Abstract
Summary
A post-menopausal Caucasian woman sustained an atypical femoral fracture (AFF) after 5-years continuous denosumab for osteoporosis without prior bisphosphonate exposure. This is only the fifth case reported of AFF in a bisphosphonate-naïve patient receiving denosumab for osteoporosis. Although rare, physicians should consider AFF in patients taking denosumab even without prior bisphosphonate exposure.
Introduction
Denosumab has demonstrated overwhelmingly favourable skeletal benefit/risk profile in managing post-menopausal osteoporosis with up to 10-year exposure in the extension of the pivotal FREEDOM randomised placebo-controlled trial. Four previous cases of atypical femoral fracture have been reported in bisphosphonate-naïve patients receiving denosumab for osteoporosis.
Methods
We present an 85-year-old Caucasian post-menopausal woman without prior fragility fracture who sustained unilateral atypical femoral fracture after 5-years continuous subcutaneous denosumab for osteoporosis. She had no prior bisphosphonate or glucocorticoid exposure and had known chronic kidney disease.
Results
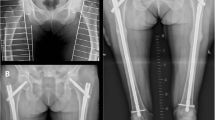
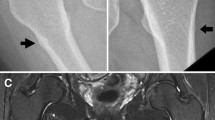
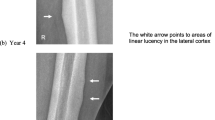
X-ray scan demonstrated complete, non-comminuted left proximal femoral shaft fracture meeting radiographic criteria for an atypical femoral fracture. Computed tomography (CT) scan lower limbs revealed unusual side-by-side appearance of proximal and distal fragments of left proximal femur. DXA BMD was artefactually elevated at lumbar spine (1.504 g/cm2, T-score + 2.5) and low-normal at right femoral neck (0.856 g/cm2, T-score -1.0). Serum biochemistry showed renal impairment at baseline (eGFR 30 mL/min/1.73m2). Low-normal serum C telopeptide of type 1 collagen (CTX) (230 ng/L) indicated therapeutic suppression of bone resorption.
Conclusion
The patient underwent intramedullary nailing of the femur and denosumab was ceased. This is only the fifth case reported of atypical femoral fracture in a bisphosphonate-naïve patient receiving denosumab for osteoporosis. Although rare, physicians should maintain a high index of suspicion for atypical femoral fracture in a patient taking denosumab even without prior bisphosphonate exposure.


Similar content being viewed by others
Data availability
No datasets were generated for this study.
References
Black DM, Abrahamsen B, Bouxsein ML et al (2019) Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr Rev 40(2):333–368. https://doi.org/10.1210/er.2018-00001
Black DM, Rosen CJ (2016) Clinical practice: postmenopausal osteoporosis. N Engl J Med 374(3):254–262. https://doi.org/10.1056/NEJMcp1513724
Shane E, Burr D, Abrahamsen B, Adler RA et al (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 29(1):1–23. https://doi.org/10.1002/jbmr.1998
Dell RM, Adams AL, Greene DF et al (2012) Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 27(12):2544–2550. https://doi.org/10.1002/jbmr.1719
Zhou W, van Rooij JGJ, Ebeling PR et al (2021) The genetics of atypical femur fractures-a systematic review. Curr Osteoporos Rep 19(2):123–130. https://doi.org/10.1007/s11914-021-00658-y
Baron R, Ferrari S, Russell RG (2011) Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48(4):677–692. https://doi.org/10.1016/j.bone.2010.11.020
Yang SP, Kim TW, Boland PJ et al (2017) Retrospective review of atypical femoral fracture in metastatic bone disease patients receiving denosumab therapy. Oncologist 22(4):438–444. https://doi.org/10.1634/theoncologist.2016-0192
Takahashi M, Ozaki Y, Kizawa R, Masuda J et al (2019) Atypical femoral fracture in patients with bone metastasis receiving denosumab therapy: a retrospective study and systematic review. BMC Cancer 19(1):980. https://doi.org/10.1186/s12885-019-6236-6
Van de Laarscot DM, McKenna MJ, Abrahamsen B et al (2020) Medical management of patients after atypical femur fractures: a systematic review and recommendations from the European Calcified Tissue Society. J Clin Endocrinol Metab 105(5):1682–1699. https://doi.org/10.1210/clinem/dgz295
Bone HG, Chapurlat R, Brandi ML et al (2013) The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 98(11):4483–4492. https://doi.org/10.1210/jc.2013-1597
Papapoulos S, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int 26(12):2773–2783. https://doi.org/10.1007/s00198-015-3234-7
Bone HG, Wagman RB, Brandi ML et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5(7):513–523. https://doi.org/10.1016/S2213-8587(17)30138-9
Saag KG, Wagman RB, Geusens P et al (2018) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 6(6):445–454. https://doi.org/10.1016/S2213-8587(18)30075-5
Goh JKM, Koh JSB, Ng ACM et al (2022) Bilateral atypical femur fractures after denosumab in a bisphosphonate naïve patient: a case report. Calcif Tissue Int. https://doi.org/10.1007/s00223-022-00952-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants for whom identifying information is included in this article.
Conflicts of interests
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Chang, R., Reyes, M. et al. Atypical femoral fracture in a bisphosphonate-naïve patient on denosumab for osteoporosis. Arch Osteoporos 17, 131 (2022). https://doi.org/10.1007/s11657-022-01166-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-022-01166-x