Abstract
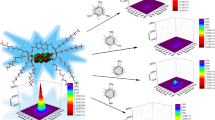
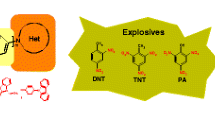
Rapid and selective detection of nitroaromatic explosives is very important for public safety, life, and environmental health. Current instrumental techniques suffer from high cost and poor site used. In order to investigate fluorescence sensing of nitroaromatics, we prepare a new small fluorescence probe derived from pamoic acid. This study covers the synthesis of Pamoic acid based [diisopropyl 4,4'-methylenebis(3-methoxy-2-naphthoate)] (2) material and characterization of its structure. The methylation of Pamoic acid ester, which we have successfully synthesized in our previous studies, was carried out in this study. Determination of the photophysical and fluorescent nitroaromatic detection properties of the compound forms the basis of the study. Structural characterization of the synthesized compound [diisopropyl 4,4'-methylenebis(3-methoxy-2-naphthoate)] (2) was characterized using spectroscopic methods. In addition, Molecular structure of the synthesized compound was determined by single crystal X-ray diffraction studies. In the final step, compounds [diisopropyl 4,4'-methylenebis(3-hydroxy-2-naphthoate)] (1) and [diisopropyl 4,4'-methylenebis(3-methoxy-2-naphthoate)] (2) were tested as fluorescent probes for the detection of some nitroaromatic explosives. It is seen that Nitrobenzene provides the best quenching effect on the compound [diisopropyl 4,4'-methylenebis(3-hydroxy-2-naphthoate)] (1) containing the -OH group, with lowest the limit of detection (LOD) value. It was observed that Picric acid provided the best quenching effect with lowest the limit of detection (LOD) value in the compound [diisopropyl 4,4'-methylenebis(3-methoxy-2-naphthoate)] (2) obtained by methylation of the -OH group in the compound [diisopropyl 4,4'-methylenebis(3-hydroxy-2-naphthoate)] (1).







Similar content being viewed by others
Availability of Data and Material
The data that supports the findings of this study are available in the supplementary material of this article.
References
Jørgensen M (1998) Quantitative determination of pamoic acid in dog and rat serum by automated ion-pair solid-phase extraction and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Appl 716:315–323. https://doi.org/10.1016/S0378-4347(98)00273-4
Şimşir T, Güngör SA, Kose M (2018) Pamoic acid-phenanthroline co-crystal and its Cu(II) complex: X-ray structures and luminescence properties. J Mol Struct 1167:88–94. https://doi.org/10.1016/j.molstruc.2018.04.093
Du M, Li CP, Zhao XJ, Yu Q (2007) Interplay of coordinative and supramolecular interactions in engineering unusual crystalline architectures of low-dimensional metal-pamoate complexes under co-ligand intervention. CrystEngComm 9:1011–1028. https://doi.org/10.1039/b707853f
González-Montiel S, Ignacio Sandoval-Chávez C, Castillo-Moreno MÁ et al (2021) Coordination from Heteroscorpionate Ligand Towards Pd(II) via Pd⋅⋅⋅Hδ−C(sp3) Interaction: Structural and Catalytic Studies. Eur J Inorg Chem 2021:2661–2668. https://doi.org/10.1002/ejic.202100351
Hunter CA, Sanders JKM (1990) The Nature of π-π Interactions. J Am Chem Soc 112:5525–5534. https://doi.org/10.1021/ja00170a016
Guckian KM, Schweitzer BA, Ren RXF et al (2000) Factors contributing to aromatic stacking in water: Evaluation in the context of DNA. J Am Chem Soc 122:2213–2222. https://doi.org/10.1021/ja9934854
Chen GH, He YP, Zhang SH, Zhang J (2019) Syntheses, crystal structures and fluorescent properties of two metal-organic frameworks based on pamoic acid. J Solid State Chem 270:335–338. https://doi.org/10.1016/j.jssc.2018.11.027
Cao T, Peng Y, Liu T et al (2014) Assembly of a series of d10 coordination polymers of pamoic acid through a mixed-ligand synthetic strategy: Syntheses, structures and fluorescence properties. CrystEngComm 16:10658–10673. https://doi.org/10.1039/c4ce01356e
Biswas S, Jena HS, Goswami S et al (2014) Synthesis and Characterization of Two Lanthanide (Gd 3+ and Dy 3+ )-Based Three-Dimensional Metal Organic Frameworks with Squashed Metallomacrocycle Type Building Blocks and Their Magnetic, Sorption, and Fluorescence Properties Study. Cryst Growth Des 14:1287–1295. https://doi.org/10.1021/cg401804e
Akhgari F, Fattahi H, Oskoei YM (2015) Recent advances in nanomaterial-based sensors for detection of trace nitroaromatic explosives. Sensors Actuators B Chem 221:867–878. https://doi.org/10.1016/j.snb.2015.06.146
Sun X, Wang Y, Lei Y (2015) Fluorescence based explosive detection: From mechanisms to sensory materials. Chem Soc Rev 44:8019–8061. https://doi.org/10.1039/c5cs00496a
Gungor O, Kose M (2018) Selective detections of nitroaromatic explosives by monomeric and polymeric Bi(III) complexes. Sensors Actuators B Chem 264:363–371. https://doi.org/10.1016/j.snb.2018.02.184
Ma Y, Wang S, Wang L (2015) Nanomaterials for luminescence detection of nitroaromatic explosives. TrAC - Trends Anal Chem 65:13–21. https://doi.org/10.1016/j.trac.2014.09.007
Ghorpade TK, Palai AK, Rath SK et al (2017) Pentiptycene-tbutylpyrene based poly(arylene-ethynylene)s: Highly sensitive and selective TNT sensor in aqueous as well as vapor phase. Sensors Actuators, B Chem 252:901–911. https://doi.org/10.1016/j.snb.2017.06.059
Zhang S, Ding L, Lü F et al (2012) Fluorescent film sensors based on SAMs of pyrene derivatives for detecting nitroaromatics in aqueous solutions. Spectrochim Acta Part A Mol Biomol Spectrosc 97:31–37. https://doi.org/10.1016/j.saa.2012.04.041
Fisher M, Cumming C, Fox M et al (2000) A man-portable chemical sniffer utilizing novel fluorescent polymers for detection of ultra-trace concentrations of explosives emanating from landmines. Response 1–10
Wan XY, Jiang FL, Liu CP et al (2015) Rapid and discriminative detection of nitro aromatic compounds with high sensitivity using two zinc MOFs synthesized through a temperature-modulated method. J Mater Chem A 3:22369–22376. https://doi.org/10.1039/c5ta04552e
Zheng B, Li Y, Tao F et al (2017) Enhanced superquenching of the hyperbranched conjugated polymer for the detection of nitroaromatic explosives. Sensors Actuators, B Chem 241:357–363. https://doi.org/10.1016/j.snb.2016.10.057
Kumar A, Chae PS (2017) New 1,8-naphthalimide-conjugated sulfonamide probes for TNP sensing in water. Sensors Actuators, B Chem 240:1–9. https://doi.org/10.1016/j.snb.2016.08.149
Ponnuvel K, Banuppriya G, Padmini V (2016) Highly efficient and selective detection of picric acid among other nitroaromatics by NIR fluorescent organic fluorophores. Sensors Actuators B Chem 234:34–45. https://doi.org/10.1016/j.snb.2016.04.129
Ding L, Bai Y, Cao Y et al (2014) Micelle-induced versatile sensing behavior of bispyrene-based fluorescent molecular sensor for picric acid and PYX explosives. Langmuir 30:7645–7653. https://doi.org/10.1021/la5011264
Yin M, Ji C (2017) Nanoscaled fluorescent films and layers for detection of environmental pollutants. In: Nanoscaled Films and Layers. InTech, p 13
Kumar V, Maiti B, Chini MK et al (2019) Multimodal fluorescent polymer sensor for highly sensitive detection of nitroaromatics. Sci Rep 9:7269. https://doi.org/10.1038/s41598-019-43836-w
Gole B, Bar AK, Mukherjee PS (2011) Fluorescent metal-organic framework for selective sensing of nitroaromatic explosives. Chem Commun 47:12137–12139. https://doi.org/10.1039/c1cc15594f
Mu Y, Ran Y, Du J et al (2017) A fluorescent lanthanide-organic framework for highly sensitive detection of nitroaromatic explosives. Polyhedron 124:125–130. https://doi.org/10.1016/j.poly.2016.12.030
Wang Y, La A, Brückner C, Lei Y (2012) FRET- and PET-based sensing in a single material: Expanding the dynamic range of an ultra-sensitive nitroaromatic explosives assay. Chem Commun 48:9903–9905. https://doi.org/10.1039/c2cc34492k
Bal M (2022) Synthesis of reduced graphene oxide-based hybrid compounds and investigation of their sensing behavior against some nitroaromatic explosives: 289. https://doi.org/10.1016/j.matchemphys.2022.126480
Gungor SA, Sahin I, Gungor O et al (2018) Pamoic acid esters and their xanthene derivatives: Flourimetric detection of nitroaromatic compounds and non-linear optical properties. Sensors Actuators, B Chem 255:3344–3354. https://doi.org/10.1016/j.snb.2017.09.161
Bal M, Tümer M, Köse M (2022) Investigation of Chemosensing and Color Properties of Schiff Base Compounds Containing a 1,2,3-triazole Group. J Fluoresc. https://doi.org/10.1007/s10895-022-03007-z
Bal M, Tümer M, Köse M (2022) Synthesis of reduced graphene oxide-based hybrid compounds and investigation of their sensing behavior against some nitroaromatic explosives. Mater Chem Phys 289:126480. https://doi.org/10.1016/j.matchemphys.2022.126480
Dolomanov OV, Bourhis LJ, Gildea RJ et al (2009) OLEX2: A complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Sebbar NK, Ellouz M, Essassi EM et al (2015) Crystal structure of 4-benzyl-2H-benzo[b][1,4]thiazin-3(4H)-one. Acta Crystallogr Sect E Crystallogr Commun 71:o999. https://doi.org/10.1107/S2056989015022276
Sheldrick GM (2015) SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr Sect A Found Crystallogr 71:3–8. https://doi.org/10.1107/S2053273314026370
Hu ML, Joharian M, Razavi SAA et al (2021) Phenolic nitroaromatics detection by fluorinated metal-organic frameworks: Barrier elimination for selective sensing of specific group of nitroaromatics. J Hazard Mater 406:124501. https://doi.org/10.1016/j.jhazmat.2020.124501
Mohan B, Kumar S, Ma S et al (2021) Mechanistic insight into charge and energy transfers of luminescent metal-organic frameworks based sensors for toxic chemicals. Adv Sustain Syst 5:1–23. https://doi.org/10.1002/adsu.202000293
Ahamad MN, Shahid M, Ahmad M, Sama F (2019) Cu(II) MOFs based on bipyridyls: topology, magnetism, and exploring sensing ability toward multiple nitroaromatic explosives. ACS Omega 4:7738–7749. https://doi.org/10.1021/acsomega.9b00715
Al-Ameer AHA (2021) Preparation, characterization and antibacterial studies of schiff base derivatives with 4-bromo-2.6-dimethylaniline and study their complexes with some transition metal ions. Int J Drug Deliv Technol 11:190–194. https://doi.org/10.25258/ijddt.11.1.35
Batool R, Riaz N, Junaid HM et al (2022) Fluorene-based fluorometric and colorimetric conjugated polymers for sensitive detection of 2,4,6-trinitrophenol explosive in aqueous medium. ACS Omega 7:1057–1070. https://doi.org/10.1021/acsomega.1c05644
Vaiana AC, Neuweiler H, Schulz A et al (2003) Fluorescence quenching of dyes by tryptophan: interactions at atomic detail from combination of experiment and computer simulation. J Am Chem Soc 125:14564–14572. https://doi.org/10.1021/ja036082j
Xiao F, Pignatello JJ (2015) π+-π Interactions between (hetero)aromatic amine cations and the graphitic surfaces of pyrogenic carbonaceous materials. Environ Sci Technol 49:906–914. https://doi.org/10.1021/es5043029
Boaz H, Rollefson GK (1950) The quenching of fluorescence. Deviations from the Stern-Volmer Law. J Am Chem Soc 72:3435–3443. https://doi.org/10.1021/ja01164a032
Lakowicz JR, Weber G (1973) Quenching of fluorescence by oxygen. a probe for structural fluctuations in macromolecules. Biochemistry 12:4161–4170. https://doi.org/10.1021/bi00745a020
Shanmugaraju S, Joshi SA, Mukherjee PS (2011) Fluorescence and visual sensing of nitroaromatic explosives using electron rich discrete fluorophores. J Mater Chem 21:9130. https://doi.org/10.1039/c1jm10406c
Funding
This work was not supported by any institution, organization or person.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MB and İŞ. Data interpretation was done by MB, İŞ and MK. The first draft of the manuscript was written by MB, İŞ and MK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bal, M., Şahin, İ. & Köse, M. Photophysical and Fluorescence Nitroaromatic Sensing Properties of Methylated Derivative of a Pamoic Acid Ester. J Fluoresc 33, 77–90 (2023). https://doi.org/10.1007/s10895-022-03038-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03038-6