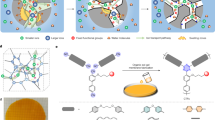
Abstract
Specific-ion selectivity is a highly desirable feature for the next generation of membranes. However, existing membranes rely on differences in charge, size and hydration energy, which limits their ability to target individual ion species. Here we demonstrate a nanocomposite ion-exchange membrane material that enables a reverse-selective transport mechanism that can selectively pass a single ion species. We demonstrate this transport mechanism with phosphate ions selectively transporting across negatively charged cation exchange membranes. Selective transport is enabled by the in situ growth of hydrous manganese oxide nanoparticles throughout a cation exchange membrane that provide a diffusion pathway via phosphate-specific, reversible outer-sphere interactions. On incorporating the hydrous manganese oxide nanoparticles, the membrane’s phosphate flux increased by a factor of 27 over an unmodified cation exchange membrane, and the selectivity of phosphorous over sulfate, nitrate and chloride reaches 47, 100 and 20, respectively. By pairing ion-specific outer-sphere interactions between the target ions and appropriate nanoparticles, these nanocomposite ion-exchange materials can, in principle, achieve selective transport for a range of ions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the plots within this paper are available via FigShare at https://doi.org/10.6084/m9.figshare.20173325. Source data are provided with this paper.
Code availability
The source code for the model is available via GitHub at https://github.com/a-iddya/Nanocomposite-membrane.
References
Duchanois, R. M. et al. Designing polymeric membranes with coordination chemistry for high-precision ion separations. Sci. Adv. 8, eabm9436 (2022).
Epsztein, R., DuChanois, R. M., Ritt, C. L., Noy, A. & Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 15, 426–436 (2020).
Luo, T., Abdu, S. & Wessling, M. Selectivity of ion exchange membranes: a review. J. Memb. Sci. 555, 429–454 (2018).
Subramonian, S. & Clifford, D. Monovalent/divalent selectivity and the charge separation concept. React. Polym. Ion Exch. Sorbents 9, 195–209 (1988).
Güler, E., van Baak, W., Saakes, M. & Nijmeijer, K. Monovalent-ion-selective membranes for reverse electrodialysis. J. Memb. Sci. 455, 254–270 (2014).
Ran, J. et al. Ion exchange membranes: new developments and applications. J. Memb. Sci. 522, 267–291 (2017).
DuChanois, R. M. et al. Designing polymeric membranes with coordination chemistry for high-precision ion separations. Sci. Adv. 8, eabm9436 (2022).
DuChanois, R. M., Porter, C. J., Violet, C., Verduzco, R. & Elimelech, M. Membrane materials for selective ion separations at the water–energy nexus. Adv. Mater. 33, 2101312 (2021).
Zuo, K. et al. Selective membranes in water and wastewater treatment: role of advanced materials. Mater. Today 50, 516–532 (2021).
Lacan, P., Guizard, C., Le Gall, P., Wettling, D. & Cot, L. Facilitated transport of ions through fixed-site carrier membranes derived from hybrid organic–inorganic materials. J. Memb. Sci. 100, 99–109 (1995).
Paltrinieri, L. et al. Hybrid polyelectrolyte-anion exchange membrane and its interaction with phosphate. React. Funct. Polym. 133, 126–135 (2018).
Li, Y. et al. Facilitated transport of small molecules and ions for energy-efficient membranes. Chem. Soc. Rev. 44, 103–118 (2015).
Ng, L. Y., Mohammad, A. W., Leo, C. P. & Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: a comprehensive review. Desalination 308, 15–33 (2013).
Kim, J. H., Won, J. & Kang, Y. S. Silver polymer electrolytes by π-complexation of silver ions with polymer containing C=C bond and their application to facilitated olefin transport membranes. J. Memb. Sci. 237, 199–202 (2004).
Park, Y. S., Won, J. & Kang, Y. S. Facilitated transport of olefin through solid PAAm and PAAm–graft composite membranes with silver ions. J. Memb. Sci. 183, 163–170 (2001).
Faiz, R. & Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 73, 261–284 (2012).
Wu, H. et al. Facilitated transport mixed matrix membranes incorporated with amine functionalized MCM-41 for enhanced gas separation properties. J. Memb. Sci. 465, 78–90 (2014).
Cheng, Y. et al. Advanced porous materials in mixed matrix membranes. Adv. Mater. 30, 1802401 (2018).
Li, Y. & Chung, T.-S. Novel Ag+-zeolite/polymer mixed matrix membranes with a high CO2/CH4 selectivity. Am. Inst. Chem. Eng. 53, 610–616 (2007).
Zhang, M. et al. Synthesis of porous UiO-66-NH2-based mixed matrix membranes with high stability, flux and separation selectivity for Ga(III). Chem. Eng. J. 421, 129748 (2021).
Li, X., Hill, M. R., Wang, H. & Zhang, H. Metal–organic framework‐based ion‐selective membranes. Adv. Mater. Technol. 6, 2000790 (2021).
Zhao, Y. et al. Metal-organic framework based membranes for selective separation of target ions. J. Memb. Sci. 634, 119407 (2021).
Zhang, H. et al. Ultrafast selective transport of alkali metal ions in metal organic frameworks with subnanometer pores. Sci. Adv. 4, eaaq0066 (2018).
Ramakrishnam Raju, M. V., Harris, S. M. & Pierre, V. C. Design and applications of metal-based molecular receptors and probes for inorganic phosphate. Chem. Soc. Rev. 49, 1090–1108 (2020).
Acelas, N. Y., Martin, B. D., López, D. & Jefferson, B. Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media. Chemosphere 119, 1353–1360 (2015).
Pan, B. et al. New strategy to enhance phosphate removal from water by hydrous manganese oxide. Environ. Sci. Technol. 48, 5101–5107 (2014).
Fransiscus, Y., Widi, R. K., Aprilasti, G. O. & Yuharma, M. D. Adsorption of phosphate in aqueous solutions using manganese dioxide. Int. J. Adv. Sci. Eng. Inf. Technol. 8, 818–824 (2018).
Yao, W. & Millero, F. J. Adsorption of phosphate on manganese dioxide in seawater. Environ. Sci. Technol. 30, 536–541 (1996).
Kawashima, M., Tainaka, Y., Hori, T., Koyama, M. & Takamatsu, T. Phosphate adsorption onto hydrous manganese(iv) oxide in the presence of divalent cations. Water Res. 20, 471–475 (1986).
Mustafa, S., Zaman, M. I. & Khan, S. Temperature effect on the mechanism of phosphate anions sorption by β-MnO2. Chem. Eng. J. 141, 51–57 (2008).
Nesbitt, H. W. & Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Mineral. 83, 305–315 (1998).
Yang, Z. et al. Vertically-aligned Mn(OH)2 nanosheet films for flexible all-solid-state electrochemical supercapacitors. J. Mater. Sci. Mater. Electron. 28, 17533–17540 (2017).
Parikh, S. J. & Chorover, J. FTIR spectroscopic study of biogenic Mn-oxide formation by Pseudomonas putida GB-1. Geomicrobiol. J. 22, 207–218 (2005).
Wang, X. & Andrews, L. Infrared spectra of M(OH)1,2,3 (M = Mn, Fe, Co, Ni) molecules in solid argon and the character of first row transition metal hydroxide bonding. J. Phys. Chem. A 110, 10035–10045 (2006).
Stenina, I., Golubenko, D., Nikonenko, V. & Yaroslavtsev, A. Selectivity of transport processes in ion-exchange membranes: relationship with the structure and methods for its improvement. Int. J. Mol. Sci. 21, 5517 (2020).
Jashni, E., Hosseini, S. M., Shen, J. N. & Van der Bruggen, B. Electrochemical characterization of mixed matrix electrodialysis cation exchange membrane incorporated with carbon nanofibers for desalination. Ionics 25, 5595–5610 (2019).
Porozhnyy, M., Huguet, P., Cretin, M., Safronova, E. & Nikonenko, V. Mathematical modeling of transport properties of proton-exchange membranes containing immobilized nanoparticles. Int. J. Hydrogen Energy 41, 15605–15614 (2016).
Zhang, B., Gao, H., Xiao, C., Tong, X. & Chen, Y. The trade-off between membrane permselectivity and conductivity: a percolation simulation of mass transport. J. Memb. Sci. 597, 117751 (2020).
Kingsbury, R. S. & Coronell, O. Modeling and validation of concentration dependence of ion exchange membrane permselectivity: significance of convection and Manning’s counter-ion condensation theory. J. Memb. Sci. 620, 118411 (2020).
Kononenko, N. et al. Porous structure of ion exchange membranes investigated by various techniques. Adv. Colloid Interface Sci. 246, 196–216 (2017).
Lopez, M., Kipling, B. & Yeager, H. L. Ionic diffusion and selectivity of a cation exchange membrane in nonaqueous solvents. Anal. Chem. 49, 629–632 (1977).
Rottiers, T., De la Marche, G., Van der Bruggen, B. & Pinoy, L. Co-ion fluxes of simple inorganic ions in electrodialysis metathesis and conventional electrodialysis. J. Memb. Sci. 492, 263–270 (2015).
White, N., Misovich, M., Yaroshchuk, A. & Bruening, M. L. Coating of Nafion membranes with polyelectrolyte multilayers to achieve high monovalent/divalent cation electrodialysis selectivities. ACS Appl. Mater. Interfaces 7, 6620–6628 (2015).
Hosseini, S. M., Jeddi, F., Nemati, M., Madaeni, S. S. & Moghadassi, A. R. Electrodialysis heterogeneous anion exchange membrane modified by PANI/MWCNT composite nanoparticles: preparation, characterization and ionic transport property in desalination. Desalination 341, 107–114 (2014).
Su, Q. et al. Fabrication of polymer-supported nanosized hydrous manganese dioxide (HMO) for enhanced lead removal from waters. Sci. Total Environ. 407, 5471–5477 (2009).
Pan, B. C. et al. Highly effective removal of heavy metals by polymer-based zirconium phosphate: a case study of lead ion. J. Colloid Interface Sci. 310, 99–105 (2007).
Acknowledgements
We thank the US Department of Agriculture (2017-67022-26135; D.J.) and the US Department of Energy (DOE) Chemical Sciences, Geosciences, and Biosciences Division under contract DE-AC02-05CH11231 (P.Z.).
Author information
Authors and Affiliations
Contributions
D.J. designed and supervised the study. A.I. designed the study, developed the material, performed the experiments, developed the mathematical model and prepared the manuscript. P.Z. developed the molecular dynamics simulations for this work. C.M.K. and S.M. assisted with the characterization and analysis of the developed material. R.K. assisted in the development of the mathematical model and provided the source code for Donnan exclusion calculations. J.W. assisted in the development of the mathematical model. E.M.V.H., I.W. and Z.J.R. contributed to the editing of the manuscript and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Qilin Li, Sukalyan Sengupta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, equations 1–26, Tables 1–3 and Sections 1 and 2.
Source data
Source Data Fig. 1
XPS and FTIR data for membrane characterization.
Source Data Fig. 2
Data files for concentration, pH and separation factor.
Source Data Fig. 4
Data files for flux and transport number.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iddya, A., Zarzycki, P., Kingsbury, R. et al. A reverse-selective ion exchange membrane for the selective transport of phosphates via an outer-sphere complexation–diffusion pathway. Nat. Nanotechnol. 17, 1222–1228 (2022). https://doi.org/10.1038/s41565-022-01209-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01209-x
This article is cited by
-
Effect of solution ions on the charge and performance of nanofiltration membranes
npj Clean Water (2024)
-
The role of ionic blockades in controlling the efficiency of energy recovery in forward bias bipolar membranes
Nature Energy (2023)
-
Specific ion selectivity with a reverse-selective mechanism
Nature Nanotechnology (2022)