Abstract
Recent studies suggested that microglia, the primary brain immune cells, can affect circuit connectivity and neuronal function1,2. Microglia infiltrate the neuroepithelium early in embryonic development and are maintained in the brain throughout adulthood3,4. Several maternal environmental factors—such as an aberrant microbiome, immune activation and poor nutrition—can influence prenatal brain development5,6. Nevertheless, it is unknown how changes in the prenatal environment instruct the developmental trajectory of infiltrating microglia, which in turn affect brain development and function. Here we show that, after maternal immune activation (MIA) in mice, microglia from the offspring have a long-lived decrease in immune reactivity (blunting) across the developmental trajectory. The blunted immune response was accompanied by changes in chromatin accessibility and reduced transcription factor occupancy of the open chromatin. Single-cell RNA-sequencing analysis revealed that MIA does not induce a distinct subpopulation but, rather, decreases the contribution to inflammatory microglia states. Prenatal replacement of microglia from MIA offspring with physiological infiltration of naive microglia ameliorated the immune blunting and restored a decrease in presynaptic vesicle release probability onto dopamine receptor type-two medium spiny neurons, indicating that aberrantly formed microglia due to an adverse prenatal environment affect the long-term microglia reactivity and proper striatal circuit development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated and analysed during this study are available from the corresponding author on reasonable request. Genomics data are available at the NCBI Gene Expression Omnibus under SuperSeries accession number GSE201817. Publicly available databases used in this manuscript include HOCOMOCO (v.11)65 (https://hocomoco11.autosome.org), ENCODE data from PECA (v.2.0; https://github.com/SUwonglab/PECA)66,67 and NHGRI GWAS catalog (v.1.0.2; https://www.ebi.ac.uk/gwas/). Source data are provided with this paper.
Code availability
Source codes are available at GitHub (https://github.com/lindsaynhayes/Hayes_2022).
References
Squarzoni, P. et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 8, 1271–1279 (2014).
Badimon, A. et al. Negative feedback control of neuronal activity by microglia. Nature 586, 417–423 (2020).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Réu, P. et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 20, 779–784 (2017).
Reed, M. D. et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 577, 249–253 (2020).
Thion, M. S. et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516 (2018).
Wendeln, A. C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018).
Liu, Y. J. et al. Microglia elimination increases neural circuit connectivity and activity in adult mouse cortex. J. Neurosci. 41, 1274–1287 (2021).
Cruz-Carrillo, G. & Camacho-Morales, A. Metabolic flexibility assists reprograming of central and peripheral innate immunity during neurodevelopment. Mol. Neurobiol. 58, 703–718 (2020).
Shin Yim, Y. et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549, 482–487 (2017).
Smolders, S., Notter, T., Smolders, S. M. T., Rigo, J. M. & Brône, B. Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain Behav. Immun. 73, 51–65 (2018).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Olah, M. et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11, 6129 (2020).
Sousa, C. et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 19, e46171 (2018).
Li, Q. et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223 (2019).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Tsafaras, G. P., Ntontsi, P. & Xanthou, G. Advantages and limitations of the neonatal immune system. Front. Pediatr. 8, 5 (2020).
Schwarz, J. M., Sholar, P. W. & Bilbo, S. D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 120, 948–963 (2012).
Negishi, H., Taniguchi, T. & Yanai, H. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harb. Perspect. Biol. 10, a028423 (2018).
Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47, D1005–D1012 (2019).
Landek-Salgado, M. A., Faust, T. E. & Sawa, A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol. Psychiatry 21, 10–28 (2016).
Owen, M. J., Sawa, A. & Mortensen, P. B. Schizophrenia. Lancet 388, 86–97 (2016).
Sherwood, R. I. et al. Discovery of directional and nondirectional pioneer transcription factors by modeling DNase profile magnitude and shape. Nat. Biotechnol. 32, 171–178 (2014).
Qu, K. et al. Individuality and variation of personal regulomes in primary human T cells. Cell Syst. 1, 51–61 (2015).
Klemm, S. L., Shipony, Z. & Greenleaf, W. J. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 20, 207–220 (2019).
Bentsen, M. et al. ATAC-seq footprinting unravels kinetics of transcription factor binding during zygotic genome activation. Nat. Commun. 11, 4267 (2020).
Karabacak Calviello, A., Hirsekorn, A., Wurmus, R., Yusuf, D. & Ohler, U. Reproducible inference of transcription factor footprints in ATAC-seq and DNase-seq datasets using protocol-specific bias modeling. Genome Biol. 20, 42 (2019).
Kang, C. et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349, aaa5612 (2015).
Mogensen, T. H. IRF and STAT transcription factors—from basic biology to roles in infection, protective immunity, and primary immunodeficiencies. Front. Immunol. 9, 3047 (2018).
Villarino, A. V., Kanno, Y. & O’Shea, J. J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 18, 374–384 (2017).
Ramirez, R. N. et al. Dynamic gene regulatory networks of human myeloid differentiation. Cell Syst. 4, 416–429 (2017).
Camp, J. G. et al. Microbiota modulate transcription in the intestinal epithelium without remodeling the accessible chromatin landscape. Genome Res. 24, 1504–1516 (2014).
Li, K. et al. Landscape and dynamics of the transcriptional regulatory network during natural killer cell differentiation. Genomics Proteomics Bioinform. 18, 501–515 (2020).
Meers, M. P., Bryson, T. D., Henikoff, J. G. & Henikoff, S. Improved CUT&RUN chromatin profiling tools. eLife 8, e46314 (2019).
Skene, P. J. & Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 6, e21856 (2017).
Luchicchi, A. et al. Maternal immune activation disrupts dopamine system in the offspring. Int. J. Neuropsychopharmacol. 19, pyw007 (2016).
Hayes, L. N. et al. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr. Bull. 40, 963–972 (2014).
Coughlin, J. M. et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl. Psychiatry 6, e777 (2016).
Conen, S. et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol. Psychiatry 26, 5398–5406 (2021).
Taneja, V. Sex hormones determine immune response. Front. Immunol. 9, 1931 (2018).
Hanamsagar, R. et al. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65, 1504–1520 (2017).
Goel, N. & Bale, T. L. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J. Neuroendocrinol. 21, 415–420 (2009).
Tamashiro, T. T., Dalgard, C. L. & Byrnes, K. R. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J. Vis. Exp. 66, e3814 (2012).
Mecha, M. An easy and fast way to obtain a high number of glial cells from rat cerebral tissue: a beginners approach. Protocol Exchange https://doi.org/10.1038/protex.2011.218 (2011).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Bennett, M. L. et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl Acad. Sci. USA 113, E1738–E1746 (2016).
Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14, 959–962 (2017).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 4, 1521 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA-seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Germain, P., Lun, A., Macnair, W. & Robinson, M. Doublet identification in single-cell sequencing data using scDblFinder. F1000 Res. https://doi.org/10.12688/f1000research.73600.1 (2021).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Ahlmann-Eltze, C. & Huber, W. glmGamPoi: fitting gamma-Poisson generalized linear models on single cell count data. Bioinformatics 36, 5701–5702 (2021).
McInnes, L., Healy, J., Saul, N. & Großberger, L. UMAP: uniform manifold approximation and projection. J. Open Source Softw. 3, 861 (2018).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Kulakovskiy, I. V. et al. HOCOMOCO: towards a complete collection of transcription factor binding models for human and mouse via large-scale ChIP-Seq analysis. Nucleic Acids Res. 46, D252–D259 (2018).
Duren, Z., Chen, X., Jiang, R., Wang, Y. & Wong, W. H. Modeling gene regulation from paired expression and chromatin accessibility data. Proc. Natl Acad. Sci. USA 114, E4914–E4923 (2017).
Duren, Z., Chen, X., Xin, J., Wang, Y. & Wong, W. H. Time course regulatory analysis based on paired expression and chromatin accessibility data. Genome Res. 30, 622–634 (2020).
Acknowledgements
We thank T. Faust, M. McCarthy and J. Ling for reading the manuscript; Y. Lema for assistance with the figures; H. Zhang for assistance with flow cytometry and cell sorting; G. Cannon for guidance with single-cell library preparation and RNA-seq; L. Jaeger (National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health; 1UL1TR001079) for support with the statistical analysis; R. Nardou for advice on electrophysiology; the staff at the Johns Hopkins Solomon H. Snyder multi-photon imaging core for microscopy and software support (NIH P30NS050274); and the staff at Plexxikon for providing the PLX5622 compound. This work was supported by NIH grants (P50MH094268, R01MH105660 and R01MH107730), and foundation grants from S-R/RUSK, the Brain & Behavior Research Foundation (NARSAD) and the Stanley Center.
Author information
Authors and Affiliations
Contributions
L.N.H. and A.S. conceived the study. L.N.H. performed experiments with help from K.A., E.C., F.L., E.V., M.P., C.T. and A.R.; L.N.H. and K.A. performed electrophysiology experiments with help from G.D.; L.N.H., E.V. and L.A.G. performed single-cell RNA-seq experiments and data analysis. L.N.H. and A.S. wrote the manuscript with input from all of the other authors. A.S. and S.K. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Vijay Kuchroo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Quality control data of adult bulk microglia RNA sequencing for Fig 1.
a, Heatmap for the gene expression of cell type specific markers for microglia, neurons, astrocytes, oligodendrocytes, and macrophages from MACS-isolated CD11b+ cells. b, Clustering of bulk microglia RNA sequencing samples from (a). One outlier identified (MSL3) and removed from subsequent analysis. Sample naming is as follows: first position M (MIA, red) or C (CON, black), second position C [cortex (Ctx), pink] or S [whole striatum (Str), purple], third position S (SAL, blue) or L (LPS, green), fourth position is animal number. c–e, Volcano plots for the DEGs determined by DESeq2 between the main effect of group (MIA versus CON), region (frontal Ctx versus whole Str), and treatment (LPS versus SAL) correcting for the other covariate variables from MACS-isolated CD11b+ cells. Black, q < 0.05. f, Heatmap for the gene expression of cell type specific markers for microglia, neurons, astrocytes, oligodendrocytes, and macrophages from FACS-isolated CD45low; CD11b+ cells from the whole Str. g, Clustering of bulk microglia RNA sequencing samples from (f). MS6 was removed from subsequent analysis due to low read count. Sample naming is as follows: first position M (MIA, red) or C (CON, black), second position S (saline, blue) or L (LPS, green), and third position is animal number
Extended Data Fig. 2 PCA and Heatmaps for DEGs represented in Fig. 1.
a, Principal component analysis (PCA) of CON and MIA microglia after SAL or LPS treatment. b, Top 25 DEGs between MIA and CON microglia after SAL treatment. c, Top DEGs between MIA and CON microglia after LPS treatment. Blunted LPS-induced genes and blunted LPS-repressed genes are indicated. d, Top DEGs between MIA and CON microglia that show an interaction between MIA and LPS (group x treatment). Blunted LPS-induced and LPS-repressed genes are indicated. e, Linear regression of the FC for the LPS-induced DEGs from Figure 1e of MACS-isolated microglia (left) in comparison with the FACS-isolated microglia (right). The black line indicates the y = x line and the blue line is the linear fit line with slope equal to 0.71 and 0.83, respectively and CCC equal to 0.81 and 0.75, respectively
Extended Data Fig. 3 Regional specificity of the diminished microglia immune response after MIA.
a–b, Regional specificity of the MIA blunted LPS response. Linear regression of the FC for the LPS-induced DEGs between microglia from the frontal Ctx (a) or whole Str (b) with slope equal to 0.71 and 0.58, respectively and CCC equal to 0.84 and 0.70, respectively. c–e, Control analysis of the LPS responsiveness between microglia from the Ctx compared with microglia from the Str (these are the mixture of both CON and MIA microglia). c, Venn diagram of DEGs (q < 0.05) between SAL and LPS in the Ctx microglia (pink) compared with the Str microglia (blue). d–e, Plot (d) and linear regression (e) of the FC for the LPS-induced DEGs in both Ctx and Str microglia (purple), only Ctx microglia (pink) or only Str microglia (light blue). Numbers indicate the total number of LPS response genes in each section. The black line indicates the y=x line and the blue line is the linear fit line (slope = 0.87 and CCC = 0.86)
Extended Data Fig. 4 Microglia density and morphology in the striatum after MIA.
a–b, Representative confocal reconstructions of microglia morphology (a) and CD68 lysosomal content (b) in CON and MIA offspring after LPS treatment. Scale bar = 10 μm c, Volume of Iba1+ (microglia) in the dorsal and ventral Str of CON (grey) and MIA (red) microglia after SAL and LPS treatment. n are individual cells. Data are analysed by a three-way ANOVA with emmeans post hoc test. nCON:SAL:DS=34 cells from 4 mice from 4 litters, nMIA:SAL:DS=19 cells from 4 mice from 3 litters, nCON:SAL:VS=25 cells from 4 mice from 4 litters, nMIA:SAL:VS=26 cells from 4 mice from 3 litters, nCON:LPS:DS=33 cells from 4 mice from 4 litters, nMIA:LPS:DS=26 cells from 3 mice from 2 litters, nCON:LPS:VS=29 cells from 4 mice from 4 litters, nMIA:LPS:VS=19 cells from 3 mice from 2 litters. Group: F(1, 206) = 1.7, p = 0.197, Trt: F(1, 206)=22, p = 5e-6, Region: F(1, 206) = 1.3, p=0.26, Interaction: F(1, 206) = 10.46, p = 0.0014, emmeans: MIAvCONLPS:DS: t(203) = 2.39, p = 0.018, MIAvCONLPS:VS: t(203)=2.20, p=0.029, MIAvCONSAL:DS: t(203) = −0.06, p = 0.95, MIAvCONSAL:VS: t(203) = −1.7, p = 0.10. d, Volume of CD68 (lysosomes) inside microglia from the dorsal and ventral Str of CON (grey) and MIA (red) offspring after SAL and LPS treatment. n are individual cells. Data are analysed by a three-way ANOVA with emmeans post hoc test. n same as in c. Group: F(1, 206) = 11.9, p = 6E-4, Trt: F(1, 206) = 1.77, p = 0.185, Region: F(1, 206) = 4.32, p = 0.039, Int: F(1, 206) = 4.44, p = 0.036, emmeans: MIAvCONLPS:DS: t(203) = 3.19, p = 0.0017, MIAvCONLPS:VS: t(203)=2.34, p = 0.020, MIAvCONSAL:DS: t(203)=0.669, p = 0.504, MIAvCONSAL:VS: t(203) = 0.727, p = 0.468. e, Microglia density in the dorsal and ventral Str of CON (grey) and MIA (red) offspring after SAL or LPS treatment. n are individual animals. Data are analysed by a three-way ANOVA with no significant difference between group, region, or treatment. nCON:SAL=4 mice from 4 litters, nCON:LPS=4 mice from 4 litters, nMIA:SAL=4 mice from 3 litters, nMIA:LPS=4 mice from 3 litters. Group: F(1, 28) = 0.036, p = 0.85, Treatment: F(1, 28) = 0.001, p = 0.98, Region: F(1, 28) = 0.363, p = 0.55. The box plots in c-e show the median (centre line), the IQR (box limits) and 1.5 × IQR (whiskers).* p < 0.05
Extended Data Fig. 5 Single cell RNA sequencing of LPS-activated striatal microglia from CON and MIA offspring with prenatal microglia replacement.
a, UMAP of ~120,000 microglia grouped into 14 clusters. Note: 3 clusters were removed due to markers for poor cell quality resulting in 14 high quality cell clusters. b, Markers enriched for each cluster. Cluster 1 is homeostatic microglia expressing P2ry12, Hexb, and Csf1r. Cluster 2 expresses Ccl3, cluster 3 expresses Apoe, cluster 4 expresses Ccl5, cluster 5 expresses Ccl12, cluster 6 expresses Saa3, cluster 7 expresses Spp1, cluster 8 expresses Lcn2, cluster 9 expresses Postn, cluster 10 expresses Arg1, cluster 11 expresses Ifit2 and Cxcl10. Cluster 12 and 13 are macrophage clusters expressing Pf4 and Ttr, respectively. Cluster 14 is a proliferative cluster expressing Mki67 and Top2a. c, Clustering distribution of microglia from CON (CON:CON, black), MIA (MIA:CON, red), prenatal microglia replacement (CON:PLX, cyan), and MIA with prenatal microglia replacement (MIA:PLX, purple). d–f, Filtering of all cells (~147,000) for low counts per cell (d, blue dots < 600 counts), low number of total genes detected per cell (f, blue dots, < 500 genes), high mitochondrial content (e, magenta, > 10% mitochondrial), doublet detection (green dots) or high read count (red dot, > 150,000). d, RNA counts per cell for each sample; cells were retained with counts > 600 and < 150,000. e, Percent mitochondrial reads per cell for each sample; cells were retained with < 10% mitochondrial content. f, Number of genes detected per cell for each sample; cells were retained with > 500 genes and < 10,000 genes detected. Cell doublets were also removed and indicated by green dots in d-f. g–m, UMAP of cells after filtering. g, UMAP of counts per cell. h, detected genes per cell. i, percent mitochondrial reads per cell. j, serum IL-6 expression quantified in each sample. k, batch effect for processing facility. l, batch effect depending on date of processing. m, individual samples processed. n are individual cells
Extended Data Fig. 6 Network analysis of ATAC and RNA-seq in MIA and CON microglia.
a, Density plot of transcription factor (TF) and target (TG) regulation directions predicated from ENCODE data and enriched in MIA and CON microglia. The direction of the regulation was determined using a Pearson’s correlation coefficient with negative values indicating a repressive network and positive values indicating an activator network. TF-TG pairs were enriched in the MIA microglia (red), CON microglia (grey) or common in both groups (blue). b, Fold change (between MIA and CON) and significance of specific TF-TG pairs in MIA microglia (red) and CON microglia (grey) determined using PECA. c, Cytoscape network of the most highly enriched TF-TG networks with colour indicating the enrichment for CON (grey) or MIA (red). The lines indicate the TF-TG network. d–e, Total concentration of DNA captured by CUT&RUN with antibodies against IRF1 (d) and STAT2 (e) IRF1: nCON=4 mice from 2 litters, nMIA=4 mice from 2 litters, STAT2: nCON=4 mice from 3 litters, nMIA=4 mice from 2 litters. f–g, Specific target DNA captured using CUT&RUN-qPCR represented as percent of total input DNA by the TF IRF2 (f), or STAT2 (g) for target genes of IRF1: Fas and IL-6 and STAT2: IL-23a. n are individual animals. Data are analysed by a two-sided t-test. n same as d-e. ITF1-Fas: t(6) = 2.5, p = 0.047, IRF1-Il-6: t(6)=3.15, p = 0.02, STAT2-IL-23: t(5) = 4.74, p = 0.005. The box plots in d–g show the median (centre line), the IQR (box limits) and 1.5 × IQR (whiskers).* p < 0.05
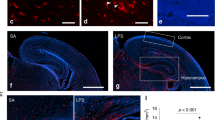
Extended Data Fig. 7 Ablation and repopulation efficiency of embryonic microglia.
a, Timeline of PLX5622 (PLX) treatment. b–c, Representative images of E12.5 (b) and E16.5 (c) brain slices with high efficiency of ablation after 3 days of ablation treatment (b) and 4 days of repopulation (c). Green= Iba1, Blue=Dapi, scale bar = 100 μm. Images are representative from 4-9 individual animals per condition. d, FACS sorting plot for quantification of repopulation of brain myeloid cells after gating for live singlets, the CD11b+, CD45+ cells were quantified relative to control animals. FSC=forward scatter, SSC=side scatter, PI=propidium iodide. e, Quantification of microglia ablation and re-infiltration. n are individual animals. Data are shown as mean ± s.e.m and analysed using a two-sided t-test. nCON:E12=6 mice from 2 litters, nPLX:E12 = 6 mice from 2 litters, nCON:E16=6 mice from 1 litter, nPLX:E16 = 6 mice from 2 litters, nCON:P1 = 7 mice from 3 litters, nPLX:P1=6 mice from 2 litters. E12: t(6)=5, p = 0.002, E16: t(8) = 5, p = 0.002, P1: t(7) = −2, p = 0.05. f, IL-6 protein expression in maternal serum 3 h after PIC or SAL injection with and without prenatal PLX treatment. n are individual animals. Data are analysed using a two-way ANOVA with Tukey post hoc test. *p < 0.05. nCON:CON=27 litters, nCON:PLX=24 litters, nMIA:CON=33 litters, nMIA:PLX=27 litters. Group: F(3, 91)=230.7, p < 2e-16, CON:PLX v CON:CON: t(91)=1.5, p=0.343, MIA:CON v CON:CON: t(91)=19.9, p < 1e-4, MIA:PLX v CON:CON: t(91)=18.6, p < 1e-4, MIA:PLX v MIA:CON: t(91)=−0.622, p=0.891. The box plots in f show the median (centre line), the IQR (box limits) and 1.5 × IQR (whiskers)
Extended Data Fig. 8 Rescue of microglia blunting in MIA offspring using prenatal microglia replacement.
a, PCA of microglia from CON:CON (black), CON:PLX (cyan), MIA:CON (red), and MIA:PLX (purple) from the whole Str. Two samples were excluded due to incorrect clustering, each labelled accordingly. b, Clustering of bulk microglia RNA sequencing samples from (a). c–e, Volcano plots for the DEGs between the main effect of group (c, among CON:CON, CON:PLX, MIA:CON, and MIA:PLX), PLX treatment (d, PLX versus CON), and LPS treatment (e, SAL versus LPS) correcting for the other covariate variables. Black = q < 0.05 using DESeq2
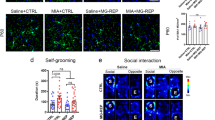
Extended Data Fig. 9 Rescue of neuronal electrophysiological phenotypes in MIA offspring using prenatal microglia replacement related to Fig. 5.
a, Two-sample KS test for the cumulative distribution of the inter-event intervals of the sEPSC frequency from Tom+ (D1R) in Fig. 5c. nCon:D1R=11 cells from 8 animals from 5 litters, nMIA:D1R=10 cells from 4 animals from 3 litters. b, sEPSC amplitude of Tom+ (D1R) and Tom- (D2R) medium spiny neurons (MSNs) in Fig. 5c. n are individual cells. Data are shown as mean ± s.e.m and analysed using t-tests. nCON:D1R=11 cells from 8 animals from 5 litters, nMIA:D1R=10 cells from 4 animals from 3 litters, nCON:D2R = 10 cells from 6 animals from 4 litters, nMIA:D2R=13 cells from 6 animals from 4 litters. c, Spine density of striatal MSNs in the ventral Str. n are individual animals. Data are shown as mean ± s.e.m and analysed using a t-test. nCon = 6, mice from 3 litters from 28 cells, nMIA = 7 mice from 3 litters from 34 cells. d, mESPC amplitudes of Tom- (D2R) MSNs analysed in Fig. 5e. n are individual cells. Data are shown as mean ± s.e.m and analysed using a t-test. nCON:D2R=16 cells from 3 animals from 2 litters, nMIA:D2R=12 cells from 3 animals from 2 litters.
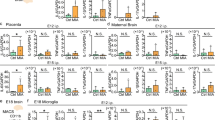
Extended Data Fig. 10 Sex effect in MIA and three-hour dose response in maternal serum after MIA.
a–b, Quantification of secreted IL-6 and TNFα from LPS-stimulated microglia in vitro at adulthood from the Str (a) and frontal Ctx (b) from Fig. 2a-b indicating the sex [male (blue) and female (pink)] of each offspring. n are independent culture experiments. Data are shown as mean ± s.e.m. with each experiment normalized to CON. Adult: nAll groups = 4 cultures each with 2 mice pooled from 3 litters for both CON and MIA. c–d, Baseline gene expression analysed by qPCR for Tnfα (c) and Aif1 (d) from bulk frontal Ctx and whole Str from CON (grey) and MIA (red) offspring indicating the sex [male (blue) and female (pink)] of each animal. n are individual animals. Data are shown as median ± IQR range with 1.5*IQR whiskers and analysed by a linear mixed-effect modelling and three-way ANOVA. Tnfα: nCON:CTX:female = 6 mice from 4 litters, nCON:CTX:male = 9 mice from 7 litters, nCON:STR:female = 6 mice from 4 litters, nCON:STR:male = 8 mice from 6 litters, nMIA:CTX:female = 5 mice from 4 litters, nMIA:CTX:male = 15 mice from 9 litters, nMIA:STR:female = 5 mice from 4 litters, nMIA:STR:male = 15 mice from 9 litters. Group: F(1, 65) = 3.78, p = 0.057, Region: F(1, 65) = 0.042, p = 0.84, Sex: F(1, 65) = 0.43, p = 0.51. Aif1: nCON:CTX:female = 6 mice from 4 litters, nCON:CTX:male = 2 mice from 2 litters, nCON:STR:female = 6 mice from 4 litters, nCON:STR:male = 2 mice from 2 litters, nMIA:CTX:female = 5 mice from 4 litters, nMIA:CTX:male = 3 mice from 2 litters, nMIA:STR:female = 5 mice from 4 litters, nMIA:STR:male = 3 mice from 2 litters. Group: F(1, 28) = 4.2, p=0.050, Region: F(1, 28) = 4.5, p=0.043, Sex: F(1, 28) = 1.5, p = 0.23. e, IL-6 protein expression in maternal serum 3 h after PIC injection. n are individual animals. Data are shown as mean ± s.e.m and analysed using a two-way ANOVA with Tukey posthoc test. *p < 0.05. ndose:0 = 81 litters, ndose:5 = 8 litters, ndose:10= 62 litters, ndose:20 = 28 litters. Group: F(3,162)=45.17, p < 2e-16, 0 v 5: t(162) = 1.7, p = 0.32, 0 v 10: t(162) = 9.8, p < 0.001, 0 v 20: t(162) = 9.4, p < 0.001, 10 v 20: t(162) = 1.7, p = 0.30. Offspring from dams with a serum response below 1,000 pg ml−1 were excluded from subsequent experiments. f. The gating strategy for FACS-isolated microglia used in Figs. 1j,k, 3 and 4a–j and Extended Data Figs. 2e, 5, 6 and 8
Supplementary information
Supplemental Information
Comprehensive statistics table with exact sample sizes, statistical test performed, information on whether the analyses were one-sided or two-sided, test statistics, exact P values, multiple-testing correction values, and centre and variance units.
Source data
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayes, L.N., An, K., Carloni, E. et al. Prenatal immune stress blunts microglia reactivity, impairing neurocircuitry. Nature 610, 327–334 (2022). https://doi.org/10.1038/s41586-022-05274-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05274-z
This article is cited by
-
Noteworthy perspectives on microglia in neuropsychiatric disorders
Journal of Neuroinflammation (2023)
-
Microbial modulation via cross-fostering prevents the effects of pervasive environmental stressors on microglia and social behavior, but not the dopamine system
Molecular Psychiatry (2023)
-
Maternal immune activation during pregnancy is associated with more difficulties in socio-adaptive behaviors in autism spectrum disorder
Scientific Reports (2023)
-
Effect of prenatal stress on the developing brain
Nature Reviews Immunology (2022)
-
Objection non-responsive! How maternal immune activation in pregnancy weakens subsequent microglial immune response
Cell Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.