- 1Department of General Surgery, Wujin Hospital Affiliated with Jiangsu University, Changzhou, China
- 2Department of General Surgery, The Wujin Clinical college of Xuzhou Medical University, Changzhou, China
- 3Department of Hepatological Surgery, Affiliated Hospital of Jiangnan University, Wuxi, China
- 4Department of General Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 5Department of Digestion, Wujin Hospital Affiliated with Jiangsu University, Changzhou, China
- 6Department of Gastrointestinal Surgery, The Fifth General Hospital of Kunming, Kunming, China
- 7Department of Gastrointestinal Surgery, The Third Affiliated Hospital of Soochow University, Changzhou, China
- 8Changzhou Key Laboratory of Molecular Diagnostics and Precision Cancer Medicine, Wujin Hospital Affiliated with Jiangsu University, Changzhou, China
Objectives: To develop a novel difficulty scoring system (NDSS) to predict the surgical difficulty of laparoscopic hepatectomy.
Patients and methods: A total of 138 patients with liver tumors performed liver resection (LLR) between March 2017 to June 2022 were selected from Affiliated Hospital of Jiangnan University and Wujin Hospital Affiliated with Jiangsu University.
Patient demographics, laboratory tests, intraoperative variables, pathological characteristics were assessed. We also assessed the Child Pugh score and the DSS-B score.
Results: Patients were divided into training and testing cohort according to their hospital. Patients in training cohort were divided into high and low difficult groups based on operation time, blood loss and conversion. Higher percentage of patients with malignant liver tumor (87.0% vs. 58.1%; P = 0.003) or history of hepatobiliary surgery (24.1% vs. 7.0%; P = 0.043) in high difficult group than in low difficult group. To improve the difficulty scoring system, we incorporated the history of hepatobiliary surgery and nature of the tumor. A novel difficulty scoring system was established. The results showed that the operation time (P < 0.001), blood loss (P < 0.001), ALT (P < 0.001) and AST (P = 0.001) were associated with the novel difficulty score significantly. Compared with DSS-B, the NDSS has a higher area under the receiver operating characteristic (AUROC) (0.838 vs. 0.814). The nomogram was established according to the NDSS. The AUROCs of the nomogram in training and testing cohort were 0.833 and 0.767. The calibration curves for the probability of adverse event showed optimal agreement between the probability as predicted by the nomogram and the actual probability.
Conclusions: We developed a nomogram with the NDSS that can predict the difficulty of LLR. This system could more accurately reflect the difficulty of surgery and help liver surgeons to make the surgical plan and ensure the safety of the operation.
Introduction
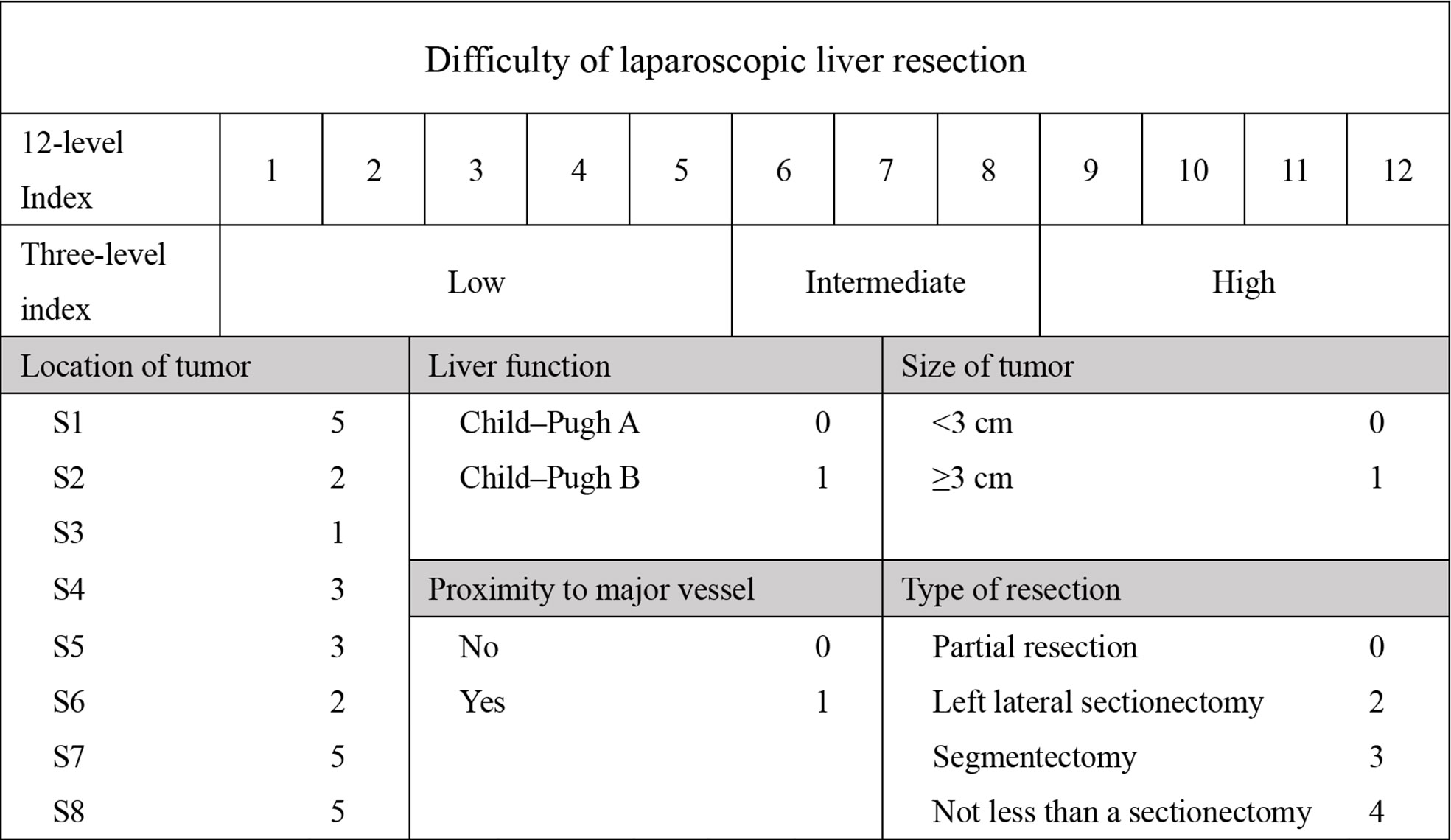
With the first case of laparoscopic liver resection (LLR) reported in 1992, LLR as a treatment for liver tumors has been developed in major centres (1, 2). In the early days after LLR was introduced, it was limited to local hepatectomy, but now expanded hemihepatectomy and laparoscopic repeat liver resection (LRLR) are no longer contraindicated (3). Compared to open liver resection (OLR), there was less blood loss, shorter hospital stays, and fewer postoperative complications (4). In 2008, the first International Consensus Conference on Laparoscopic Hepatectomy (ICCLLR) was held in the United States, where LLR was identified as a safe and effective treatment for liver disease (5). And in 2014, the second ICCLLR was held in Japan, where the surgical indications were expanded and highlighted the assessment of surgical difficulty was believed important (6). The most used difficulty scoring system was Ban Difficulty Scoring System (DSS-B), which was developed by Japanese scientists Ban in 2014 (7). The scoring system included five factors: the extent of liver resection, tumor location, tumor size, proximity to major blood vessels, and Child-Pugh score of liver function. With the development of LLR around the world in recent years, some other factors affecting the difficulty of LLR have been gradually found. For instance, Uchida et al. assessed the surgical outcomes of LLR in patients with liver cirrhosis with specific reference to a difficulty scoring system (8). Kinoshita et al. investigate the predictive factors and classifications for the difficulty of laparoscopic repeated liver resection (LRLR) in patients with recurrent hepatocellular carcinoma (9). In addition, Takase et al. found that the operation time of LRLR was longer than that of laparoscopic primary liver resection (LPLR). Moreover, there was no score for caudate lobe tumors in DSS-B. Based on the above, we believed that some other factors including the history of hepatectomy may also increase the difficulty of LLR. Therefore, we intend to develop a novel difficulty scoring system (NDSS) to predict the surgical difficulty of laparoscopic hepatectomy.
Methods
Patients
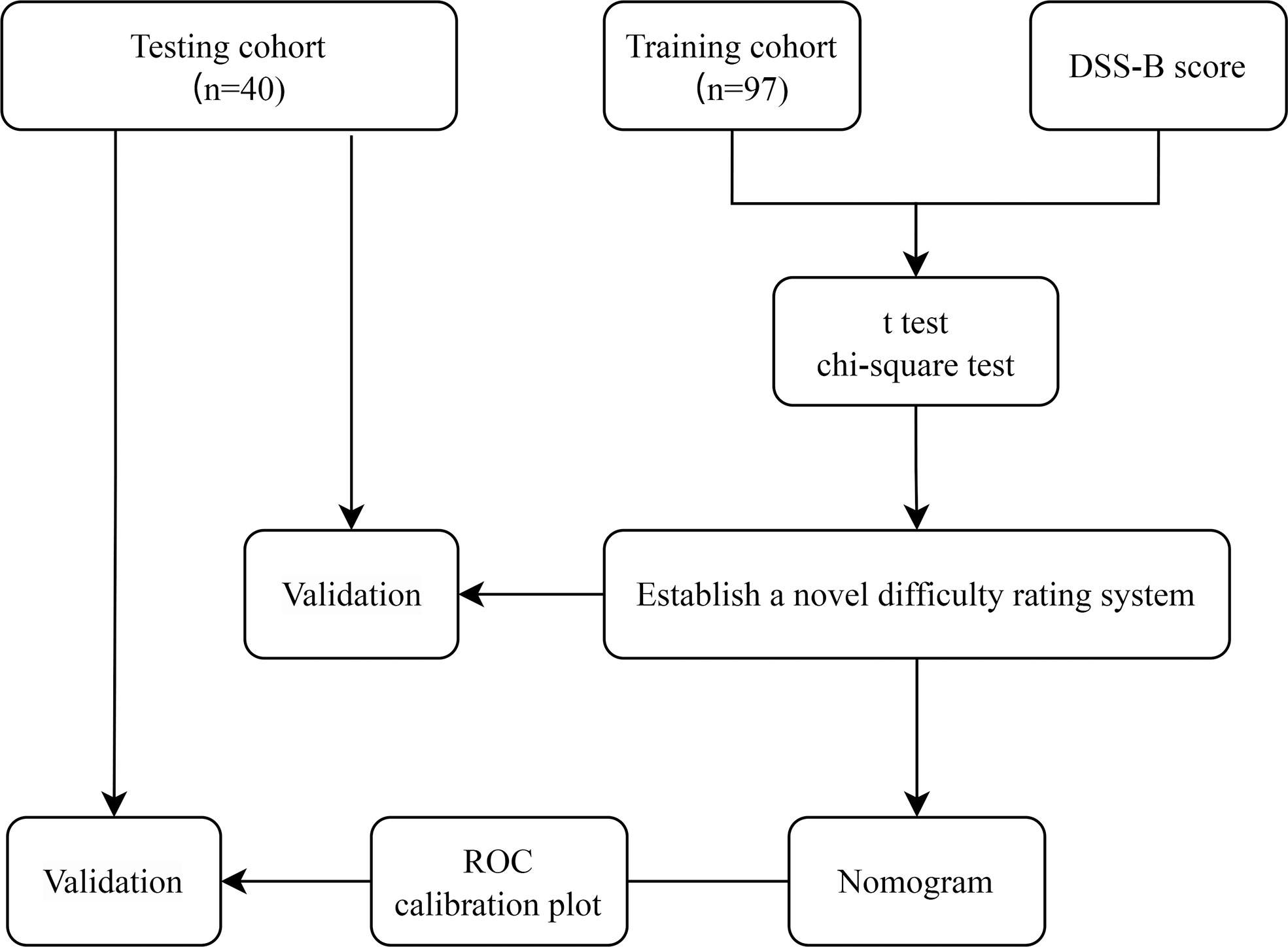
From December 2020 to March 2022, 97 patients (training cohort) who performed LLR for liver tumor were selected at the Department of Hepatological Surgery, Affiliated Hospital of Jiangnan University. From March 2017 to June 2022, 41 patients (testing cohort) who performed LLR for liver tumor were selected at the Department of General Surgery, Wujin Hospital Affiliated with Jiangsu University. Patients who had also undergone lymph node dissection or other organ resection (except cholecystectomy) were excluded. This research was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University (LS2021078) and Wujin Hospital Affiliated with Jiangsu University (2022-SR-084).
Data collection
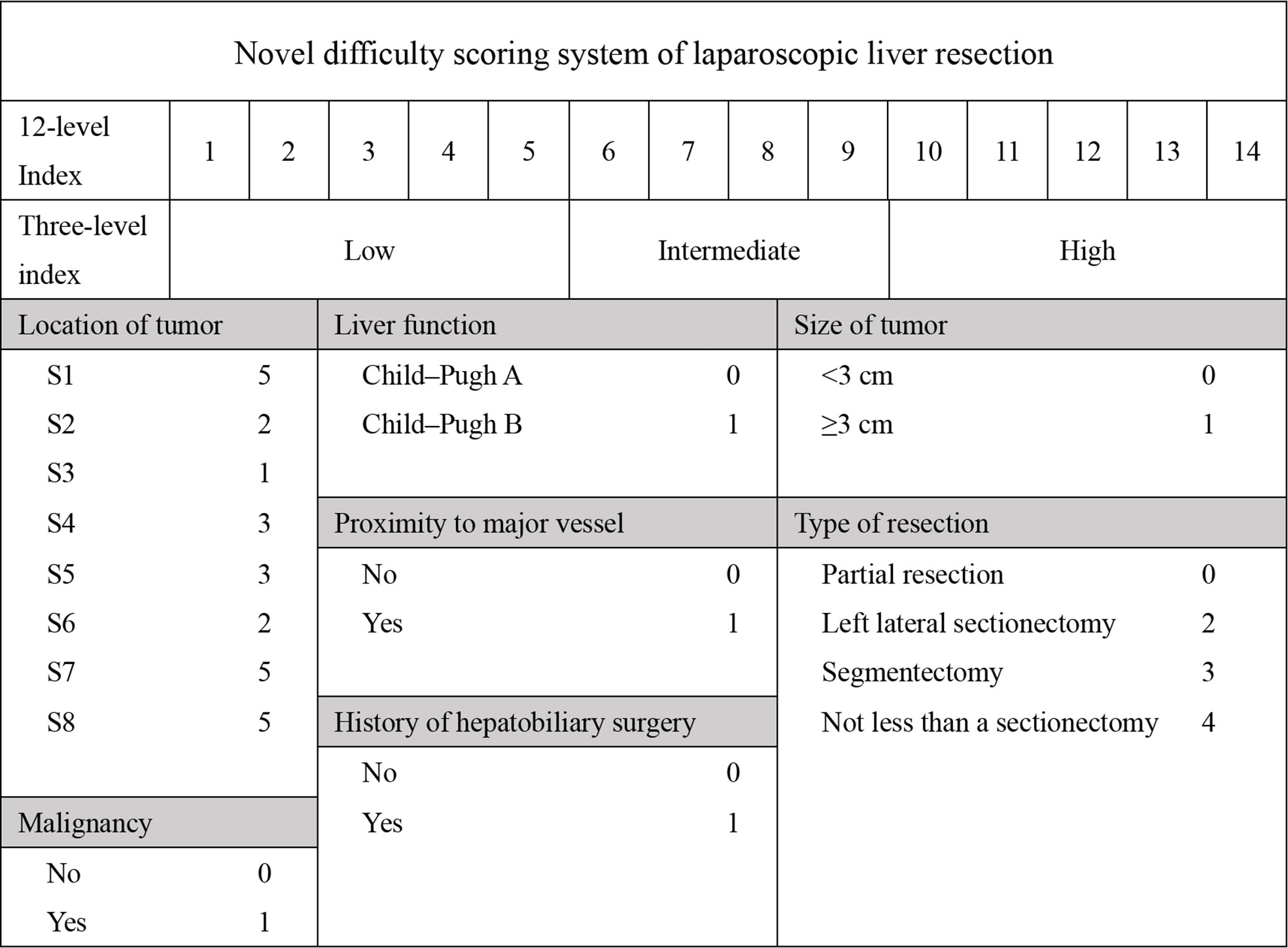
Patient demographics included age, gender, comorbidity, and history of surgery. Laboratory tests included alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), prothrombin time (PT), total bilirubin (TB), white blood cell count (WBC), C-reactive protein (CRP). Intraoperative variables included operation time, blood loss, blood transfusion and postoperative stay (POS). Pathological characteristics included tumor size, tumor position, and pathological pattern. We also assessed the Child-Pugh score and the DSS-B score (7, 10). To accommodate all patients, we rated caudate lobe tumors at 5 points. The detailed grading is shown in Figure 1. Postoperative complications included haemorrhage, bile leakage, ileus, pneumonia, pleural effusion, abdominal infection, liver failure, and incision infection. Postoperative hospitalization days were also recorded.
Statistical analysis
Statistical analyses were conducted with Prism 9.0.1 (GraphPad Software, LLC). For continuous variables, data were expressed as mean ± standard deviation (SD), and the differences between the two groups were analyzed by the two independent samples Student t-test and Mann Whitney test. The differences among groups (more than two) were analyzed by one-factor analysis of variance (One-Way ANOVA). For categorical variables, the differences between groups were analyzed by the chi-square test, Chi-square with Yates’ correction, and Fisher’s exact test according to the sample size. Linear regression was used to predict the correlation between variables. The accuracy of different difficulty scoring systems was compared by the receiver operating characteristic (ROC) curve. Calibration plot for incidence of high difficulty was generated to assess the performance characteristics of the constructed difficulty scoring systems. The nomograms were established by the “rms” package in R version 4.2.0. We also draw the calibration plots for the adverse event rate were generated to assess the performance characteristics of the constructed nomograms. Bootstraps with 1000 resample were used for validation of the nomogram and C-index. The ROC curve and calibration plot were drawn by RStudio software (Version 1.4.1103).
Results
Patient characteristics and surgical outcomes
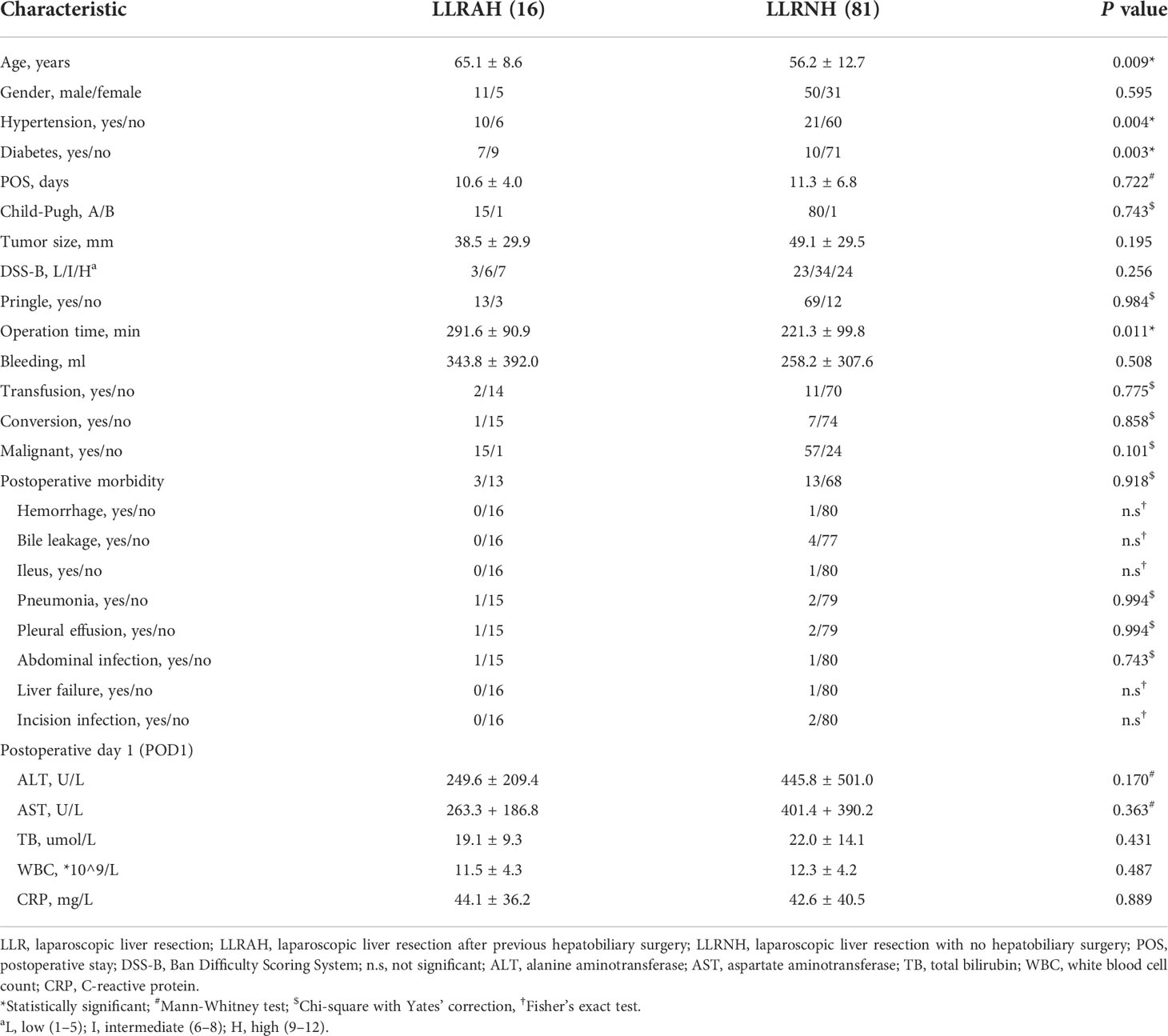
The flow chart of the study is shown in Figure 2. Among the 97 patients in training cohort, 40 (41.2%) patients had a history of abdominal surgery and 16 (16.5%) patients had a history of hepatobiliary surgery in them. Based on the preoperative history of hepatobiliary surgery, we classified patients into two groups: laparoscopic liver resection after previous hepatobiliary surgery (LLRAH) and laparoscopic liver resection with no hepatobiliary surgery (LLRNH). The characteristics and surgical outcomes of LLR between LLRAH and LLRNH groups were shown in Table 1. The results showed that patients in the LLRAH group were older and had more comorbidities compared to those in the LLRNH group (65.1 ± 8.6 vs. 56.2 ± 12.7; P = 0.009). And the operation time of LLR for patients in the LLRAH group was longer than that in the LLRNH group. Therefore, we believed that the history of hepatobiliary surgery was one of the important factors affecting the difficulty of LLR.
Establish and validate a novel difficulty rating system
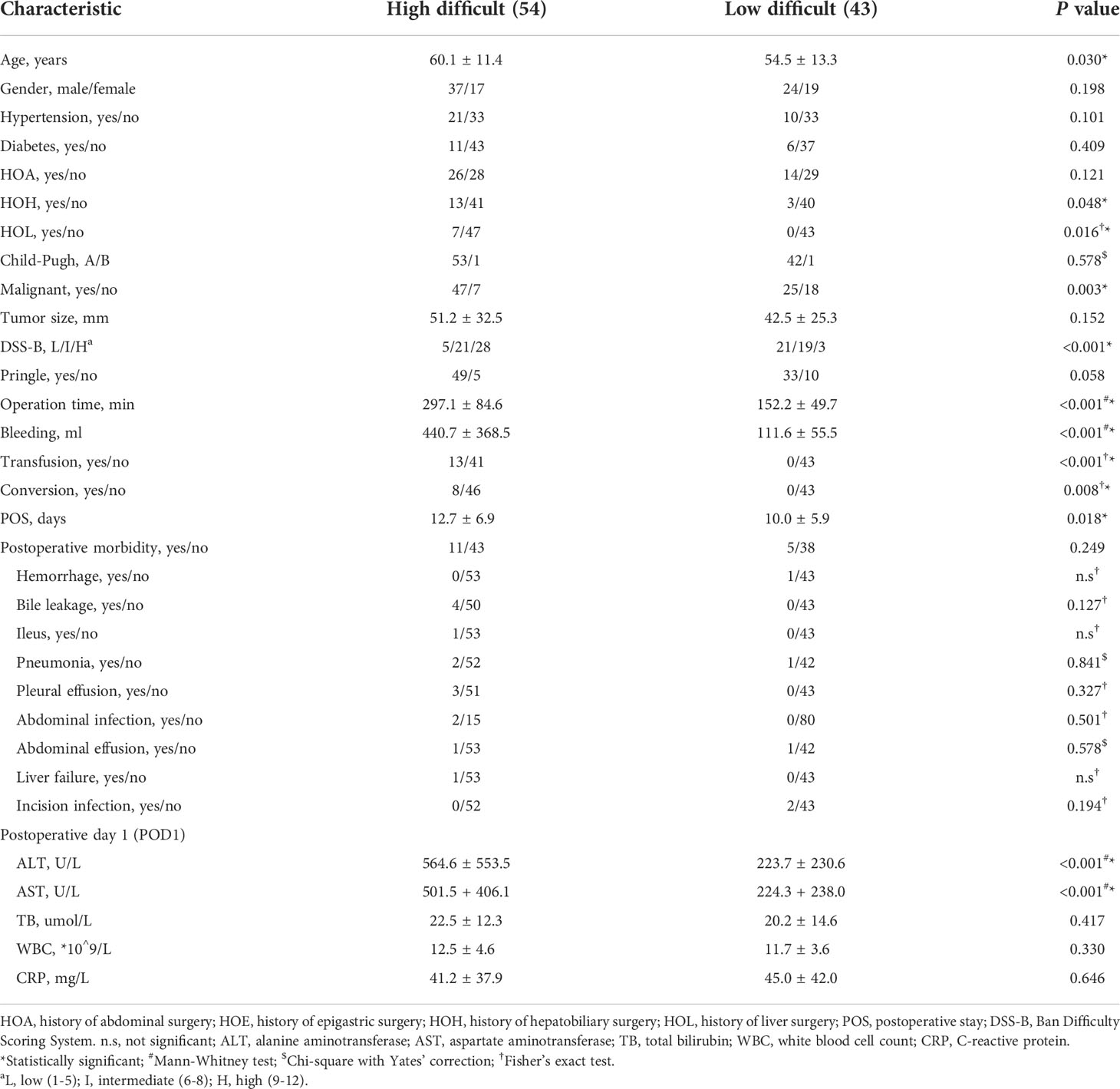
It is well known that the operation time, blood loss and conversion to open surgery reflect surgical difficulty. An adverse event was defined when the operation time exceeded 240 minutes or the blood loss exceeded 400 ml, or when the operation was switched to open surgery. Therefore, we believed that the operation was difficult when adverse event occurred. Based on this, patients were divided into high and low difficult group. The characteristics and surgical outcomes of LLR between high difficult and low difficult groups were shown in Table 2. The results showed that patients in high difficult group were older (60.1 ± 11.4 vs. 54.5 ± 13.3; P = 0.030) and had higher difficult score (DSS-B, high difficult, 51.9% vs. 7.0%, P < 0.001) compared to those in low difficult group. And higher percentage of patients with malignant liver tumor (87.0% vs. 58.1%; P = 0.003) or history of hepatobiliary surgery (24.1% vs. 7.0%; P = 0.043) in high difficult group than in low difficult group. To improve the difficulty scoring system, we incorporated the history of hepatobiliary surgery and the nature of the tumor. A novel difficulty scoring system was established as shown in Figure 3. A history of hepatobiliary surgery or malignancy was each assigned a score of 1. The NDSS for a liver tumor in this study ranged from 1 to 13 (Table 3; Figure 4). The results showed that the operation time (P < 0.001), blood loss (P < 0.001), transfusion (P < 0.001), ALT (P < 0.001) and AST (P = 0.001) were associated with the novel difficulty score significantly. The area under the receiver operating characteristic (AUROC) was used to verify the accuracy of the NDSS in predicting surgical difficulty of LLR for patients with a liver tumor. Compared with DSS-B, the NDSS has a higher AUROC (0.838 vs. 0.814, Figure 5A). The C-index of the DSS-B was 0.814 (95% CI: 0.731-0.897). The C-index of the NDSS was 0.838 (95% CI: 0.762-0.914). Additionally, the calibration plots of DSS-B and NDSS had a good coherence between the predictions and actual values in predicting surgical difficulty, as shown in Figures 5B, C.
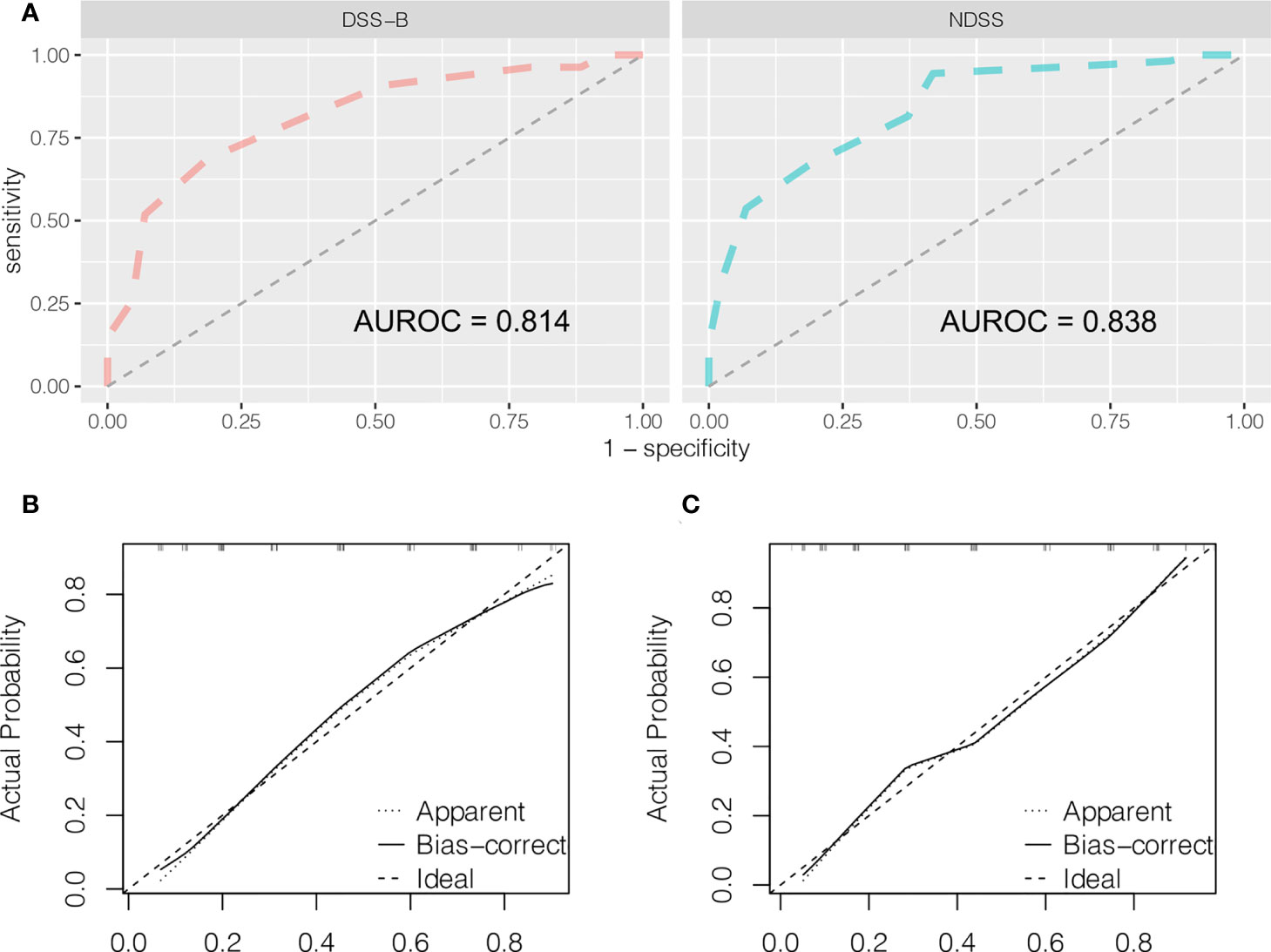
Figure 5 Predictive accuracy comparison of DSS-B and NDSS by ROC curve analyses (A). The calibration curves for predicting surgical difficulty by DSS-B (B) and NDSS (C).
Subgroup analysis
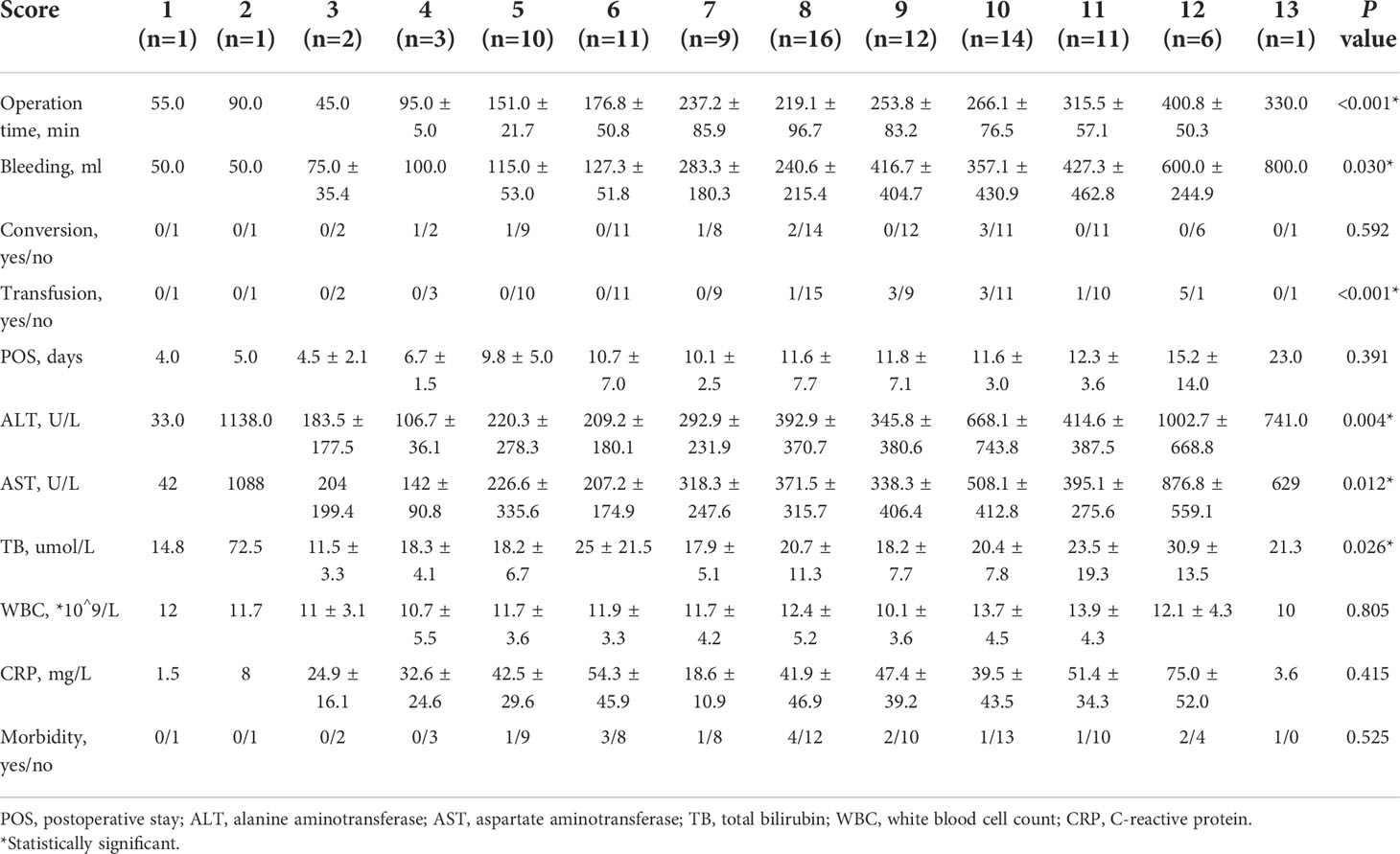
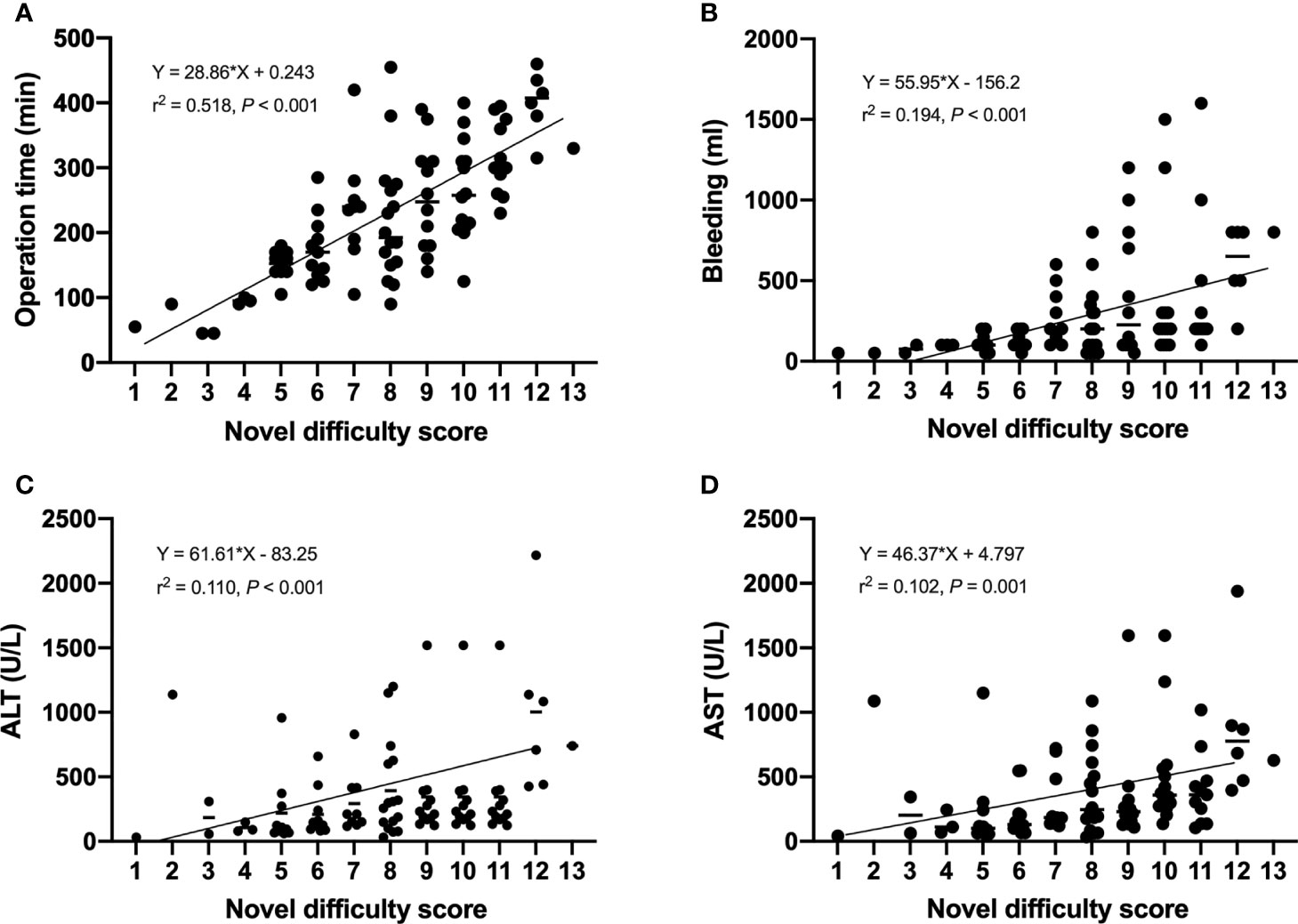
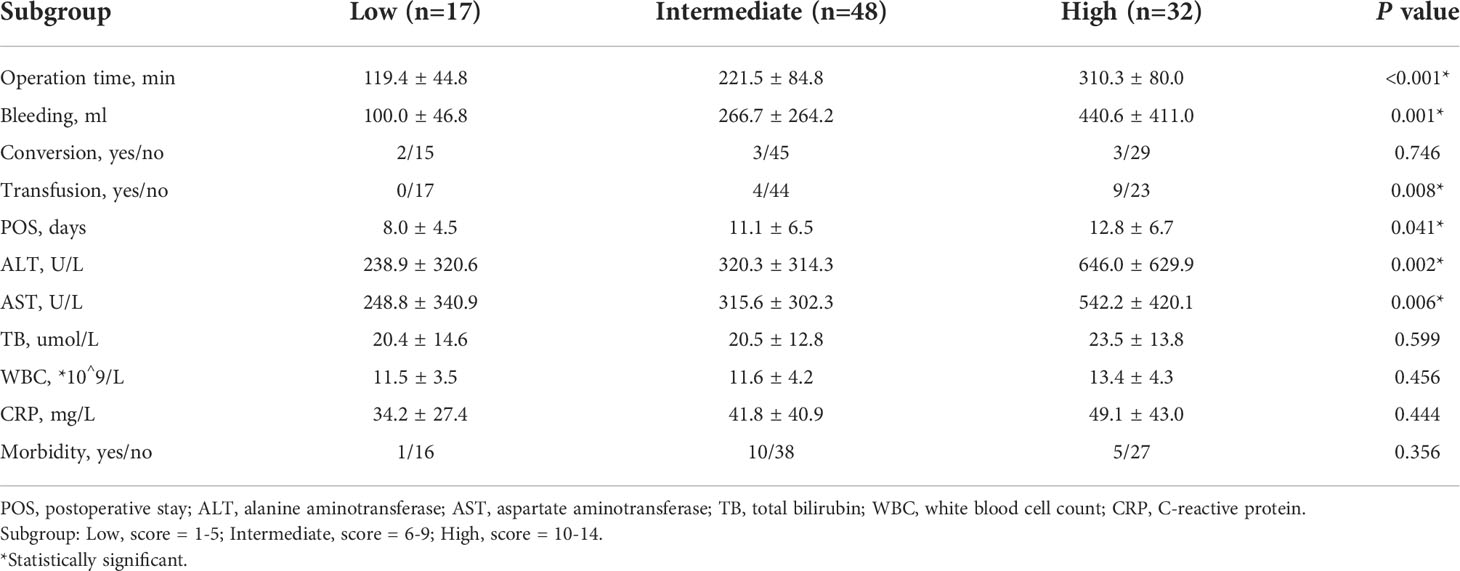
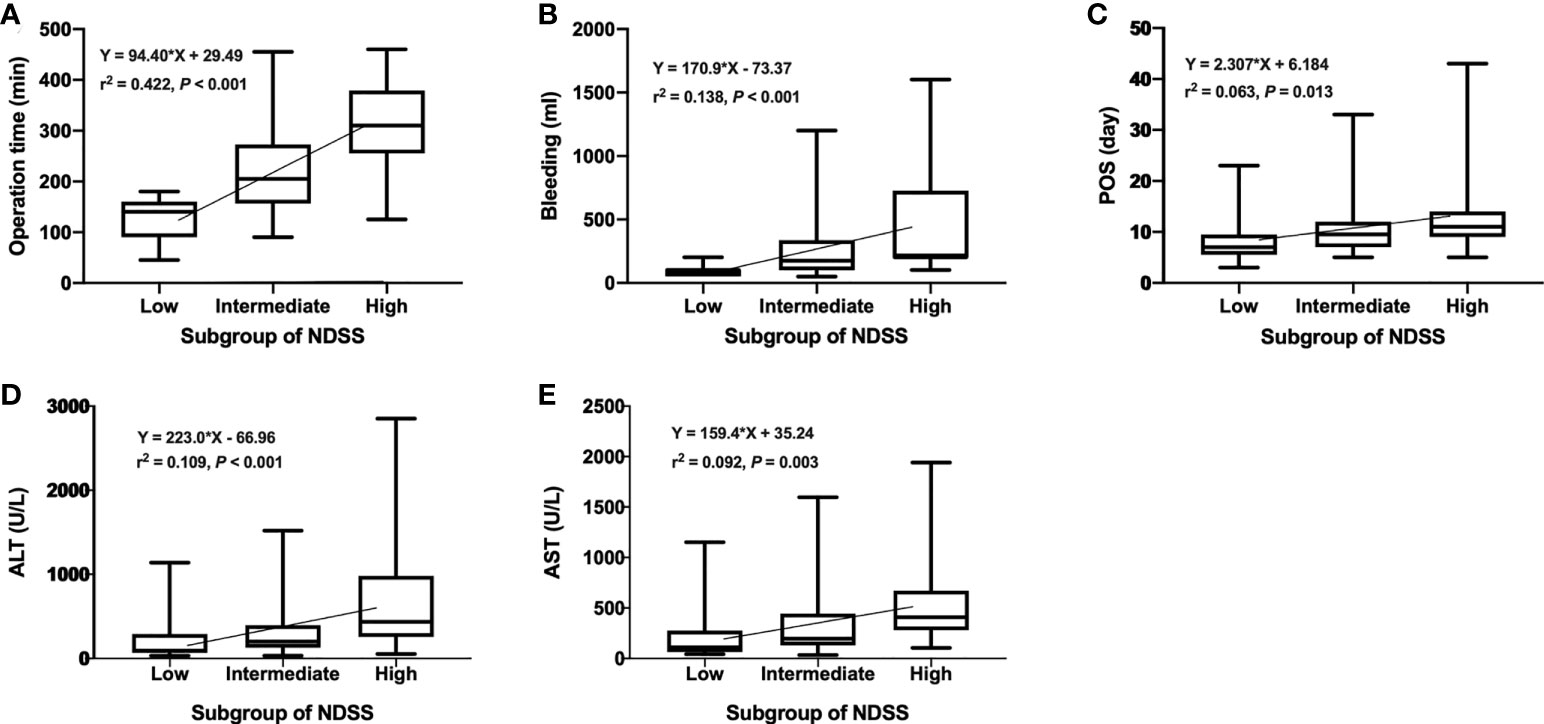
We compared the intraoperative outcomes among cases classified as low (NDSS 1-5), intermediate (NDSS 6-9), high (NDSS 10-14) difficulty. The results showed that the operation time (P < 0.001), blood loss (P = 0.001), transfusion (P = 0.008), POS (P = 0.041), ALT (P = 0.002) and AST (P = 0.006) were significantly different among these subgroups (Table 4). And its correlation was shown in Figure 6.
Development and validation the nomogram of adverse event
The NDSS were incorporated into the nomograms (Figure 7A). In the training cohort, the AUROC of the nomograms was 0.833 (Figure 7B). To validate the nomogram, 41 patients (testing cohort) who performed LLR were selected from Wujin Hospital Affiliated with Jiangsu University. The characteristics between training and testing cohort were shown in Table 5. In the testing cohort, the AUROC of the nomogram for predicting the adverse event (the degree of surgical difficulty) was 0.767 (Figure 7C). The calibration plots of the nomogram had a good coherence between the predictions and actual values in the probability of adverse event in both training and testing cohorts, as shown in Figures 7D–E.
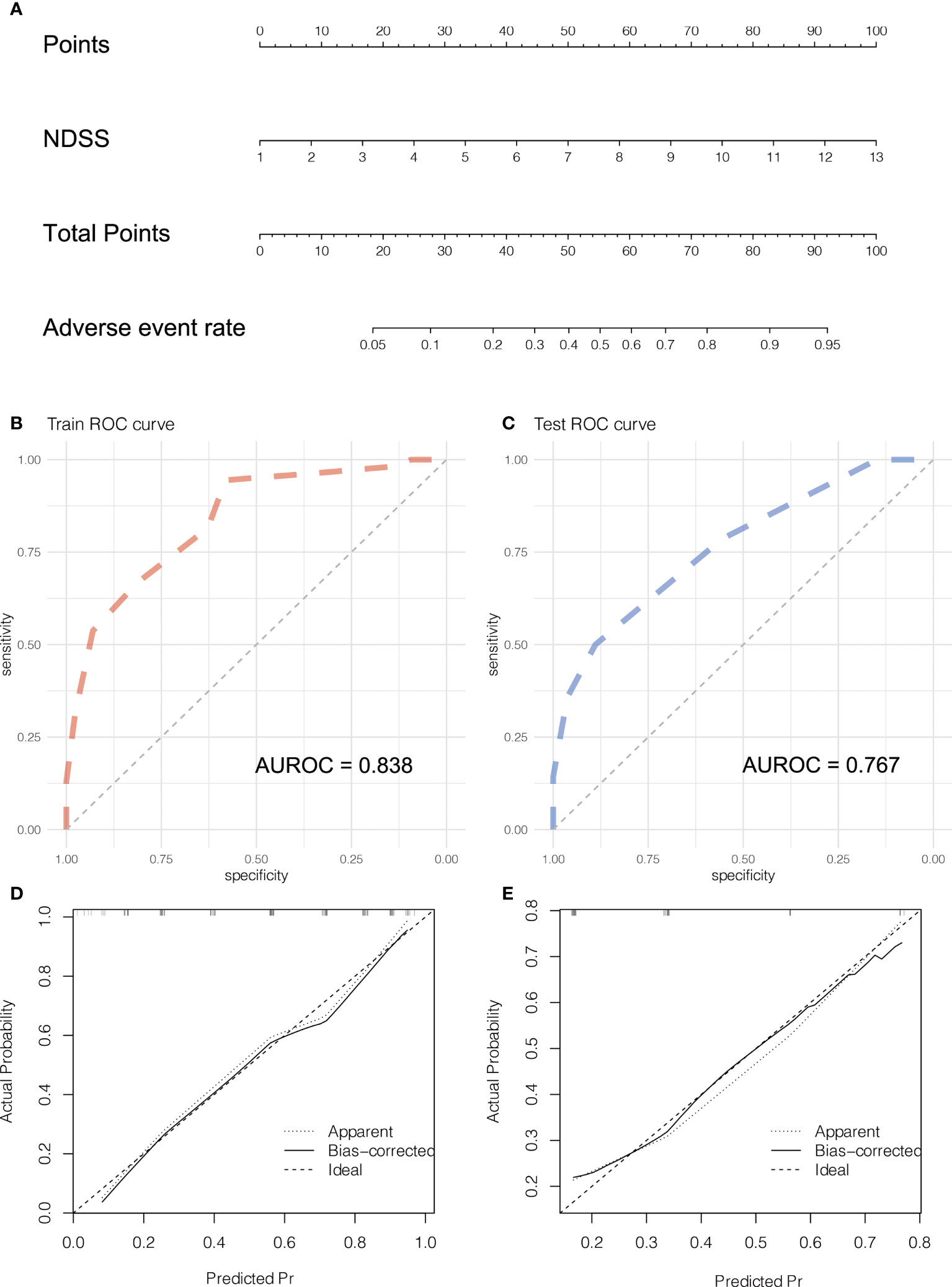
Figure 7 Development and validation the Nomogram of adverse event. The nomogram (A) of adverse event. The ROC curve of the nomogram in training (B) and testing (C) cohort. The calibration plots of the nomogram in training (D) and testing (E) cohorts.
Discussion
LLR has rapidly become widespread all over the world (11). In recent years, more and more surgical centres have included laparoscopic hepatectomy in the routine treatment of liver tumors, and the proportion is gradually increasing, and is up to 30.8% of liver resection (LR) (3). At the European Guidelines Meeting for Laparoscopic Liver Surgery, it was noted that LLR was a complex surgical skill that must be mastered in a progressive manner (12). The conventional advice is to start with a small or left lateral lobectomy and then perform a major resection as the experience increases. In addition, this simplification overlooked factors that affect laparoscopic liver resection difficulties difficulty, such as the relationship between neoplasms and large vessels and the history of liver resection. Hence a simple, objective, and robust preoperative difficulty scoring system could help surgeons master the procedure step by step.
For the past few years, a difficulty grading system of LLR has been proposed by experts (7, 13–17). Ban et al. analyzed clinical data and difficulty index of 30 patients, screened out 5 independent risk factors affecting LLR difficulty, and established the DSS-B using a linear regression model (7). The impact of different types of laparoscopic surgery was not considered in DSS-B, such as total laparoscopic hepatectomy, hand-assisted laparoscopic hepatectomy, on the difficulty of LLR, making the difficulty score incomplete. Therefore, some scholars improved DSS-B and launched an upgraded version of the IWATE Criteria for difficulty scoring (13). Different from DSS-B, Hasegawa et al. used operation time as the indicator of surgical difficulty, evaluated the influence of preoperative factors on operation time through multiple linear regression analysis, and included BMI as a difficulty scoring factor for the first time (14). In 2018, Kawaguchi et al. developed a difficulty scoring system based on the extent of resection (DSS-ER) (15). For the first time, operation time, blood loss and conversion rate were used as difficulty criteria, and the median was used as the cutoff value. However, this scoring system was based on the intraoperative results and ignored the preoperative and postoperative factors, and the verification was carried out by postoperative results, which might have a certain bias. In addition, it only considered the type of resection and ignored the influence of factors such as the general state of the patients and tumors on the operation, so its accuracy might be affected. In the same year, Halls et al. reported on a difficulty scoring system based on data from seven centres (16). For the first time, previous open liver surgery history and preoperative neoadjuvant chemotherapy were factored into the scoring system. However, the definition of preoperative neoadjuvant chemotherapy in this study was very vague, and there was a lack of data about the time and cycle of neoadjuvant chemotherapy, so the actual prediction results might be biased to some extent. Subsequently, Tong et al. proposed Sir Run Run Shaw Hospital (SRRSH) risk models based on conversion and complication. For the first time, this scoring system included the American society of anesthesiologists (ASA), ALT, cirrhosis and other indicators that reflect the general situation of patients and was a prediction model for the feasibility and safety of LLR. However, most of the cases in this study were small-scale hepatectomy, which might lead to selection bias. Therefore, more cases were needed to prove the application of SRRSH score in large-scale hepatectomy. The difficulty scoring system reported previously did not contain all the factors affecting surgery, which affects their accuracy. In addition to the above factors, Guilbaud et al. reported that an estimated parenchymal transection surface area ≥ 100 cm2 was a relevant indicator of surgical difficulty and postoperative complications in LLR (18).
In the present study, the high difficulty outcome events were identified as blood loss > 400 ml, and operation time > 240 min or conversions. The measures of surgical difficulty were similar to DSS-ER (15). But the specific reference values were different. This difference may have to do with the different measures used by different centres. Through correlation analysis, age, history of hepatobiliary surgery (HOH), history of liver surgery (HOL), and malignant and DSS-B were closely related to surgical difficulty. Based on clinical experience and the results of other centres, age was not a direct factor affecting the difficulty of LLR. The treatment of malignant tumors requires radical excision, while benign tumors can be excised close to the tumor without worrying about positive margins. Moreover, malignant tumors are often accompanied by changes in liver texture, such as hepatocellular carcinoma, which is often accompanied by hepatitis, hepatic fatty degeneration, or alcoholic liver. These changes were not sufficient to cause significant liver function damage and affected Child-Pugh score but increased operation time during surgery. There was an overlap between patients with HOH and patients with HOL. So, we selected HOH, malignant and DSS-B to build the novel difficulty scoring system. The novel system was an improvement of the classical model DSS-B. And the novel system was better than DSS-B according to the ROC curve.
Although there was no correlation between surgical difficulty and postoperative complications in this study, some studies have shown that highly difficult LLR might increase the incidence of postoperative complications (9, 15). High difficult LLR may lead to longer operation time and more blood loss, resulting in a higher incidence of postoperative complications. In these patients, laparoscopic hepatectomy should be carefully determined and recommended only in high-volume centres with an experienced team. Thus, more difficult cases would be taken over by more qualified surgeons (19). In addition to postoperative complications, the relationship between the difficulty grade of laparoscopic liver resection for malignant tumor and the long-term outcomes is of great concern to scientists (20). A growing body of evidence indicates that postoperative complications, which in our series increased along with LLR difficulty, trigger the systemic proinflammatory cascade through the release of cytokines such as IL-1b, IL-6, TNF-a, oxidative stress, and immunosuppression and consequently promote tumorigenesis and metastatic spread (21–23). Postoperative complications have a negative impact on overall survival and disease-free survival in all types of malignancies (24, 25). In addition, failure or delayed administration of adjuvant therapy due to postoperative complications may increase tumor recurrence and affect survival. In addition, with the increase of LLR difficulty, the significant increase in intraoperative blood loss and transfusion ratio was also a risk factor for poor short-term and long-term prognosis of various malignant tumors (26).
In addition to liver tumors, laparoscopic liver resection can also be used for intrahepatic duct (IHD) stones. Kim et al. developed a modified difficulty scoring system for IHD stones (10). The technical requirements of laparoscopic hepatectomy for IHD stones appear to be higher than for tumors, as the liver inflammation associated with IHD stones can lead to perihepatic adhesion and anatomic distortion. In addition, additional choledochoscopy of the remaining biliary tract is often required intraoperatively, which increases surgical complexity and prolongs surgical time. Therefore, under the same circumstances, laparoscopic liver resection for IHD stones is more difficult than liver tumors, and the two are not applicable to the same difficulty scoring system.
The use of surgical robots in liver surgery is growing almost daily. The robot offers a three-dimensional image with instruments of seven degrees of freedom (27). Compared with laparoscopic surgery, the main advantages of the robot are its ergonomic design, superior flexibility and visualization, which may better simulate open surgery and solve some operational difficulties in laparoscopic hepatectomy. However, the robotic hepatectomy is still a cutting-edge technology for liver surgeons, which requires a certain learning process. It is not clear whether the previous difficulty scoring system is suitable for robotic hepatectomy. Therefore, Chong et al. validated the DSS-B in robotic hepatectomy and to compare the outcomes of robotic hepatectomy and conventional laparoscopic hepatectomy among different difficulty levels (28). The results suggest that the benefits of the robotic platform may be minimal in moderate-to-low difficulty hepatectomy. However, robotic approaches make high difficulty liver resection more minimally invasive.
There are multiple advantages to the present study. Firstly, history of previous abdominal surgery was included in the evaluation system of surgical difficulty of LLR. Additionally, this study is the first to develop a nomogram related to the difficulty of laparoscopic hepatectomy. However, the limitations of this study include its retrospective nature, and the lack of subgroup analysis of malignancies. The liver texture of hepatocellular carcinoma is different from that of metastatic liver tumors. In addition, some studies have shown that preoperative neoadjuvant chemotherapy also has a certain impact on liver resection for metastatic liver cancer, which was not reflected in this study (20, 29).
Conclusion
In conclusion, we improved the DSS-B and proposed a new classification system of LLRs according to their surgical difficulty. This system provides 3 difficult levels of LLRs: low difficulty, intermediate difficulty, and high difficulty. This classification could more accurately reflect the difficulty of surgery and help liver surgeons to make the surgical plan and ensure the safety of the operation. As surgeons gain experience, they can choose appropriate patients and gradually progress from a low level of expertise to an advanced level of expertise.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Affiliated Hospital of Jiangnan University ethics committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CX and MZ: Study design, Data collection, Writing. TJ and YT: Study design, Data collection, Revision. LZ, ZY and ZZ: Study design, Data analysis. LX and ZL: Study design and Data analysis. WD, XX and WX: Study design, Data collection, Data analysis, Writing, Revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Changzhou Sci&Tech Program (CJ20210013, CJ20220008), Young Talent Development Plan of Changzhou Health Commission (CZQM2020118, CZQM2021028), the Development Foundation of Affiliated Hospital of Xuzhou Medical University (XYFY2020016), Medical Research Project of Jiangsu Health Commission (No. Z2019027), Changzhou High-Level Medical Talents Training Project.
Acknowledgments
The authors acknowledge for colleagues in the Department of Hepatobiliary Surgery, Affiliated Hospital of Jiangnan University. Thanks for the support of Changzhou High-Level Medical Talents Training Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Salky B, Bauer J, Easter DW, Cuschieri A, Lavelle-Jones M, Nathanson L, et al. 1992 Scientific session of the society of American gastrointestinal surgeons (Sages) Washington, D.C., USA, April 11–12, 1992. Surg Endoscopy (1992) 6(2):85–110. doi: 10.1007/BF02281090
2. Chen JF, Fu XT, Gao Z, Shi YH, Tang Z, Liu WR, et al. Laparoscopic vs. open repeat hepatectomy for recurrent liver tumors: A propensity score-matched study and meta-analysis. Front Oncol (2021) 11:646737. doi: 10.3389/fonc.2021.646737
3. Ban D, Tanabe M, Kumamaru H, Nitta H, Otsuka Y, Miyata H, et al. Safe dissemination of laparoscopic liver resection in 27,146 cases between 2011 and 2017 from the national clinical database of Japan. Ann Surg (2021) 274(6):1043–50. doi: 10.1097/SLA.0000000000003799
4. Nomi T, Hirokawa F, Kaibori M, Ueno M, Tanaka S, Hokuto D, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: A multi-centre propensity score-based analysis. Surg Endosc (2020) 34(2):658–66. doi: 10.1007/s00464-019-06812-z
5. Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: The Louisville statement, 2008. Ann Surg (2009) 250(5):825–30. doi: 10.1097/sla.0b013e3181b3b2d8
6. Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in morioka. Ann Surg (2015) 261(4):619–29. doi: 10.1097/SLA.0000000000001184
7. Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci (2014) 21(10):745–53. doi: 10.1002/jhbp.166
8. Uchida H, Iwashita Y, Tada K, Saga K, Takayama H, Hirashita T, et al. Laparoscopic liver resection in cirrhotic patients with specific reference to a difficulty scoring system. Langenbecks Arch Surg (2018) 403(3):371–7. doi: 10.1007/s00423-018-1671-4
9. Kinoshita M, Kanazawa A, Kodai S, Shimizu S, Murata A, Nishio K, et al. Difficulty classifications of laparoscopic repeated liver resection in patients with recurrent hepatocellular carcinoma. Asian J Endosc Surg (2020) 13(3):366–74. doi: 10.1111/ases.12746
10. Kim J, Cho JY, Han HS, Yoon YS, Choi Y, Lee JS, et al. Validation of a difficulty scoring system for laparoscopic liver resection in hepatolithiasis. Surg Endosc (2021) 35(3):1148–55. doi: 10.1007/s00464-020-07479-7
11. Schmelzle M, Krenzien F, Schoning W, Pratschke J. Laparoscopic liver resection: Indications, limitations, and economic aspects. Langenbecks Arch Surg (2020) 405(6):725–35. doi: 10.1007/s00423-020-01918-8
12. Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, et al. The Southampton consensus guidelines for laparoscopic liver surgery: From indication to implementation. Ann Surg (2018) 268(1):11–8. doi: 10.1097/SLA.0000000000002524
13. Wakabayashi G. What has changed after the morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr (2016) 5(4):281–9. doi: 10.21037/hbsn.2016.03.03
14. Hasegawa Y, Wakabayashi G, Nitta H, Takahara T, Katagiri H, Umemura A, et al. A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg Endosc (2017) 31(12):5356–63. doi: 10.1007/s00464-017-5616-8
15. Kawaguchi Y, Fuks D, Kokudo N, Gayet B. Difficulty of laparoscopic liver resection: Proposal for a new classification. Ann Surg (2018) 267(1):13–7. doi: 10.1097/SLA.0000000000002176
16. Halls MC, Berardi G, Cipriani F, Barkhatov L, Lainas P, Harris S, et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg (2018) 105(9):1182–91. doi: 10.1002/bjs.10821
17. Tong Y, Li Z, Ji L, Wang Y, Wang W, Ying J, et al. A novel scoring system for conversion and complication in laparoscopic liver resection. Hepatobiliary Surg Nutr (2018) 7(6):454–65. doi: 10.21037/hbsn.2018.10.09
18. Guilbaud T, Scemama U, Sarran A, Tribillon E, Nassar A, Gayet B, et al. Predictive ability of preoperative ct scan for the intraoperative difficulty and postoperative outcomes of laparoscopic liver resection. Surg Endosc (2021) 35(6):2942–52. doi: 10.1007/s00464-020-07734-x
19. Ivanecz A, Plahuta I, Mencinger M, Perus I, Magdalenic T, Turk S, et al. The learning curve of laparoscopic liver resection utilising a difficulty score. Radiol Oncol (2021) 56(1):111–8. doi: 10.2478/raon-2021-0035
20. Holowko W, Triantafyllidis I, Neuberg M, Tabchouri N, Beaussier M, Bennamoun M, et al. Does the difficulty grade of laparoscopic liver resection for colorectal liver metastases correlate with long-term outcomes? Eur J Surg Oncol (2020) 46(9):1620–7. doi: 10.1016/j.ejso.2020.05.019
21. Lippitz BE, Harris RA. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology (2016) 5(5):e1093722. doi: 10.1080/2162402X.2015.1093722
22. Murata M. Inflammation and cancer. Environ Health Prev Med (2018) 23(1):50. doi: 10.1186/s12199-018-0740-1
23. Bent R, Moll L, Grabbe S, Bros M. Interleukin-1 beta-a friend or foe in malignancies? Int J Mol Sci (2018) 19(8):2155. doi: 10.3390/ijms19082155
24. Dorcaratto D, Mazzinari G, Fernandez M, Munoz E, Garces-Albir M, Ortega J, et al. Impact of postoperative complications on survival and recurrence after resection of colorectal liver metastases: Systematic review and meta-analysis. Ann Surg (2019) 270(6):1018–27. doi: 10.1097/SLA.0000000000003254
25. Kong J, Li G, Chai J, Yu G, Liu Y, Liu J. Impact of postoperative complications on long-term survival after resection of hepatocellular carcinoma: A systematic review and meta-analysis. Ann Surg Oncol (2021) 28(13):8221–33. doi: 10.1245/s10434-021-10317-2
26. Marincola Smith P, Baechle J, Solorzano CC, Tan M, Lopez-Aguiar AG, Dillhoff M, et al. Impact of perioperative blood transfusion on survival in pancreatic neuroendocrine tumor patients: Analysis from the us neuroendocrine study group. HPB (Oxford) (2020) 22(7):1042–50. doi: 10.1016/j.hpb.2019.10.2441
27. Giulianotti PC, Bianco FM, Daskalaki D, Gonzalez-Ciccarelli LF, Kim J, Benedetti E. Robotic liver surgery: Technical aspects and review of the literature. Hepatobiliary Surg Nutr (2016) 5(4):311–21. doi: 10.21037/hbsn.2015.10.05
28. Chong CCN, Lok HT, Fung AKY, Fong AKW, Cheung YS, Wong J, et al. Robotic versus laparoscopic hepatectomy: Application of the difficulty scoring system. Surg Endosc (2020) 34(5):2000–6. doi: 10.1007/s00464-019-06976-8
29. Halls MC, Cherqui D, Taylor MA, Primrose JN, Abu Hilal M. Collaborators of the difficulty of laparoscopic liver surgery s. are the current difficulty scores for laparoscopic liver surgery telling the whole story? an international survey and recommendations for the future. HPB (Oxford) (2018) 20(3):231–6. doi: 10.1016/j.hpb.2017.08.028
Keywords: difficulty scoring system, laparoscopic surgery, liver resection, liver tumor, outcome
Citation: Xi C, Zhu M, Ji T, Tan Y, Zhuang L, Yuan Z, Zhang Z, Xu L, Liu Z, Xu X, Xue W and Ding W (2022) A novel difficulty scoring system of laparoscopic liver resection for liver tumor. Front. Oncol. 12:1019763. doi: 10.3389/fonc.2022.1019763
Received: 15 August 2022; Accepted: 02 September 2022;
Published: 29 September 2022.
Edited by:
Qizhao Huang, Southern Medical University, ChinaReviewed by:
Yan He, the Affiliated Hospital of Guizhou Medical University, ChinaLifan Xu, Army Medical University, China
Copyright © 2022 Xi, Zhu, Ji, Tan, Zhuang, Yuan, Zhang, Xu, Liu, Xu, Xue and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Ding, dwdoctor@163.com
†These authors have contributed equally to this work
 Cheng Xi1,2†
Cheng Xi1,2† Xuezhong Xu
Xuezhong Xu Wei Ding
Wei Ding