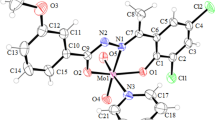
CoL3·DMF, [CoCdCl2L3]·0.5H2O, and [CoMnCl2L3]·H2O complexes, where HL is a product of the condensation of o-vanillin and methylamine have been obtained. The crystal structure of the latter complex has been determined by X-ray structural analysis. The study of the antioxidant activity of these complexes in a model reaction of radical-chain oxidation of benzyl alcohol with an atmospheric oxygen has proved a significant inhibition of this process at concentrations of the complexes of 10–7-10–6 mol/L. It is established that the inhibitory activity of the compounds depends on the nature of the metal ions present in the complex.





Similar content being viewed by others
References
J. C. Pessoa and I. Correia, Coord. Chem. Rev., 388, 227-247 (2019).
X. Wang, N. Ling, Y.-W. Zhang, et al., Inorg. Chem. Commun., 110, 107585 (2019).
S. S. Shah, D. Shah, I. Khan, et al., Biointerface Res. Appl. Chem., 10, 6936-6963 (2020).
O. O. Bozhko, O. D. Kachkovskyi, L. Ye. Kalashnikova, et al., Kataliz i Neftekhimiya, No. 27, 25-32 (2018).
M. Andruh, ChemComm., 54, 3559-3577 (2018).
O. V. Nesterova, K. V. Kasyanova, E. A. Buvaylo, et al., Catalysts, 9, 209 (2019).
N. A. Davidenko and V. N. Kokozay, Theor. Exp. Chem., 53, 69-92 (2017).
O. V. Nesterova, K. V. Kasyanova, V. G. Makhankova, et al., Appl. Catal. A., 560, 171-184 (2018).
G. M. Sheldrick, Acta Crystallogr. C., 71, 3-8 (2015).
G. A. Kovtun, T. M. Kameneva, V. V. Pavlischuk, et al., Kataliz i Neftekhimiya, No. 14, 91-94 (2006).
G. Yadav, S. Tiwari, and M. L. Jain, Mater. Today, 5, No. 1, Part 1, 248-253 (2018).
S. D. Chatziefthimiou, Y. G. Lazarou, E. Hadjoudis, et al., J. Phys. Chem. B., 110, 23701-23709 (2006).
E. A. Buvaylo, O. Nesterova, V. N. Kokozay, et al., Cryst. Growth Des., 12, 3200-3208 (2012).
O. Y. Vassilyeva, E. A. Buvaylo, V. N. Kokozay, et al., Dalton Trans., 50, 2841-2853 (2021).
A. Bottcher, T. Takeuchi, K. I. Hardcastle, et al., Inorg. Chem., 36, 2498-2504 (1997).
V. V. Goncharuk, G. L. Kamalov, G. A. Kovtun, et al., Catalysis. Mechanisms of Homogeneous and Heterogeneous catalysis, Cluster Approaches [in Russian], Naukova Dumka, Kyiv (2002).
Acknowledgements
The work was performed with the support of the Ministry of Education and Science of Ukraine (project 22BP037-13, contract No. BF/30-2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 58, No. 3, pp. 193-198, May-June, 2022.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kokozay, V.N., Polunkin, E.V., Vassilyeva, O.Y. et al. Homo- and Heteronuclear Cobalt(III)-Based Complexes with 2-Methoxy-6-[(Methylimino)Methyl]Phenol: Structure and Antioxidant Properties. Theor Exp Chem 58, 213–219 (2022). https://doi.org/10.1007/s11237-022-09738-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-022-09738-1