Abstract
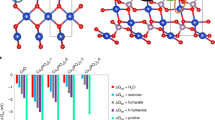
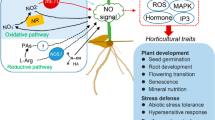
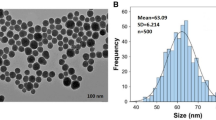
Nanobiotechnology approaches to engineering crops with enhanced stress tolerance may be a safe and sustainable strategy to increase crop yield. Under stress conditions, cellular redox homeostasis is disturbed, resulting in the over-accumulation of reactive oxygen species (ROS) that damage biomolecules (lipids, proteins and DNA) and inhibit crop growth and yield. Delivering ROS-scavenging nanomaterials to plants has been shown to alleviate abiotic stress. Here we review the current state of knowledge of using ROS-scavenging nanomaterials to enhance plant stress tolerance. When present below a threshold level, ROS can mediate redox signalling and defence pathways that foster plant acclimatization against stress. We find that ROS-triggering nanomaterials, such as nanoparticulate silver and copper oxide, have the potential to be judiciously applied to crop species to stimulate the defence system, prime stress responses and subsequently increase the stress resistance of crops.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lata, R., Chowdhury, S., Gond, S. K. & White, J. F. Jr. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 66, 268–276 (2018).
Acevedo, M. et al. A scoping review of adoption of climate-resilient crops by small-scale producers in low- and middle-income countries. Nat. Plants 6, 1231–1241 (2020).
Choudhury, F. K., Rivero, R. M., Blumwald, E. & Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 90, 856–867 (2017).
Kundu, P. et al. in Advancement in Crop Improvement Techniques (eds Tuteja, N. et al.) 241–262 (Woodhead, 2020).
Wu, J. et al. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 48, 1004–1076 (2019).
Korsvik, C., Patil, S., Seal, S. & Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 1056–1058 (2007).
Giraldo, J. P. et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 13, 400–408 (2014).
Mittler, R., Zandalinas, S. I., Fichman, Y. & Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-022-00499-2 (2022).
Castro, B. et al. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 7, 403–412 (2021).
Ray, P. D., Huang, B.-W. & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990 (2012).
Corpas, F. J., Gupta, D. K. & Palma, J. M. in Reactive Oxygen Species and Oxidative Damage in Plants Under Stress (eds Gupta, D. K. et al.) 1–22 (Springer, 2015).
Sachdev, S., Ansari, S. A., Ansari, M. I., Fujita, M. & Hasanuzzaman, M. Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10, 277 (2021).
Hasanuzzaman, M. et al. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9, 681 (2020).
Mhamdi, A. & Van Breusegem, F. Reactive oxygen species in plant development. Development 145, dev164376 (2018).
You, J. & Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 6, 1092 (2015).
Tripathy, B. C. & Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 7, 1621–1633 (2012).
Chapman, J. M., Muhlemann, J. K., Gayomba, S. R. & Muday, G. K. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem. Res. Toxicol. 32, 370–396 (2019).
Wu, H., Tito, N. & Giraldo, J. P. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 11, 11283–11297 (2017).
Wu, H., Shabala, L., Shabala, S. & Giraldo, J. P. Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environ. Sci. Nano 5, 1567–1583 (2018).
Djanaguiraman, M., Nair, R., Giraldo, J. P. & Prasad, P. V. V. Cerium oxide nanoparticles decrease drought-induced oxidative damage in sorghum leading to higher photosynthesis and grain yield. ACS Omega 3, 14406–14416 (2018).
Liu, Y. et al. Foliar-applied cerium oxide nanomaterials improve maize yield under salinity stress: reactive oxygen species homeostasis and rhizobacteria regulation. Environ. Pollut. 299, 118900 (2022).
Liu, J. et al. Cerium oxide nanoparticles improve cotton salt tolerance by enabling better ability to maintain cytosolic K(+)/Na(+) ratio. J. Nanobiotechnol. 19, 153–153 (2021).
Gohari, G. et al. Protective effects of cerium oxide nanoparticles in grapevine (Vitis vinifera L.) cv. flame seedless under salt stress conditions. Ecotoxicol. Environ. Saf. 220, 112402 (2021).
Mohammadi, M. H. Z. et al. Cerium oxide nanoparticles (CeO2-NPs) improve growth parameters and antioxidant defense system in Moldavian balm (Dracocephalum moldavica L.) under salinity stress. Plant Stress 1, 100006 (2021).
Wong, E. L. S., Vuong, K. Q. & Chow, E. Nanozymes for environmental pollutant monitoring and remediation. Sensors 21, 408 (2021).
Yao, J. et al. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem. Sci. 9, 2927–2933 (2018).
Lu, L. et al. Mn3O4 nanozymes boost endogenous antioxidant metabolites in cucumber (Cucumis sativus) plant and enhance resistance to salinity stress. Environ. Sci. Nano 7, 1692–1703 (2020).
Zhang, Y. et al. Star polymers with designed reactive oxygen species scavenging and agent delivery functionality promote plant stress tolerance. ACS Nano 16, 4467–4478 (2022).
Wang, Y. et al. Alleviation of nitrogen stress in rice (Oryza sativa) by ceria nanoparticles. Environ. Sci. Nano 7, 2930–2940 (2020).
Chen, Z. et al. Graphene enhances photosynthesis and the antioxidative defense system and alleviates salinity and alkalinity stresses in alfalfa (Medicago sativa L.) by regulating gene expression. Environ. Sci. Nano 8, 2731–2748 (2021).
Zhou, H. et al. Molecular basis of cerium oxide nanoparticle enhancement of rice salt tolerance and yield. Environ. Sci. Nano 8, 3294–3311 (2021).
An, J. et al. Emerging investigator series: molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ. Sci. Nano 7, 2214–2228 (2020).
Khan, M. N. et al. Nanoceria seed priming enhanced salt tolerance in rapeseed through modulating ROS homeostasis and α-amylase activities. J. Nanobiotechnol. 19, 276 (2021).
Suzuki, N. et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25, 3553–3569 (2013).
Avramova, Z. Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 83, 149–159 (2015).
Bruce, T. J. A., Matthes, M. C., Napier, J. A. & Pickett, J. A. Stressful ‘memories’ of plants: evidence and possible mechanisms. Plant Sci. 173, 603–608 (2007).
Nair, A. U., Bhukya, D. P. N., Sunkar, R., Chavali, S. & Allu, A. D. Molecular basis of priming-induced acquired tolerance to multiple abiotic stresses in plants. J. Exp. Bot. 73, 3355–3371 (2022).
He, W., Zhou, Y. T., Wamer, W. G., Boudreau, M. D. & Yin, J. J. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 33, 7547–7555 (2012).
Foldbjerg, R., Dang, D. A. & Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 85, 743–750 (2011).
Khan, I. et al. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): the oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 156, 221–232 (2020).
Li, Y. et al. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 91, 509–519 (2017).
Yan, X. et al. Rice exposure to silver nanoparticles in a life cycle study: effect of dose responses on grain metabolomic profile, yield, and soil bacteria. Environ. Sci. Nano 9, 2195–2206 (2022).
Paparella, S. et al. Seed priming: state of the art and new perspectives. Plant Cell Rep. 34, 1281–1293 (2015).
Zhou, X. et al. AgNPs seed priming accelerated germination speed and altered nutritional profile of Chinese cabbage. Sci. Total Environ. 808, 151896 (2022).
Acharya, P., Jayaprakasha, G. K., Crosby, K. M., Jifon, J. L. & Patil, B. S. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci Rep. 10, 5037 (2020).
Guha, T., Das, H., Mukherjee, A. & Kundu, R. Elucidating ROS signaling networks and physiological changes involved in nanoscale zero valent iron primed rice seed germination sensu stricto. Free Radic. Biol. Med. 171, 11–25 (2021).
Saleem, M., Fariduddin, Q. & Castroverde, C. D. M. Salicylic acid: a key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 168, 381–397 (2021).
El-Shetehy, M. et al. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 16, 344–353 (2021).
Ma, C. et al. Advanced material modulation of nutritional and phytohormone status alleviates damage from soybean sudden death syndrome. Nat. Nanotechnol. 15, 1033–1042 (2020).
Zhao, L. et al. Activation of antioxidant and detoxification gene expression in cucumber plants exposed to a Cu(OH)2 nanopesticide. Environ. Sci. Nano 4, 1750–1760 (2017).
Avellan, A. et al. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 13, 5291–5305 (2019).
Shen, Y. et al. Role of foliar biointerface properties and nanomaterial chemistry in controlling Cu transfer into wild-type and mutant Arabidopsis thaliana leaf tissue. J. Agric. Food Chem. 70, 4267–4278 (2022).
Hong, J. et al. Foliar application of nanoparticles: mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 8, 1196–1210 (2021).
Su, Y. et al. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: a critical review and data analysis. Environ. Sci. Nano 6, 2311–2331 (2019).
Wu, H. & Li, Z. Nano-enabled agriculture: how do nanoparticles cross barriers in plants? Plant Commun. https://doi.org/10.1016/j.xplc.2022.100346 (2022).
Chen, L. et al. CeO2 nanoparticles improved cucumber salt tolerance is associated with its induced early stimulation on antioxidant system. Chemosphere 299, 134474 (2022).
Lv, J., Christie, P. & Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges. Environ. Sci. Nano 6, 41–59 (2018).
Amritha, M. S., Sridharan, K., Puthur, J. T. & Dhankher, O. P. Priming with nanoscale materials for boosting abiotic stress tolerance in crop plants. J. Agric. Food Chem. 69, 10017–10035 (2021).
Elhaj Baddar, Z. & Unrine, J. M. Effects of soil pH and coatings on the efficacy of polymer coated ZnO nanoparticulate fertilizers in wheat (Triticum aestivum). Environ. Sci. Technol. 55, 13532–13540 (2021).
Kasote, D. M., Lee, J. H. J., Jayaprakasha, G. K. & Patil, B. S. Seed priming with iron oxide nanoparticles modulate antioxidant potential and defense-linked hormones in watermelon seedlings. ACS Sustain. Chem. Eng. 7, 5142–5151 (2019).
Naseer, M. et al. Nano-enabled improvements of growth and colonization rate in wheat inoculated with arbuscular mycorrhizal fungi. Environ. Pollut. 295, 118724 (2022).
Mickelbart, M. V., Hasegawa, P. M. & Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 16, 237–251 (2015).
Ma, W. et al. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 13, 529 (2022).
Lee, D. et al. Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat. Commun. 11, 1838 (2020).
Wu, D. et al. Engineering Fe–N doped graphene to mimic biological functions of NADPH oxidase in cells. J. Am. Chem. Soc. 142, 19602–19610 (2020).
Acknowledgements
L.Z. acknowledges grants from the National Science Foundation of China (21876081 and 21906081).
Author information
Authors and Affiliations
Contributions
L.Z. and J.C.W. discussed and wrote the review; T.B. assisted in reference collection and designed the figures; and H.W., J.L.G.-T. and A.K. helped with the critical review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Food thanks Honghong Wu, Om Parkash Dhankher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, L., Bai, T., Wei, H. et al. Nanobiotechnology-based strategies for enhanced crop stress resilience. Nat Food 3, 829–836 (2022). https://doi.org/10.1038/s43016-022-00596-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43016-022-00596-7
This article is cited by
-
Impact of seed priming with Selenium nanoparticles on germination and seedlings growth of tomato
Scientific Reports (2024)
-
Nanopriming with magnesium oxide nanoparticles enhanced antioxidant potential and nutritional richness of radish leaves grown in field
Clean Technologies and Environmental Policy (2024)
-
Chiral nanomaterial-enabled bactericide and nanopesticide for sustainable agriculture and food safety
Journal of Nanoparticle Research (2023)
-
Nano-enabled crop resilience against pathogens: potential, mechanisms and strategies
Crop Health (2023)
-
Molecular insights of nanozymes from design to catalytic mechanism
Science China Chemistry (2023)