6, and TNF-alpha estimation were received from Krishgen Biosystems, Mumbai. ELISA kits for serotonin (5-HT, 5-hydroxy tryptamine) and norepinephrine from Bioassay Technology Laboratory, Shanghai. All other chemicals and reagents were acquired from SRL Pvt. Ltd., India, and Sigma-Aldrich, USA. 18β-glycyrrhetinic acid and FXT were diluted in distilled water with 0.5 percent sodium–carboxymethylcellulose. In psychiatric patients, oral therapy of 18βGA and FXT was chosen as the most favored form of administration.
Animals
Wistar rats (male and female, 9–12 weeks, 140–160 g) were bought from the NIPERS’s Central Animal Facility in Mohali, Punjab, India. Rodents were maintained in a light–dark cycle of 12 h each, with a temperature environment of 24 ± 2 °C. A commercial pellet chow meal (Ashirwad Industries Ltd., Chandigarh, India) and unlimited water from the tap were available. Before beginning the studies, the rats were given at least 1 week to adapt to laboratory settings. All techniques were accomplished with the approval of the Institutional Animal Ethics Committee and strict adherence to ethics and guidelines established by the CPCSEA, Government of India.
CUMS Exposure, Body Weight, and Treatment
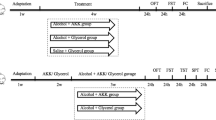
Animals were subjected to a range of moderate psychological challenges, as previously reported [6, 54], with slight changes in accordance with Table 1’s timeline. Three rats were housed in each cage, and the animals were given free access to food and water. The rats in the CUMS and CUMS plus 18βGA/FXT-administered groups, on the other hand, were segregated in separate cages for 36 days and subjected to the following ten types of stressors: (1) clipping the tail for 3 min (1 cm from the tail’s tip); (2) water denial for 24 h; (3) cage slanting for 24 h (45°); (4) 5 min of warm swimming at 40 °C; (5) 24 h of fasting; (6) night-time lighting; (7) 10 min overhang; (8) 5 min cold swimming (8 °C); (9) wet cushion overnight; (10) physical restraint for 3 h. Every day between 9:00 AM and 6:00 PM, these stressors were used (except for the 24 h stressors) in a changeable random sequence for 36 days, with each stressor repeated three times. However, 24 h of food deprivation and overhang (10 min) were repeated six times. By avoiding exposure to the same stressful situation for two days, prophecy and adjustment to that stressor were halted.
Further, eight groups of 48 rats were chosen at randomly (n = 6) after acclimating to the laboratory conditions: (1) non-CUMS (Control) + Vehicle; (2) non-CUMS + 18β-glycyrrhetinic acid (50 mg/kg); (3) non-CUMS + FXT (10 mg/kg); (4) CUMS group: Vehicle; (5–7) three 18βGA-treated groups (10, 20, and 50 mg/kg of 18βGA plus CUMS); and (8) CUMS plus 10 mg/kg of clinically used medicine FXT intragastrically. 18βGA and FXT doses were established based on earlier research [6, 54, 55]. To administer medication intragastrically, the stomach was first identified as being in the upper left quadrant of the belly. The stomach was then carefully accessed by delicately slipping the feeding needle through the abdominal wall into the stomach. Over the course of the last seven days of the CUMS challenges, 18βGA and FXT were given once a day (from day 30 to day 36). From day 30 to day 36, the non-CUMS control group and CUMS rats were administered the same dose of vehicle (0.5 percent of Na-CMC, intragastrically). Further, the weekly body weight of the animals was monitored for 6 weeks.
Behavioral Evaluation
After the CUMS procedure was completed, trials were conducted using the typical behavior paradigm in a soundproofed environment indicated in Table 2, namely, open-field test (OFT) on day 37, SPT on days 38 and 39, and FST on days 42 and 43. One test was conducted daily, with a 24-h gap in SPT and FST. A skilled observer who was unaware of the groupings conducted the behavioral experiments. Because FXT is often used to manage depressive behaviors produced by CUMS [56], we utilized FXT as a positive control. Following the results of behavioral testing, animals were slaughtered for cytokines, ACTH, CORT, and neurotransmitter analysis. Using Western blotting and IHC, the relative expression of the TrkB and BDNF proteins in the PFC and hippocampus tissues was examined. Table 2 shows the complete timeline for the experimental illustration.
Open-Field Test
OFT measured the rats’ locomotor and exploratory activity under bright ambient light [56, 57]. The rats (n = 6) were put singly in the central area and given 5 min to investigate the new environment. The total squares crossed number, the rearing number, grooming events (coat washing), total distance traveled, and frequency of entering the center were recorded using Orchid’s video tracking system. Following each rat’s exposure, the OFT system was wiped with 50% ethanol to eliminate olfactory nodding.
Sucrose Preference Test
The sucrose preference test (SPT) was utilized to examine anhedonic behavior and was carried out with slight adjustments as previously described [6, 52]. The SPT measures the decrease in sucrose liking and ingestion to determine the severity of depression in rats. This indicates alterations in the susceptibility of rats to incentives [58]. One percent sucrose solution in two bottles per rat was available during the initial training. Then rats are restricted to food and regular drinking water for the next 24 h. Each rat in all groups (n = 6) was given two bottles of one percent sucrose solution and a bottle of water for unrestricted drinking for 3 h. Sucrose preference (percent) = (sucrose intake/total fluid consumption) × 100.
Forced Swim Test
FST is used to detect the severity of despair in animals, and the procedure is based on past research [59, 60]. Animals (n = 6) were trained for the first 15 min before the actual test in a cylinder tank (50 × 20 cm) with water (25 ± 2 °C, 30 cm deep). The immobility period during which an individual animal was compelled to swim for 5 min was assessed 24 h later. After each rat’s test, the water was changed to fresh, clean water.
Tissue and Blood Samples
The blood sample from the sinuses of the retro-orbital region was collected in ethylene-diamine-tetraacetate-coated vials to measure inflammatory cytokines after the behavioral analysis on day 44. Commercial ELISA kits were used to detect plasma cytokine levels in accordance with the manufacturer’s recommendations. The IL-1beta, IL-6, and TNF-alpha were measured in picograms per milliliter (pg/mL) in each group having six animals. At 450 nm, each well’s absorbance was evaluated using a microplate reader (BioTek in Germany). Following that, the serum was collected and kept at − 20 °C after centrifugation at 1500 g for 15 min at 4 °C. Commercial ACTH, CORT for stress, ALT, AST, and ALP kits were used to measure the liver enzymes in each group having six animals. Further, animals were euthanized; half of the animals (n = 3) were used for IHC while the other half (n = 3) for Western blot analysis and neurotransmitter estimation.
Serotonin and Norepinephrine Estimation in Brain Tissues
PFC and hippocampal tissue samples from both hemispheres were utilized for neurotransmitter estimates in half of the rats (n = 3). After homogenization, the tissues were centrifuged for 20 min at 4 °C, 8000 rpm. As directed by the manufacturer, kits were used to quantify serotonin and norepinephrine in the supernatant (Bioassay Technology Laboratory, Shanghai).
Immunohistochemistry
As described earlier, hippocampal and PFC BDNF levels were examined using immunohistochemistry [61]. Cold 0.9% NaCl (150 ml) and fixative (150 ml, cold 4% paraformaldehyde) were administered to half of the animals (n = 3) transcardially. Following detachment, the brains were postfixed in 4% PFA at a temperature of 4 °C. Using a vibratome, each brain was coronally sectioned at 10 μm (Leica Instruments, Germany). BDNF (1:200) was utilized as the principal antibody. 3,3′-diamiobenzidine was added after the goat anti-rabbit secondary antibody (1:500) and incubated for 10 min. The color development time was determined by observing slides under a microscope. After the development was complete, slices were socked in distilled water to halt the coloring, then counterstained with hematoxylin. Images of stained tissues were obtained (Leica, Germany). The optical density of BDNF expression in CA1 and PFC was measured using ImageJ software (NIH, USA).
Western Blot Analysis
Three animals from each group’s hippocampi and PFC were completely mashed in phosphatase and protease inhibitor-containing RIPA buffer. (Sigma-Aldrich). Lysates were centrifuged at 4 °C for 15 min at 15,000 revolutions per minute (rpm). Using a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), the samples were divided, and the proteins were then transferred to PVDF membranes (Millipore, USA). These membranes were incubated with the primary antibodies for BDNF (Santa Cruz Biotechnology, Inc., USA; 1:1000), TrkB (Santa Cruz Biotechnology, Inc., USA; 1:1000), and anti-β-actin (Santa Cruz Biotechnology, Inc., USA; 1:1000) overnight at 4 °C after being blocked in 5% BSA for 3 h at room temperature. At a temperature of 25 °C, the membranes were treated with the secondary antibodies that had been conjugated to horseradish peroxidase for 2 h. To see the signals, a more advanced chemiluminescence detecting apparatus was used (Bio-Rad, USA). The relative quantification of protein bands was calculated using Image J software from the National Institutes of Health in the United States. The ratio of band intensities to β-actin was calculated to estimate the relative protein expression level.
Analytical Statistics
The data were statistically evaluated and reported as mean ± S.E.M. using GraphPad Prism version 9.00, California, USA. Posthoc one- and two-way analysis of variance Tukey’s multiple comparison (TMC) tests were used for the data analysis and bar graph creation. The p-value cutoff for statistical significance was chosen at 0.05.
Results
18βGA Treatment’s Effect in OFT and Body Weight
In the OFT, substantial variations in the square crossing numbers (F (7, 14) = 33.14, p < 0.0001), rearing number (F (7, 14) = 17.67, p < 0.0001), grooming number (F (7, 14) = 7.701, p < 0.0001), total distance travelled (F (7, 14) = 20.02, p < 0.0001), and frequency of entering in the center (F (14, 120) = 23.58, p < 0.0001) were found using a one-way analysis of variance posthoc TMC testing. CUMS group in OFT exhibited a substantial lessening in the squares traversed number (F (7, 14) = 33.14, p < 0.0001; Fig. 1A; one-way ANOVA), rearing number (F (7, 14) = 17.67, p < 0.0001; Fig. 1B; one-way ANOVA), grooming number (F (7, 14) = 7.701, p < 0.001; Fig. 1C; one-way ANOVA), the total travel distance (F (7, 14) = 20.02, p < 0.0001; Fig. 1D; One-way ANOVA), and the frequency of entering in the center (F (7, 14) = 14.36, p < 0.0001; Fig. 1E; One-way ANOVA) than non-CUMS control rats. On comparison with CUMS animals, 18βGA at dose of 20 and 50 mg/kg, intragastrically and FXT at dose of 10 mg/kg, intragastrically for seven days substantially increased the square crossings (F (7, 14) = 33.14, p < 0.05, 0.001, and 0.0001; Fig. 1A), rearing number (F (7, 14) = 17.67, p < 0.001, 0.0001, 0.0001; Fig. 1B), grooming number (F (7, 14) = 7.701, p < 0.01, 0.001, 0.0001; Fig. 1C). 18βGA (50 mg/kg) and FXT (10 mg/kg) also significantly altered total distance travelled (F (7, 14) = 20.02, p < 0.0001, and 0.0001; Fig. 1D), and frequency of entering in center (F (7, 14) = 14.36, p < 0.0001, and 0.001; Fig. 1E) in the OFT correspondingly. In contrast, 18βGA (10 mg/kg, intragastrically) had no significant impact on the number of squares crossed (F (7, 14) = 33.14, p > 0.05; Fig. 1A), rearing number (F (7, 14) = 17.67, p > 0.05; Fig. 1B), grooming number (F (7, 14) = 7.701, p > 0.05; Fig. 1C), total distance travelled (F (7, 14) = 20.02, p > 0.05; Fig. 1D), and frequency of entering in center (F (7, 14) = 14.36, p > 0.05; Fig. 1E) versus the CUMS group. In addition, versus vehicle-treated non-CUMS control group, 18βGA administration (50 mg/kg, intragastrically, 7 days) and FXT in non-CUMS rats, had no effect on the number of square crossed [F (7, 14) = 33.14, p > 0.05 (18βGA), p > 0.05 (FXT); Fig. 1A], rearing number (F (7, 14) = 17.67, p > 0.05, and 0.05; Fig. 1B), grooming number (F (7, 14) = 7.701, p > 0.05 (18βGA), p > 0.05 (FXT); Fig. 1C), total distance travelled (F (7, 14) = 20.02, p > 0.05, and 0.05; Fig. 1D), and frequency of entering in center (F (7, 14) = 14.36, p > 0.05 (18βGA), p > 0.05 (FXT); Fig. 1E).
In the OFT, 18βGA therapy influences square crossing numbers (A), rearing number (B), grooming counts (C), total distance traveled (D), frequency of entering the center (E) in the CUMS and non-CUMS rats. Percent weekly weight changes (F) after 18βGA therapy are also presented in the CUMS and non-CUMS rats. The results are displayed as mean ± S.E.M. of 6 animals in each group with ###p < 0.001, ####p < 0.0001 vs non-CUMS rats and *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs CUMS rats, “one-way (A–E) and two-way (F) analysis of variance TMC test”. CUMS stands for chronic unpredictable mild stress; 18βGA stands for 18β-glycyrrhetinic acid; OFT is for the open field test; i.g. stands for intragastrically
Further, we measured weight gain weekly since it is also an alternative sign of depression. The CUMS group gained considerably less body weight than the control group at weeks 5 and 6, as seen in Fig. 1F (p < 0.001 and 0.0001, respectively). In contrast, the CUMS + 18βGA (20, 50 mg/kg, intragastrically) and FXT (10 mg/kg, intragastrically) groups depicted significant larger percent weight gain than the CUMS group (p < 0.05, 0.0001, 0.0001) on weeks 6 and 7. There was no discernible difference in the rate of body weight growth between the CUMS, 18βGA (10 mg/kg, intragastrically)-treated CUMS rats (all p > 0.05). There was no significant percent weight increase in all groups from week 1 to week 4 of the study.
18βGA’s Effects on FST
One-way ANOVA demonstrated significant variations in immobility duration across the groups in the FST (Fig. 2A) [F (7, 40) = 39.88, p < 0.0001]. Posthoc TMC tests revealed a substantial increase in immobility duration (F (7, 40) = 39.88, p < 0.0001) on exposure of CUMS as demonstrated in Fig. 2A. In the FST rat model, chronic treatment with 18βGA (20, 50 mg/kg, intragastrically, Fig. 2A) and FXT (10 mg/kg, intragastrically, Fig. 2A) substantially reduced immobility length in CUMS rats (F (7, 40) = 39.88, p < 0.05, 0.0001, and 0.0001). However, 18βGA (10 mg/kg, intragastrically, Fig. 2A) did not influence the duration of immobility in CUMS rats (F (7, 40) = 39.88, p > 0.05). Similarly, versus non-CUMS control group, 18βGA (50 mg/kg, intragastrically, Fig. 2A) or FXT (Fig. 2A) administration for 7 days did not influence immobility time (F (7, 40) = 39.88, p > 0.05 (18βGA), p > 0.05) in non-CUMS animals. Compared to FLX in the non-CUMS animals, FXT did not affect the immobility duration in the CUMS group (p > 0.05).
The effects of CUMS and 18βGA on the FST immobility time (A) and sucrose preference % (B). Rats were exposed to the FST and SPT after receiving 18βGA (10, 20, and 50 mg/kg, intragastrically), FXT (10 mg/kg, intragastrically), or vehicle. The results are displayed as mean ± S.E.M. of 6 animals in each group with ###p < 0.001, ####p < 0.0001 vs non-CUMS rats and *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs CUMS rats, “one-way analysis of variance then TMC test”. CUMS stands for chronic unpredictable mild stress; 18βGA stands for 18β-glycyrrhetinic acid; FST stands for forced swimming test; SPT is for sucrose preference test; i.g. stands for intragastrically
Effects of 18βGA on Percentage Sucrose Preference
The percent of sucrose ingested varied significantly across the treatment groups, as shown in Fig. 2B [F (7, 40) = 9.587, p < 0.0001]. Sucrose consumption was considerably lower in the CUMS rats (F (7, 40) = 9.587, p < 0.001) on a one-way analysis of variance posthoc TMC tests vs. without CUMS control animals. On the contrary, 18βGA (20 and 50 mg/kg, intragastrically) and FXT depicted increase in sucrose consumption in CUMS animals considerably (F (7, 40) = 9.587, p < 0.05, 0.01, and 0.001) than vehicle-treated CUMS group. Nevertheless, there was no significant difference in % sucrose intake between the 18βGA (10 mg/kg, intragastrically)-treated CUMS rats and the vehicle-treated CUMS rats (F (7, 40) = 9.587, p > 0.05). Additionally, 18βGA administration (50 mg/kg, 7 days) or FXT in non-CUMS rats, had no effect on the % sucrose intake (F (7, 40) = 9.587, p > 0.05 (18βGA), p > 0.05) versus non-CUMS control group. FXT had no impact on the CUMS group’s sucrose consumption compared to FLX in the non-CUMS animals (p > 0.05).
18βGA’s Effects on Hepatic Biomarkers, ACTH, and CORT Levels
The influence of 18βGA on hepatic markers was investigated and shown in Fig. 3A. Significant variations in the liver markers ALT, AST, and ALP levels [F (7, 120) = 21.33, p < 0.0001, 0.0001, and 0.01] were found in CUMS rats versus non-CUMS control group, according to a two-way analysis of variance. On comparison to CUMS rats, 18βGA or FXT treatment substantially lowered the high levels of liver enzymes ALT (F (7, 120) = 21.33, p < 0.001 (18βGA, 20), p < 0.001 (18βGA, 50), p > 0.05 (FXT, 10), AST (F (7, 120) = 21.33, p < 0.01 (18βGA, 20), p < 0.05 (18βGA, 50), p > 0.05 (FXT, 10) and ALP (F (7, 120) = 21.33, p < 0.01 (18βGA, 20), p < 0.0001 (18βGA, 50), p < 0.01(FXT, 10). However, 18GβA (10 mg/kg, intragastrically) significantly unchanged AST, ALT, or ALP values in the CUMS animals (p > 0.05). Furthermore, 18βGA administration (50 mg/kg, intragastrically, 7 days) or FXT in non-CUMS rats did not affect the levels of ALT, AST, or ALP (F (7, 120) = 21.33, p > 0.05) versus non-CUMS control group. FXT also failed to correct ALT and AST concentrations in the CUMS rats.
Effects of CUMS and 18βGA on serum markers of the liver (A), ACTH (B), and CORT (C). The results are displayed as mean ± S.E.M. of 6 animals in each group with ##p < 0.01, ####p < 0.0001 vs non-CUMS rats and *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs CUMS rats, “one-way (ACTH and CORT) and two-way (liver markers) analysis of variance then TMC test”. ALP stands for alkaline phosphatase; CUMS stands for chronic unpredictable mild stress; ALT stands for alanine aminotransferase; 18βGA stands for 18β-glycyrrhetinic acid; i.g. stands for intragastrically; AST stands for aspartate aminotransferase; ACTH stands adrenocorticotropic hormone and CORT for corticosterone
Further, ACTH levels in the serum varied significantly across the treatment groups, as shown in Fig. 3B [F (7, 40) = 9.658, p < 0.0001]. ACTH level in the serum was considerably increased in the CUMS rats (F (7, 40) = 9.658, p < 0.0001) on a one-way analysis of variance posthoc TMC tests vs. without CUMS control animals. On the contrary, 18βGA (50 mg/kg, intragastrically) and FXT depicted a decrease in ACTH levels in CUMS animals considerably (F (7, 40) = 9.658, p < 0.001 and 0.05) than vehicle-treated CUMS group. Nevertheless, there was no significant difference in ACTH levels between the 18βGA (10 and 20 mg/kg, intragastrically)-treated CUMS rats and the vehicle-treated CUMS rats (F (7, 40) = 9.658, p > 0.05). Additionally, 18βGA administration (50 mg/kg, 7 days) or FXT in non-CUMS rats, had no effect on the ACTH levels (F (7, 40) = 9.658, p > 0.05 (18βGA), p > 0.05) versus non-CUMS control group.
Furthermore, as shown in Fig. 3C, the CORT level in the serum was altered significantly across the treatment groups, as shown in Fig. 3C [F (7, 40) = 11.66, p < 0.0001]. CORT level in the serum was considerably increased in the CUMS rats (F (7, 40) = 11.66, p < 0.0001) on a one-way analysis of variance posthoc TMC tests vs. without CUMS control animals. 18βGA (50 mg/kg, intragastrically) and FXT depicted a decrease in CORT levels in CUMS animals considerably (F (7, 40) = 11.66, p < 0.0001 and 0.05) than the vehicle-treated CUMS group. However, there was no significant difference in CORT levels between the 18βGA (10 and 20 mg/kg, intragastrically)-treated CUMS rats and the vehicle-treated CUMS rats (F (7, 40) = 11.66, p > 0.05). Additionally, 18βGA administration (50 mg/kg, 7 days) or FXT in non-CUMS rats, had no effect on the CORT levels (F (7, 40) = 11.66, p > 0.05 (18βGA), p > 0.05) versus non-CUMS control group. In contrast to FLX in the non-CUMS animals, FXT did not affect the ALT, ALP, ACTH, or CORT levels in the CUMS group (p > 0.05), except for AST (p < 0.05).
18βGA’s Effects on Cytokines
The impact of 18βGA on cytokine levels in the plasma was then investigated and illustrated in Fig. 4. In the plasma of rats, two-way ANOVA displayed differences in the cytokines [F (7, 120) = 23.11, p < 0.0001]. The IL-1beta (F (7, 120) = 23.11, p < 0.0001), IL-6 (F (7, 120) = 23.11, p < 0.0001), and TNF-alpha (F (7, 120) = 23.11, p < 0.0001) levels in the plasma were significantly elevated when the rats were exposed to chronic CUMS, according to the posthoc TMC test. On comparison to CUMS rats, treatment with 18βGA (50 mg/kg, intragastrically) and FXT (10 mg/kg, intragastrically) significantly reduced IL-1beta (F (7, 120) = 23.11, p < 0.0001 (18βGA, 50 mg/kg), p < 0.01 (FXT, 10 mg/kg), IL-6 (F (7, 120) = 23.11, p < 0.05 (18βGA, 50 mg/kg), p < 0.01(FXT, 10 mg/kg), and TNF-alpha (F (7, 120) = 23.11, p < 0.0001 (18βGA, 50 mg/kg), p < 0.0001(FXT, 10 mg/kg) levels. However, 18βGA (10 and 20 mg/kg, intragastrically) had no effect on pro-inflammatory cytokines in the CUMS rats (F (7, 120) = 23.11, p > 0.05, p > 0.05). Similarly, 1-week 18βGA administration (50 mg/kg, intragastrically) or FXT in non-CUMS rats, had little influence on IL-1beta (F (7, 120) = 23.11, p > 0.05), IL-6 (F (7, 120) = 23.11, p > 0.05), and TNF-alpha (F (7, 120) = 23.11, p > 0.05) levels compared to the non-CUMS control group.
The effects of CUMS and 18βGA on IL-1beta, IL-6, and TNF-alpha in plasma are shown in Fig. 4. The results are presented as mean ± S.E.M. of 6 animals in each group with ####p < 0.0001 vs non-CUMS rats and *p < 0.05, **p < 0.01, ****p < 0.0001 vs CUMS rats, “two-way analysis of variance then TMC test”. TNF-alpha, tumor necrosis factor; CUMS stands for chronic unpredictable mild stress; 18βGA stands for 18β-glycyrrhetinic acid; IL-1beta stands for interleukin-1 beta; IL-6, interleukin-6; i.g. stands for intragastrically
Influence of 18βGA on Norepinephrine Levels in the Hippocampal and PFC Tissues
Two-way analysis of variance showed substantial alterations in norepinephrine levels in the PFC and the hippocampus [F (7, 32) = 52.69, p < 0.0001] across the treatment groups, as shown in Fig. 5A. Posthoc TMC test revealed a substantial decrease in norepinephrine levels in the hippocampus (F (7, 32) = 51.09, p < 0.0001) and PFC (F (7, 32) = 51.09, p < 0.0001) in CUMS rats versus non-CUMS control group. On the contrary, 18βGA (20 and 50 mg/kg) or FXT administration for 7 days increased norepinephrine levels in the hippocampus [F (7, 32) = 51.09, p < 0.01 (18βGA, 20), p < 0.0001 (18βGA, 50), p < 0.05 (FXT, 10)] and PFC [F (7, 32) = 51.09, p < 0.05 (18βGA, 20), p < 0.0001 (18βGA, 50), p < 0.05 (FXT, 10)] in CUMS rats. Further, 18βGA (10 mg/kg, intragastrically) had no effect on the norepinephrine levels in the CUMS rats in the hippocampus (F (7, 32) = 51.09, p > 0.05) and PFC (F (7, 32) = 51.09, p > 0.05) versus vehicle-treated CUMS rats. Furthermore, 18βGA administration (50 mg/kg, intragastrically, 7 days) or FXT (10 mg/kg, intragastrically, 7 days) in non-CUMS rats, had nonsignificant influence on norepinephrine in the hippocampus (F (7, 32) = 51.09, p > 0.05, and > 0.05) and PFC (F (7, 32) = 51.09, p > 0.05, and > 0.05) versus non-CUMS control group.
The effects of 18βGA on norepinephrine (A) and serotonin (B) levels in the PFC and the hippocampus in CUMS and non-CUMS rats. The results are displayed as mean ± S.E.M. of 3 animals in each group with ####p < 0.0001 vs non-CUMS rats and *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs CUMS rats, “two-way analysis of variance then TMC test”. CUMS stands for chronic unpredictable mild stress; 18βGA stands for 18β-glycyrrhetinic acid; i.g. stands for intragastrically
18βGA’s Effects on the Levels of Serotonin in the PFC and Hippocampus
Substantial alterations were detected using a two-way analysis of variance in 5-HT levels in the PFC and the hippocampal tissues [F (7, 32) = 28.23, p < 0.0001] across the treatment groups, as revealed in Fig. 5B. In the comparison of CUMS rats to non-CUMS controls, posthoc TMC test revealed a substantial fall in hippocampal 5-HT levels (F (7, 32) = 28.23, p < 0.0001) and PFC (F (7, 32) = 28.23, p < 0.0001). 18βGA (20 and 50, intragastrically, 7 days) or FXT administration in the CUMS rats improved 5-HT levels in the hippocampus [F (7, 32) = 28.23, p < 0.01 (18βGA, 20), p < 0.0001 (18βGA, 50), p < 0.0001 (FXT, 10)] and PFC [F (7, 32) = 28.23, p < 0.01 (18βGA, 20 mg/kg), p < 0.001 (18βGA, 50), p < 0.0001 (FXT, 10)] versus vehicle-treated CUMS rats, demonstrating antidepressant-like effects. Conversely, 18βGA (10 mg/kg, intragastrically) had no influence on 5-HT levels in the hippocampal (F (7, 32) = 28.23, p > 0.05) and PFC (F (7, 32) = 28.23, p > 0.05) in CUMS group. Interestingly, in non-CUMS rats, treatment with 18βGA (50 mg/kg, intragastrically) or FXT showed no impact on 5-HT levels in the hippocampus (F (7, 32) = 28.23, p > 0.05, and 0.05) or PFC (F (7, 32) = 28.23, p > 0.05, and 0.05) versus non-CUMS control group. Compared to FLX in the non-CUMS animals, FXT did not affect the serotonin and noradrenaline levels in the CUMS group (p > 0.05).
Impact of 18βGA on the Hippocampal and PFC BDNF Quantitation in Immunohistochemistry
The BDNF signaling protein was studied by immunohistochemistry to assess the probable mechanism of 18βGA. In the immunohistochemistry experiment, the effects of 10, 20, and 50 mg/kg doses of 18βGA administration and FXT on the expressions of BDNF in the CA1 area (Fig. 6) and PFC (Fig. 7) were examined. One-way analysis of variance revealed substantial variances in BDNF levels in the hippocampal tissue [F (5, 12) = 39.05, p < 0.0001] and PFC [F (5, 12) = 21.74, p < 0.0001] across the treatment groups, as shown in Figs. 6 and 7. The findings showed that CUMS dramatically reduced BDNF levels in the CA1 region (F (5, 12) = 39.05, p < 0.0001; Fig. 6) and in the PFC (F (5, 12) = 21.74, p < 0.0001; Fig. 7) versus non-CUMS control group. 18βGA administration (50 mg/kg) and FXT considerably reversed these changes in the CA1 region (F (5, 12) = 39.05, p < 0.0001, and 0.0001; Fig. 6) and in PFC (F (5, 12) = 21.74, p < 0.05, and 0.01; Fig. 7) respectively. Furthermore, the findings of FXT-treated rats were comparable to those of 18βGA administration (50 mg/kg) in the CA1 area. 18βGA administration (20 mg/kg) also significantly reversed these alterations in the CA1 region (F (5, 12) = 39.05, p < 0.05; Fig. 6) while failed to alter expression in the PFC (F (5, 12) = 21.74, p > 0.05; Fig. 7). Nonetheless, on comparison to CUMS rats, 18βGA (10 mg/kg) did not influence BDNF levels in the CA1 area (F (5, 12) = 39.05, p > 0.05; Fig. 6) and PFC (F (5, 12) = 21.74, p > 0.05; Fig. 7) in the CUMS animals.
Effects of CUMS and 18βGA at doses 10, 20, and 50 mg/kg intragastrically and FXT at dose 10 mg/kg intragastrically on relative BDNF protein expression by IHC staining in the CA1 area. The scale bar represents 25 μm. The results are displayed as mean ± S.E.M. of 3 animals in each group with ####p < 0.0001 vs. non-CUMS rats and *p < 0.05, ****p < 0.0001 vs. CUMS rats, “one-way analysis of variance then TMC test.” CUMS stands for chronic unpredictable mild stress; 18βGA stands for 18β-glycyrrhetinic acid; BDNF is a brain-derived neurotrophic factor; i.g. stands for intragastrically
Effects of CUMS and 18βGA on the PFC’s relative BDNF protein expression by immunohistochemistry staining. The scale bar represents 25 μm. The results are displayed as mean ± S.E.M. of 3 animals in each group with ####p < 0.0001 vs. non-CUMS rats and *p < 0.05, **p < 0.01 vs. CUMS rats, “one-way analysis of variance post-hoc TMC test.” CUMS stands for chronic unpredictable mild stress; 18βGA stands for 18β-glycyrrhetinic acid; BDNF is a brain-derived neurotrophic factor; i.g. stands for intragastrically
Effects of 18βGA on BDNF and TrkB Expression in the Hippocampus and PFC as per Western Blot Analysis
Western blot analysis was used to determine how 18βGA treatment (10, 20, and 50, intragastrically) and FXT (10) affected the expressions of BDNF in the CA1 region and PFC (Fig. 8). The results of the two-way analysis of variance specified statistically substantial alterations in relative BDNF protein expression [F (5, 24) = 27.40, p < 0.0001] and TrkB expression [F (5, 24) = 19.87, p < 0.0001] in the PFC and the hippocampus across the treatment groups, as shown in Fig. 8. The analytical results suggested that CUMS significantly diminished BDNF relative protein expression in the CA1 area (F (5, 24) = 27.40, p < 0.0001) and in the PFC (F (5, 24) = 27.40, p < 0.0001) compared to the non-CUMS control group (Fig. 8A). When compared to the non-CUMS control group, the relative expression of TrkB protein is lowered in the CA1 area of the hippocampus (F (5, 24) = 19.87, p < 0.0001) and in the PFC (F (5, 24) = 19.87, p < 0.001) in the CUMS group (Fig. 8B). 18βGA (50) and FXT (10) considerably reversed relative BDNF expression in the CA1 region of hippocampus (F (5, 24) = 27.40, p < 0.01, 0.05) and in PFC (F (5, 24) = 27.40, p < 0.05, p < 0.05) respectively (Fig. 8A). Furthermore, 18βGA (50) and FXT significantly also reversed relative TrkB expression in the CA1 region (F (5, 24) = 19.87, p < 0.05, and 0.05) and in PFC (F (5, 24) = 19.87, p < 0.05, and 0.05) correspondingly compared to CUMS group (Fig. 8B). The treatment with 18βGA (20) also significantly reversed relative TrkB expression in the CA1 region [F (5, 24) = 19.87, (p < 0.05)]. Furthermore, administration of 10 mg/kg intragastrically of 18βGA resulted in insignificant changes in the relative expression of BDNF and TrkB in PFC and the hippocampus (Fig. 8) in CUMS group of rats.
Effects of CUMS and 18βGA at doses 10, 20, and 50 mg/kg, intragastrically and FXT at dose 10 mg/kg, intragastrically on BDNF (A) and TrkB (B) protein expression by Western blotting analysis in the PFC and the hippocampus. Representative immunoblot images of BDNF, TrkB, and β-actin. The results are displayed as mean ± S.E.M. of 3 animals in each group with ####p < 0.0001 vs. non-CUMS rats and *p < 0.05, **p < 0.01 vs. CUMS rats, “two-way analysis of variance post-hoc TMC test.” CUMS stands for chronic unpredictable mild stress; 18βGA stands for 18β-glycyrrhetinic acid; BDNF is a brain-derived neurotrophic factor; TrkB stands for tropomyosin receptor kinase B; i.g. stands for intragastrically
Discussion
The current study focused on 18βGA’s antidepressant mechanism and signaling pathway. Further, we confirm 18GA’s antidepressant effects on CUMS-induced distress for the first time via upregulation of the BDNF/TrkB signaling pathway.
CUMS may better imitate some key signs of human sadness, such as loss of curiosity, pleasure, and incentive, by exposing animals to unexpected and unpleasant everyday situations [52, 62]. As a result, the CUMS model was chosen as the evaluation model. According to our results, animals showed exposure to CUMS, enhanced distress, inflammation, elevation in hepatic enzymes, and lowered 5-HT, norepinephrine in the PFC and the hippocampus of rats. Further, all these alterations were effectively reversed by 18βGA therapy.
Further, the SPT has previously shown anhedonia (a reduction in attention to rewards) and has been linked to depression. Rats in the SPT were offered the option of consuming water or a highly favored sucrose solution. Sucrose preference declines after weeks of exposure to CUMS stressors; however, it is possible to return it to normal levels with long-term antidepressant therapy [58]. Furthermore, we discovered that giving rats 18βGA (20 and 50) or FXT (10) for 1 week augmented sucrose liking and reduced behaviors of depression in CUMS group of rats.
To further confirm, we studied the antidepressant-like effects of the 18βGA using the FST, a trustworthy animal model with excellent concurrent accuracy. In the FST, rodents exposed to CUMS displayed behaviors indicative of hopelessness, and our study’s findings align with prior research [59, 63]. 18βGA (20 and 50 mg/kg for seven days) or FXT (7 days) declined time spent immobile in the forced swim test considerably, demonstrating the antidepressant influence of 18βGA. However, the CUMS group of rats showed no response to 10 mg/kg of 18βGA. Consequently, the findings revealed that 18βGA had antidepressant-like action in chronic stress situations.
Moreover, we investigated whether the anti-immobility effects of the 18βGA treatment were the result of an antidepressant effect or a locomotor stimulating effect. As a result, we evaluated locomotor and exploratory behaviors using the OFT animal models [56, 57]. The square crossing number, rearing number, grooming number, total distance traveled, and center entering frequency in the OFT significantly reduced CUMS rats in this study. 18βGA (20 and 50 mg/kg) or FXT treatment of CUMS rats recovered their locomotion. It is interesting to note that none of the dosages of 18βGA administered had any effect on the movement of the non-CUMS rats, which suggests that the doses of 18βGA do not have any influence on the locomotor activity of rats. Additionally, we observed that the CUMS technique decreased rat body weight, which was consistent with earlier studies [64, 65]. Further, we revealed that 18βGA (20 and 50) or FXT (10) for 1 week recovered the loss of body weight in CUMS group of rats.
Furthermore, earlier studies have displayed that chronic stress is linked to the onset of depression, related inflammation, and neurotransmitter disruption in the brain [53, 66, 67]. Increased inflammatory cytokines in the brain have been linked to the genesis of depression in a number of studies [68]. Since peripherally generated cytokines may affect the CNS, the effect of cytokines on neurotransmitters like serotonin has been widely researched in animals [69, 70] and clinical [71] research. Cytokines induce indoleamine 2,3 dioxygenase to restrict 5-HT production. This route reduces 5-HT, which causes depression.
Consequently, we chose to measure the levels of inflammatory cytokines and neurotransmitters in the CUMS and non-CUMS groups after administering 18βGA and FXT. Our study confirms that exposure to CUMS significantly increased cytokine and depressive behaviors. These findings align with those reported by CUMS [72] using comparable scenarios. We hypothesized that 18βGA may reduce cytokines linked to 5-HT and norepinephrine deficiency in CUMS group. The most frequently studied cytokines as strong mediators of inflammation and accompanying depression are IL-1beta, IL-6, and TNF-alpha. The ability of cytokines to infiltrate the brain through the leaky area of the blood–brain barrier has previously been linked to depression pathogenesis [73]. The current investigation examined to understand whether a 1-week treatment with 18βGA has the capability to control cytokine levels. This is the first evidence of 18βGA’s ability to reduce IL-1beta, IL-6, and TNF-alpha levels in CUMS-related inflammatory disorders. Therefore, we hypothesize that the antidepressant effects of 18βGA could be attributable to a decrease in neuroinflammatory cytokines.
Furthermore, the pathophysiology of depression, which is an illness that affects several neurotransmitters as well as different regions of the brain, is still not fully understood. However, it is well acknowledged that persistent stress contributes significantly to the development of depression. Chronic long-term stress may result in HPA axis dysfunction and increased corticosteroid release, which are thought to play a key role in the etiology of human depression [74]. One of the many neurobiological processes that contribute to the incidence and progression of depression is the HPA axis. The paraventricular nucleus of the hypothalamus releases CRH during the stress response, which encourages the pituitary to generate ACTH. The HPA axis gets input through the glucocorticoid receptor as a result of the adrenal cortex producing glucocorticoids. HPA axis activity is notably high in patients with depression. Cortisol levels are often raised, which ultimately negatively undermines the HPA axis’s ability to regulate itself [75]. As a result, serum levels of CORT and ACTH were assessed. It was revealed that CUMS rats had considerably higher levels of CORT and ACTH, which was in line with studies showing that chronic stress may elevate CORT and ACTH levels [56]. The serum levels of CORT and ACTH in CUMS rats were markedly reduced after 18βGA treatment. These findings imply that a key mechanism behind the antidepressant action of 18βGA may also be the regulation of HPA axis hyperfunction.
Furthermore, the monoaminergic neurons in the hippocampus and PFC may get damaged by excessive HPA axis stimulation, reducing monoamine neurotransmitters’ release [76, 77]. Studies have shown that disrupting the HPA axis’s negative feedback control would ultimately decrease neurotransmitters and worsen depression. It has been proposed that a lack of monoamines causes depressive disorders. Most antidepressants now available act by raising 5-HT or norepinephrine levels in the brain [78]. 5-HT and norepinephrine levels in the PFC and the hippocampal tissues of CUMS rats were likewise shown to be significantly lower in the current investigation. Norepinephrine is also reported to have a tonic antiinflammatory effect in the CNS by suppressing microglial and astrocytic activation. Therefore, norepinephrine may have a neuroprotective function in CNS illnesses where inflammatory processes play a role in pathogenesis [79, 80]. However, our study has limitations because we have not evaluated 18βGA’s effects on microglial and astrocytic activation, which researchers may further study. Furthermore, 1 week of 18βGA (20 and 50 mg/kg) or FXT treatment significantly increased 5-HT and norepinephrine levels in the PFC and the hippocampus of CUMS rats, implying that 18βGA’s antidepressant influence may be related to improved levels of key monoamine 5-HT and norepinephrine. The outcomes of 18βGA were equivalent to those of FXT, a standard medicine.
Moreover, chronic stress may severely affect the immunological, digestive, and neurological systems by stimulating the hypothalamic–pituitary–adrenal (HPA) axis. To understand the link between chronic stress, neuroinflammation, and changed liver markers, we evaluated how CUMS altered liver enzymes and how 18βGA affected the altered liver markers. In our study, a 1-week therapy with 18βGA (50 mg/kg) significantly diminished the concentration of hepatic enzymes ALT, AST, and ALP increased by chronic stress, proving that 18βGA may also repair hepatic damage. Our findings corroborate earlier studies on hepatic enzyme increase during CUMS [46, 81, 82]. Therefore, depression might be connected to liver damage, and that 18βGA can alleviate depressive-like behavior by suppressing the liver-brain inflammatory axis.
Additionally, BDNF/TrkB signaling pathway, in particular, is gaining popularity as a possible novel target for managing depression [11, 47]. The BDNF/TrkB signaling pathway has also been linked to central nervous system activities such as neurogenesis and the protection of neurons from oxidative damage. CUMS stimulation significantly decreased the protein expression of BDNF & TrkB in the rat PFC and the hippocampus. In this research, standard medicine FXT was revealed to have the capacity to offset BDNF/TrkB protein levels in the PFC and the hippocampus, indicating that FXT promotes the expression of the BDNF/TrkB pathway. These FXT-related data are mostly in line with the current investigation findings. Furthermore, the present research discovered that the expression of BDNF, which had been decreased by CUMS, had increased after being treated with 18βGA for 1 week. Additionally, our findings revealed that CUMS reduced TrkB levels which was also reversed on the administration of 18βGA. The rise in the PFC and the hippocampus BDNF levels after 18βGA therapy was considerably similar to that seen in rats given FXT. Moreover, enhanced BDNF expression is linked to higher levels of 5-HT and norepinephrine, which amplifies the antidepressant effects of 18βGA. All of these findings show that 18βGA has the ability to treat depression via upregulating the BDNF-TrkB signaling pathway. BDNF via TrkB receptors enhances intracellular tyrosine kinase activity and activates both the MAPK pathway and the cAMP-REBP pathway, which further boosts the survival rate of nerve cells, synaptic plasticity, and neurogenesis [83]. BDNF-TrkB impacts neurons’ existence, growth, and activities and stimulates synapse formation and transmission capability [84]. The establishment of antidepressant effects is heavily influenced by BDNF/TrkB signaling.
Overall, the findings supported our prediction that 18βGA reversed depressive-like behavior, ameliorates neuroinflammation, liver biomarkers, stress hormones, and improves body weight, brain neurotransmitter concentration, and its effect on BDNF/TrkB signaling pathway in both PFC and hippocampus. 18βGA could be a potential depression treatment medication, but further study is required before it can be used in patients. Future research should establish if the HPA axis is a viable new avenue for using 18βGA in treating depression. Additionally, a more advanced and precise network pharmacological analysis could be used to investigate further the functional impact of neuroinflammation in depression with 18βGA.
Data Availability
This article has all the data generated or analyzed during this investigation.
Abbreviations
- 18βGA:
-
18β-Glycyrrhetinic acid
- ACTH:
-
Adrenocorticotropic hormone
- CORT:
-
Corticosterone
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- ANOVA:
-
Analysis of variance
- AST:
-
Aspartate aminotransferase
- BDNF:
-
Brain-derived neurotrophic factor
- CPCSEA:
-
Committee for the purpose of control and supervision of experiments on animals
- CUMS:
-
Chronic unpredictable mild stress
- ELISA:
-
Enzyme-linked immunosorbent assay
- FST:
-
Forced swimming test
- FXT:
-
Fluoxetine
- IHC:
-
Immunohistochemistry
- IL-1beta:
-
Interleukin-1beta
- IL-6:
-
Interleukin-6
- OFT:
-
Open field test
- PFC:
-
Prefrontal cortex
- SEM:
-
Standard error of the mean
- SPT:
-
Sucrose preference test
- TNF-alpha:
-
Tumor necrosis factor-alpha
- TrkB:
-
Tropomyosin receptor kinase B
References
Zhu XL, Chen JJ, Han F et al (2018) Novel antidepressant effects of paeonol alleviate neuronal injury with concomitant alterations in BDNF, Rac1 and RhoA levels in chronic unpredictable mild stress rats. Psychopharmacology. https://doi.org/10.1007/s00213-018-4915-7
Santomauro DF, Mantilla Herrera AM, Shadid J et al (2021) Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398:1700–1712. https://doi.org/10.1016/s0140-6736(21)02143-7
Eshel N, Roiser JP (2010) Reward and punishment processing in depression. Biol Psychiatry 68:118–124
Li ZQ, Yan ZY, Lan FJ et al (2018) Suppression of NLRP3 inflammasome attenuates stress-induced depression-like behavior in NLGN3-deficient mice. Biochem Biophys Res Commun 501:933–940. https://doi.org/10.1016/j.bbrc.2018.05.085
Kokare DM, Singru PS, Dandekar MP et al (2008) Involvement of alpha-melanocyte stimulating hormone (α-MSH) in differential ethanol exposure and withdrawal related depression in rat: neuroanatomical-behavioral correlates. Brain Res 1216:53–67. https://doi.org/10.1016/j.brainres.2008.03.064
Fernandes J, Gupta GL (2019) N-acetylcysteine attenuates neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Behav Brain Res 364:356–365. https://doi.org/10.1016/J.BBR.2019.02.025
Montejo AL, Montejo L, Navarro-Cremades F (2015) Sexual side-effects of antidepressant and antipsychotic drugs. Curr Opin Psychiatry 28:418–423. https://doi.org/10.1097/YCO.0000000000000198
Yan K, Chen Y-B, Wu J-R et al (2018) Current rapid-onset antidepressants and related animal models. Curr Pharm Des. https://doi.org/10.2174/1381612824666180727115222
Pandarakalam JP (2018) Challenges of treatment-resistant depression. Psychiatr Danub 30:273–284. https://doi.org/10.24869/psyd.2018.273
Berk M, Williams LJ, Jacka FN et al (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 11:200. https://doi.org/10.1186/1741-7015-11-200
Zhang J, Yao W, Hashimoto K (2016) Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol 14:721–731. https://doi.org/10.2174/1570159x14666160119094646
Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27:24–31. https://doi.org/10.1016/j.it.2005.11.006
Liu YM, Shen JD, Xu LP et al (2017) Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int Immunopharmacol 45:128–134. https://doi.org/10.1016/j.intimp.2017.02.007
Zhang FF, Peng W, Sweeney JA et al (2018) Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther 24:994–1003. https://doi.org/10.1111/cns.12835
McEwen BS, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41:3–23. https://doi.org/10.1038/npp.2015.171
Kim SH, Hong J, Lee JE, Lee YC (2017) 18β-Glycyrrhetinic acid, the major bioactive component of glycyrrhizae radix, attenuates airway inflammation by modulating Th2 cytokines, GATA-3, STAT6, and Foxp3 transcription factors in an asthmatic mouse model. Environ Toxicol Pharmacol 52:99–113. https://doi.org/10.1016/j.etap.2017.03.011
Kao TC, Shyu MH, Yen GC (2010) Glycyrrhizic acid and 18β-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3β signaling and glucocorticoid receptor activation. J Agric Food Chem 58:8623–8629. https://doi.org/10.1021/jf101841r
Wu S, Cui S, Wang L et al (2018) 18β-Glycyrrhetinic acid protects against alpha-naphthylisothiocyanate-induced cholestasis through activation of the Sirt1/FXR signaling pathway. Acta Pharmacol Sin 39:1865–1873. https://doi.org/10.1038/s41401-018-0110-y
Wu S, Lu H, Wang W et al (2021) Prevention of D-GalN/LPS-induced ALI by 18β-glycyrrhetinic acid through PXR-mediated inhibition of autophagy degradation. Cell Death Dis. https://doi.org/10.1038/s41419-021-03768-8
Mahmoud AM, Al Dera HS (2015) 18β-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: potential role of PPARγ and Nrf2 upregulation. Genes Nutr. https://doi.org/10.1007/s12263-015-0491-1
Shetty AV, Thirugnanam S, Dakshinamoorthy G et al (2011) 18Α-Glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int J Oncol 39:635–640. https://doi.org/10.3892/ijo.2011.1061
Luo YH, Wang C, Xu WT et al (2021) 18β-Glycyrrhetinic acid has anti-cancer effects via inducing apoptosis and G2/M cell cycle arrest, and inhibiting migration of a549 lung cancer cells. Onco Targets Ther 14:5131–5144. https://doi.org/10.2147/OTT.S322852
Cai H, Chen X, Zhang J, Wang J (2018) 18β-Glycyrrhetinic acid inhibits migration and invasion of human gastric cancer cells via the ROS/PKC-α/ERK pathway. J Nat Med 72:252–259. https://doi.org/10.1007/s11418-017-1145-y
Kalaiarasi P, Kaviarasan K, Pugalendi KV (2009) Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats. Eur J Pharmacol 612:93–97. https://doi.org/10.1016/j.ejphar.2009.04.003
Zhang M, Chang Z, Zhang P et al (2019) Protective effects of 18β-glycyrrhetinic acid on pulmonary arterial hypertension via regulation of Rho A/Rho kinsase pathway. Chem Biol Interact. https://doi.org/10.1016/j.cbi.2019.108749
Gupta P, Das PK, Ukil A (2015) Antileishmanial effect of 18β-glycyrrhetinic acid is mediated by toll-like receptor-dependent canonical and noncanonical p38 activation. Antimicrob Agents Chemother 59:2531–2539. https://doi.org/10.1128/AAC.03997-14
Hardy ME, Hendricks JM, Paulson JM, Faunce NR (2012) 18 -Glycyrrhetinic acid inhibits rotavirus replication in culture. Virol J. https://doi.org/10.1186/1743-422X-9-96
Zhang Q, Xiong Y, Cheng J et al (2022) Synthesis and biological evaluation of ruthenium polypyridine complexes with 18β-glycyrrhetinic acid as antibacterial agents against Staphylococcus aureus. Dalton Trans 51:1099–1111. https://doi.org/10.1039/d1dt02692e
Long DR, Mead J, Hendricks JM et al (2013) 18β-Glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob Agents Chemother 57:241–247. https://doi.org/10.1128/AAC.01023-12
Wang D, Zhang Y, Wang C et al (2016) 18β-Glycyrrhetinic acid, a novel naturally derived agent, suppresses prolactin hyperactivity and reduces antipsychotic-induced hyperprolactinemia in in vitro and in vivo models. Neurochem Res 41:2233–2242. https://doi.org/10.1007/s11064-016-1938-5
Chu S, Wang W, Zhang N et al (2021) Protective effects of 18β-glycyrrhetinic acid against myocardial infarction: involvement of PI3K/Akt pathway activation and inhibiting Ca 2+ influx via L-type Ca 2+ channels. Food Sci Nutr 9:6831–6843. https://doi.org/10.1002/FSN3.2639
Qing C, Ziyun L, Xuefei Y et al (2022) Protective effects of 18β-glycyrrhetinic acid on neonatal rats with hyperoxia exposure. Inflammation. https://doi.org/10.1007/S10753-021-01616-7
Feng Y, Mei L, Wang M et al (2021) Anti-inflammatory and pro-apoptotic effects of 18beta-glycyrrhetinic acid in vitro and in vivo models of rheumatoid arthritis. Front Pharmacol. https://doi.org/10.3389/FPHAR.2021.681525
Cheng X, Qiu L, Wang F (2020) 18α-Glycyrrhetinic acid (GA) ameliorates fructose-induced nephropathy in mice by suppressing oxidative stress, dyslipidemia and inflammation. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2019.109702
Su L, Wang Z, Huang F et al (2018) 18β-Glycyrrhetinic acid mitigates radiation-induced skin damage via NADPH oxidase/ROS/p38MAPK and NF-κB pathways. Environ Toxicol Pharmacol 60:82–90. https://doi.org/10.1016/j.etap.2018.04.012
Rashid S, Nafees S, Siddiqi A et al (2017) Partial protection by 18β glycrrhetinic acid against cisplatin induced oxidative intestinal damage in wistar rats: possible role of NFkB and caspases. Pharmacol Rep 69:1007–1013. https://doi.org/10.1016/j.pharep.2017.02.013
Zhou J, Cai W, Jin M et al (2015) 18Β-Glycyrrhetinic acid suppresses experimental autoimmune encephalomyelitis through inhibition of microglia activation and promotion of remyelination. Sci Rep. https://doi.org/10.1038/srep13713
Jin L, Zhu Z, Hong L et al (2022) ROS-responsive 18β-glycyrrhetic acid-conjugated polymeric nanoparticles mediate neuroprotection in ischemic stroke through HMGB1 inhibition and microglia polarization regulation. Bioact Mater 19:38–49. https://doi.org/10.1016/J.BIOACTMAT.2022.03.040
Tian H, Cheng Y, Zhang Y et al (2021) 18β-Glycyrrhetinic acid alleviates demyelination by modulating the microglial M1/M2 phenotype in a mouse model of cuprizone-induced demyelination. Neurosci Lett. https://doi.org/10.1016/J.NEULET.2021.135871
Oztanir MN, Ciftci O, Cetin A et al (2014) The beneficial effects of 18β-glycyrrhetinic acid following oxidative and neuronal damage in brain tissue caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Neurol Sci 35:1221–1228. https://doi.org/10.1007/s10072-014-1685-9
Caglayan C, Kandemir FM, Ayna A et al (2022) Neuroprotective effects of 18β-glycyrrhetinic acid against bisphenol A-induced neurotoxicity in rats: involvement of neuronal apoptosis, endoplasmic reticulum stress and JAK1/STAT1 signaling pathway. Metab Brain Dis. https://doi.org/10.1007/S11011-022-01027-Z
Murck H, Lehr L, Hahn J et al (2020) Adjunct therapy with glycyrrhiza glabra rapidly improves outcome in depression—a pilot study to support 11-beta-hydroxysteroid dehydrogenase type 2 inhibition as a new target. Front Psychiatry. https://doi.org/10.3389/fpsyt.2020.605949
Dhingra D, Sharma A (2006) Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog Neuro-Psychopharmacol Biol Psychiatry 30:449–454. https://doi.org/10.1016/j.pnpbp.2005.11.019
Geng C, Guo Y, Wang C et al (2020) Systematic impacts of chronic unpredictable mild stress on metabolomics in rats. Sci Rep 101(10):1–11. https://doi.org/10.1038/s41598-020-57566-x
Yang XH, Song SQ, Xu Y (2017) Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr Dis Treat 13:2727–2736. https://doi.org/10.2147/NDT.S150028
Zhang M, Wu W, Huang C et al (2022) Shuxie-1 decoction alleviated CUMS -induced liver injury via IL-6/JAK2/STAT3 signaling. Front Pharmacol. https://doi.org/10.3389/FPHAR.2022.848355
Jiang N, Wang H, Li C et al (2021) The antidepressant-like effects of the water extract of Panax ginseng and Polygala tenuifolia are mediated via the BDNF-TrkB signaling pathway and neurogenesis in the hippocampus. J Ethnopharmacol 267:113625. https://doi.org/10.1016/j.jep.2020.113625
Wei L, Kan LY, Zeng HY et al (2018) BDNF/TrkB pathway mediates the antidepressant-like role of H2S in CUMS-exposed rats by inhibition of hippocampal ER stress. NeuroMol Med 20:252–261. https://doi.org/10.1007/s12017-018-8489-7
Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical Implications. Arch Med Sci 11:1164–1178. https://doi.org/10.5114/aoms.2015.56342
Kao TC, Shyu MH, Yen GC (2009) Neuroprotective effects of glycyrrhizic acid and 18β-glycyrrhetinic acid in PC12 cells via modulation of the PI3K/Akt pathway. J Agric Food Chem 57:754–761. https://doi.org/10.1021/jf802864k
Ma X, Chen H, Cao L et al (2021) 18β-Glycyrrhetinic acid improves high-intensity exercise performance by promoting glucose-dependent energy production and inhibiting oxidative stress in mice. Phyther Res 35:6932–6943. https://doi.org/10.1002/ptr.7310
Willner P (2017) The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress 6:78–93. https://doi.org/10.1016/j.ynstr.2016.08.002
Gaignier F, Legrand-Frossi C, Stragier E et al (2018) A model of chronic exposure to unpredictable mild socio-environmental stressors replicates some spaceflight-induced immunological changes. Front Physiol. https://doi.org/10.3389/fphys.2018.00514
Yang G, Wang L, Yu X et al (2017) Protective effect of 18 β -glycyrrhetinic acid against triptolide-induced hepatotoxicity in rats. Evid Based Complement Altern Med. https://doi.org/10.1155/2017/3470320
MacHado DG, Cunha MP, Neis VB et al (2012) Fluoxetine reverses depressive-like behaviors and increases hippocampal acetylcholinesterase activity induced by olfactory bulbectomy. Pharmacol Biochem Behav 103:220–229. https://doi.org/10.1016/j.pbb.2012.08.024
Wang C, Gan D, Wu J et al (2018) Honokiol exerts antidepressant effects in rats exposed to chronic unpredictable mild stress by regulating brain derived neurotrophic factor level and hypothalamus–pituitary–adrenal axis activity. Neurochem Res 43:1519–1528. https://doi.org/10.1007/s11064-018-2566-z
Li H, Xiang Y, Zhu Z et al (2021) Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J Neuroinflamm 18:1–18. https://doi.org/10.1186/s12974-021-02303-y
He L, Zeng L, Tian N et al (2020) Optimization of food deprivation and sucrose preference test in SD rat model undergoing chronic unpredictable mild stress. Anim Model Exp Med 3:69–78. https://doi.org/10.1002/ame2.12107
Rosas-Sánchez GU, German-Ponciano LJ, Rodríguez-Landa JF (2022) Considerations of pool dimensions in the forced swim test in predicting the potential antidepressant activity of drugs. Front Behav Neurosci. https://doi.org/10.3389/fnbeh.2021.757348
Hu C, Luo Y, Wang H et al (2017) Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS ONE. https://doi.org/10.1371/journal.pone.0185129
Song Y, Sun R, Ji Z et al (2018) Perilla aldehyde attenuates CUMS-induced depressive-like behaviors via regulating TXNIP/TRX/NLRP3 pathway in rats. Life Sci 206:117–124. https://doi.org/10.1016/j.lfs.2018.05.038
Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J (2019) Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev 99:101–116. https://doi.org/10.1016/j.neubiorev.2018.12.002
Lee EH, Park JY, Kwon HJ, Han PL (2021) Repeated exposure with short-term behavioral stress resolves pre-existing stress-induced depressive-like behavior in mice. Nat Commun 12:1–18. https://doi.org/10.1038/s41467-021-26968-4
Xu P, Wang KZ, Lu C et al (2016) Antidepressant-like effects and cognitive enhancement of the total phenols extract of Hemerocallis citrina Baroni in chronic unpredictable mild stress rats and its related mechanism. J Ethnopharmacol 194:819–826. https://doi.org/10.1016/j.jep.2016.09.023
Liu CY, Chen JB, Liu YY et al (2022) Saikosaponin D exerts antidepressant effect by regulating Homer1-mGluR5 and mTOR signaling in a rat model of chronic unpredictable mild stress. Chin Med. https://doi.org/10.1186/S13020-022-00621-8
Adzic M, Brkic Z, Mitic M et al (2017) Therapeutic strategies for treatment of inflammation-related depression. Curr Neuropharmacol. https://doi.org/10.2174/1570159X15666170828163048
Beurel E, Toups M, Nemeroff CB (2020) The bidirectional relationship of depression and inflammation: double trouble. Neuron 107:234–256. https://doi.org/10.1016/j.neuron.2020.06.002
Lee CH, Giuliani F (2019) The role of inflammation in depression and fatigue. Front Immunol 10:1696. https://doi.org/10.3389/fimmu.2019.01696
Dunn AJ, Wang J (1995) Cytokine effects on CNS biogenic amines. NeuroImmunoModulation 2:319–328. https://doi.org/10.1159/000097211
Anisman H, Matheson K (2005) Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev 29:525–546. https://doi.org/10.1016/j.neubiorev.2005.03.007
Obermanns J, Krawczyk E, Juckel G, Emons B (2021) Analysis of cytokine levels, T regulatory cells and serotonin content in patients with depression. Eur J Neurosci 53:3476–3489. https://doi.org/10.1111/ejn.15205
Afridi R, Suk K (2021) Neuroinflammatory basis of depression: learning from experimental models. Front Cell Neurosci 15:242. https://doi.org/10.3389/fncel.2021.691067
Felger JC, Lotrich FE (2013) Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246:199. https://doi.org/10.1016/J.NEUROSCIENCE.2013.04.060
Jia Y, Liu L, Sheng C et al (2019) Increased serum levels of cortisol and inflammatory cytokines in people with depression. J Nerv Ment Dis 207:271–276. https://doi.org/10.1097/NMD.0000000000000957
Keller J, Gomez R, Williams G et al (2017) HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry 22:527–536. https://doi.org/10.1038/MP.2016.120
Zhao J, Niu C, Wang J et al (2018) The depressive-like behaviors of chronic unpredictable mild stress-treated mice, ameliorated by Tibetan medicine Zuotai: involvement in the hypothalamic-pituitary-adrenal (HPA) axis pathway. Neuropsychiatr Dis Treat 14:129–141. https://doi.org/10.2147/NDT.S151107
Li H, Li Y, Zhang X et al (2021) The combination of Aquilaria sinensis (Lour.) Gilg and Aucklandia costus Falc. volatile oils exerts antidepressant effects in a CUMS-induced rat model by regulating the HPA axis and levels of neurotransmitters. Front Pharmacol. https://doi.org/10.3389/FPHAR.2020.614413
Harmer CJ, Duman RS, Cowen PJ (2017) How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 4:409. https://doi.org/10.1016/S2215-0366(17)30015-9
O’Sullivan JB, Ryan KM, Curtin NM et al (2009) Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol 12:687–699. https://doi.org/10.1017/S146114570800967X
Hinojosa AE, Caso JR, García-Bueno B et al (2013) Dual effects of noradrenaline on astroglial production of chemokines and pro-inflammatory mediators. J Neuroinflamm 10:1–10. https://doi.org/10.1186/1742-2094-10-81
Gao X, Liang M, Fang Y et al (2018) Deciphering the differential effective and toxic responses of Bupleuri Radix following the induction of chronic unpredictable mild stress and in healthy rats based on serum metabolic profiles. Front Pharmacol 8:995. https://doi.org/10.3389/FPHAR.2017.00995/BIBTEX
Gupta GL, Fernandes J (2019) Protective effect of Convolvulus pluricaulis against neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Biomed Pharmacother 109:1698–1708. https://doi.org/10.1016/j.biopha.2018.11.046
Yang T, Nie Z, Shu H et al (2020) The role of BDNF on neural plasticity in depression. Front Cell Neurosci 14:82. https://doi.org/10.3389/fncel.2020.00082
Zagrebelsky M, Tacke C, Korte M (2020) BDNF signaling during the lifetime of dendritic spines. Cell Tissue Res 382:185–199. https://doi.org/10.1007/s00441-020-03226-5
Funding
This study was financially supported by the Science and Engineering Research Board (SERB), which is part of the Department of Science and Technology under the Government of India (SR-FT/LS–072012).
Author information
Authors and Affiliations
Contributions
GLG conceived, designed, and analyzed data. GLG, LS, and MA conducted experiments. GLG and MA wrote the manuscript. The article was reviewed and approved by each of the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Ethical Approval
All techniques were accomplished after approval of the Institutional Animal Ethics Committee and strict adherence to ethics and guidelines established by the CPCSEA, Government of India. Further, no author’s research involving human subjects is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, G.L., Sharma, L. & Sharma, M. 18β-Glycyrrhetinic Acid Ameliorates Neuroinflammation Linked Depressive Behavior Instigated by Chronic Unpredictable Mild Stress via Triggering BDNF/TrkB Signaling Pathway in Rats. Neurochem Res 48, 551–569 (2023). https://doi.org/10.1007/s11064-022-03779-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03779-7