Abstract
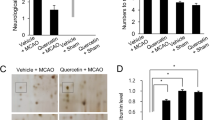
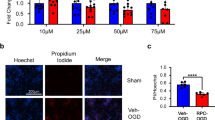
Ischemic stroke is a neurological disease that causes brain damage by increasing oxidative stress and ion imbalance. Retinoic acid is a major metabolite of vitamin A and regulates oxidative stress, calcium homeostasis, and cell death. Intracellular calcium is involved in neuronal growth and synaptic plasticity. Parvalbumin is a calcium-binding protein that is mainly expressed in brain. In this study, we investigated whether retinoic acid has neuroprotective effects by controlling intracellular calcium concentration and parvalbumin expression in ischemic brain damage. Middle cerebral artery occlusion (MCAO) was performed to induce cerebral ischemia. Retinoic acid (5 mg/kg) or vehicle was injected into the abdominal cavity for four days before surgery and cerebral cortices were collected 24 h after MCAO for further studies. MCAO damage induced neurological deficits and histopathological changes and decreased parvalbumin expression. However, retinoic acid treatment alleviated these changes. In cultured neurons, glutamate (5 mM) exposure induced neuronal cell death, increased intracellular calcium concentration, and decreased parvalbumin expression. Retinoic acid treatment attenuated these changes against glutamate toxicity in a dose-dependent manner. It also regulates glutamate induced change in bcl-2 and bax expression. The mitigation effects of retinoic acid were greater under non-transfection conditions than under parvalbumin siRNA transfection conditions. Our findings showed that retinoic acid modulates intracellular calcium concentration and parvalbumin expression and prevents apoptosis in ischemic brain injury. In conclusion, retinoic acid contributes to the preservation of neurons from ischemic stroke by controlling parvalbumin expression and apoptosis-related proteins.








Similar content being viewed by others
Data Availability
Data will be made available on reasonable request.
Code Availability
Not applicable.
References
Mistry EA, Dumont AS (2020) Medium vessel occlusion and acute ischemic stroke: a call for treatment paradigm reappraisal. Stroke 51:3200–3202
Pappachan J, Kirkham FJ (2008) Cerebrovascular disease and stroke. Arch Dis Child 93:890–898
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188
Sims NR, Muyderman H (2010) Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 1802:80–91
Song M, Yu SP (2014) Ionic regulation of cell volume changes and cell death after ischemic stroke. Transl Stroke Res 5:17–27
Yang C, Hawkins KE, Doré S, Candelario-Jalil E (2019) Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol 316:C135–C153
Baker KD, Edwards TM, Rickard NS (2013) The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci Biobehav Rev 37:1211–1239
Weber JT, Rzigalinski BA, Ellis EF (2001) Traumatic injury of cortical neurons causes changes in intracellular calcium stores and capacitative calcium influx. J Biol Chem 276:1800–1807
Ludhiadch A, Sharma R, Muriki A, Munshi A (2022) Role of calcium homeostasis in ischemic stroke: a review. CNS Neurol Disord Drug Targets 21:52–61
Chung JW, Ryu WS, Kim BJ, Yoon BW (2015) Elevated calcium after acute ischemic stroke: association with a poor short-term outcome and long-term mortality. J Stroke 17:54–59
Marambaud P, Dreses-Werringloer U, Vingtdeux V (2009) Calcium signaling in neurodegeneration. Mol Neurodegener 4:1–15
Cates MS, Berry MB, Ho EL, Li Q, Potter JD, Phillips GN Jr (1999) Metal-ion affinity and specificity in EF-hand proteins: coordination geometry and domain plasticity in parvalbumin. Structure 7:1269–1278
Bjerke IE, Yates SC, Laja A, Witter MP, Puchades MA, Bjaalie JG, Leergaard TB (2020) Densities and numbers of calbindin and parvalbumin positive neurons across the rat and mouse brain. iScience 24:101906
Reid RE (1985) The functional nature of calcium binding units in calmodulin, troponin C and parvalbumin. J Theor Biol 114:353–374
Kahl CR, Means AR (2003) Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev 24:719–736
Koledova VV, Khalil RA (2006) Ca2+, calmodulin, and cyclins in vascular smooth muscle cell cycle. Circ Res 98:1240–1243
Ruden JB, Dugan LL, Konradi C (2021) Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacology 46:279–287
Lanoue AC, Blatt GJ, Soghomonian JJ (2013) Decreased parvalbumin mRNA expression in dorsolateral prefrontal cortex in Parkinson’s disease. Brain Res 1531:37–47
Park DJ, Kang JB, Shah FA, Koh PO (2021) Quercetin attenuates the reduction of parvalbumin in middle cerebral artery occlusion animal model. Lab Anim Res 37:9
Wöhr M, Orduz D, Gregory P, Moreno H, Khan U, Vörckel KJ, Wolfer DP, Welzl H, Gall D, Schiffmann SN, Schwaller B (2015) Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl Psychiatry 5:e525
Dräger UC (2006) Retinoic acid signaling in the functioning brain. Sci STKE 2006:pe10
Yu S, Levi L, Siegel R, Noy N (2012) Retinoic acid induces neurogenesis by activating both retinoic acid receptors (RARs) and peroxisome proliferator-activated receptor β/δ (PPARβ/δ). J Biol Chem 287:42195–42205
Wołoszynowska-Fraser MU, Kouchmeshky A, McCaffery P (2020) Vitamin A and retinoic acid in cognition and cognitive disease. Annu Rev Nutr 40:247–272
Lee HP, Casadesus G, Zhu X, Lee HG, Perry G, Smith MA, Gustaw-Rothenberg K, Lerner A (2009) All-trans retinoic acid as a novel therapeutic strategy for Alzheimer’s disease. Expert Rev Neurother 9:1615–1621
Cai W, Wang J, Hu M, Chen X, Lu Z, Bellanti JA, Zheng SG (2019) All trans-retinoic acid protects against acute ischemic stroke by modulating neutrophil functions through STAT1 signaling. J Neuroinflammation 16:175
Kang JB, Park DJ, Shah MA, Koh PO (2021) Retinoic acid exerts neuroprotective effects against focal cerebral ischemia by preventing apoptotic cell death. Neurosci Lett 757:135979
Aoto J, Nam CI, Poon MM, Ting P, Chen L (2008) Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron 60:308–320
Lenz M, Kruse P, Eichler A, Straehle J, Beck J, Deller T, Vlachos A (2021) All-trans retinoic acid induces synaptic plasticity in human cortical neurons. Elife 10:e63026
Chatzi C, Brade T, Duester G (2011) Retinoic acid functions as a key GABAergic differentiation signal in the basal ganglia. PLoS Biol 9:e1000609
Håglin S, Berghard A, Bohm S (2020) Increased retinoic acid catabolism in olfactory sensory neurons activates dormant tissue-specific stem cells and accelerates age-related metaplasia. J Neurosci 40:4116–4129
Riancho J, Berciano MT, Ruiz-Soto M, Berciano J, Landreth G, Lafarga M (2016) Retinoids and motor neuron disease: Potential role in amyotrophic lateral sclerosis. J Neurol Sci 360:115–120
Kang JB, Park DJ, Shah MA, Koh PO (2022) Quercetin ameliorates glutamate toxicity-induced neuronal cell death by controlling calcium-binding protein parvalbumin. J Vet Sci 23:e26
Kong L, Wang Y, Wang XJ, Wang XT, Zhao Y, Wang LM, Chen ZY (2015) Retinoic acid ameliorates blood-brain barrier disruption following ischemic stroke in rats. Pharmacol Res 99:125–136
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC (2000) Cognitive deficits after focal cerebral ischemia in mice. Stroke 31:1939–1944
Takeshita H, Yamamoto K, Nozato S, Inagaki T, Tsuchimochi H, Shirai M, Yamamoto R, Imaizumi Y, Hongyo K, Yokoyama S, Takeda M, Oguro R, Takami Y, Itoh N, Takeya Y, Sugimoto K, Fukada SI, Rakugi H (2017) Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci Rep 7:42323
Markgraf CG, Green EJ, Hurwitz BE, Morikawa E, Dietrich WD, McCabe PM, Ginsberg MD, Schneiderman N (1992) Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res 575:238–246
Kim DH, Kim DW, Jung BH, Lee JH, Lee H, Hwang GS, Kang KS, Lee JW (2019) Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J Ginseng Res 43:326–334
Brossaud J, Roumes H, Moisan MP, Pallet V, Redonnet A, Corcuff JB (2013) Retinoids and glucocorticoids target common genes in hippocampal HT22 cells. J Neurochem 125:518–531
Kao JP, Harootunian AT, Tsien RY (1989) Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem 264:8179–8184
Ahlemeyer B, Bauerbach E, Plath M, Steuber M, Heers C, Tegtmeier F, Krieglstein J (2001) Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic Biol Med 30:1067–1077
Kitamura M, Ishikawa Y, Moreno-Manzano V, Xu Q, Konta T, Lucio-Cazana J, Furusu A, Nakayama K (2002) Intervention by retinoic acid in oxidative stress-induced apoptosis. Nephrol Dial Transplant 9:84–87
Xing HY, Meng EY, Xia YP, Peng H (2015) Effect of retinoic acid on expression of LINGO-1 and neural regeneration after cerebral ischemia. J Huazhong Univ Sci Technolog Med Sci 35:54–57
Sato Y, Meller R, Yang T, Taki W, Simon RP (2008) Stereo-selective neuroprotection against stroke with vitamin A derivatives. Brain Res 1241:188–192
Baimbridge KG, Celio MR, Rogers JH (1992) Calcium-binding proteins in the nervous system. Trends Neurosci 15:303–308
Bischop DP, Orduz D, Lambot L, Schiffmann SN, Gall D (2012) Control of neuronal excitability by calcium binding proteins: a new mathematical model for striatal fast-spiking interneurons. Front Mol Neurosci 5:78
Baev AY, Vinokurov AY, Novikova IN, Dremin VV, Potapova EV, Abramov AY (2022) Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells 11:706
Koh PO (2012) Melatonin regulates the calcium-buffering proteins, parvalbumin and hippocalcin, in ischemic brain injury. J Pineal Res 53:358–365
Kristián T, Siesjö BK (1998) Calcium in ischemic cell death. Stroke 29:705–718
Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P (2012) Mitochondrial Ca(2+) and apoptosis. Cell Calcium 52:36–43
Fairless R, Williams SK, Diem R (2019) Calcium-binding proteins as determinants of central nervous system neuronal vulnerability to disease. Int J Mol Sci 20:2146
Ouh IO, Kim YM, Gim SA, Koh PO (2013) Focal cerebral ischemic injury decreases calbindin expression in brain tissue and HT22 cells. Lab Anim Res 29:156–161
Yuan HH, Chen RJ, Zhu YH, Peng CL, Zhu XR (2013) The neuroprotective effect of overexpression of calbindin-D(28k) in an animal model of Parkinson’s disease. Mol Neurobiol 47:117–122
Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A (2000) Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci 97:13372–13377
Kostandy BB (2012) The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol Sci 33:223–237
Guo H, Camargo LM, Yeboah F, Digan ME, Niu H, Pan Y, Reiling S, Soler-Llavina G, Weihofen WA, Wang HR, Shanker YG, Stams T, Bill A (2017) A NMDA-receptor calcium influx assay sensitive to stimulation by glutamate and glycine/D-serine. Sci Rep 7:11608
Wang YZ, Christakos S (1995) Retinoic acid regulates the expression of the calcium binding protein, calbindin-D28K. Mol Endocrinol 9:1510–1521
Sakamoto K, Hiraiwa M, Saito M, Nakahara T, Sato Y, Nagao T, Ishii K (2010) Protective effect of all-trans retinoic acid on NMDA-induced neuronal cell death in rat retina. Eur J Pharmacol 635:56–61
Guerra MT, Fonseca EA, Melo FM, Andrade VA, Aguiar CJ, Andrade LM, Pinheiro AC, Casteluber MC, Resende RR, Pinto MC, Fernandes SO, Cardoso VN, Souza-Fagundes EM, Menezes GB, de Paula AM, Nathanson MH, Leite Mde F (2011) Mitochondrial calcium regulates rat liver regeneration through the modulation of apoptosis. Hepatology 54:296–306
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government [MEST][NRF-2021R1F1A105878711].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection, analysis, and manuscript writing were performed by JK. Material preparation and data collection were performed by DP. PK wrote and supervised the research. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, JB., Park, DJ. & Koh, PO. Retinoic Acid Prevents the Neuronal Damage Through the Regulation of Parvalbumin in an Ischemic Stroke Model. Neurochem Res 48, 487–501 (2023). https://doi.org/10.1007/s11064-022-03769-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03769-9