Abstract
Although several drugs have been proposed and used to treat the COVID-19 virus, but recent clinical trials have concentrated on ivermectin. It appears that ivermectin can potentially act against COVID-19 and stop the development in its infancy. The purpose of this study was to determine the effect of ivermectin on the recovery of outpatients with COVID-19. In this cross-sectional study, we compared the symptoms reduction in COVID-19 disease in two groups of patients by administering ivermectin. A total of 347 mild outpatients in the Iranian provinces of Qazvin and Khuzestan with a confirmed PCR were enrolled. The symptoms of outpatients with COVID-19 were analyzed using SPSS (V23). In this cross-sectional study, the sex ratio was 0.64 (female/male: 37.9/59.8) and most patients were under 50 years old (72.8%). The results of this study demonstrated a significant decrease in several COVID-19 disease symptoms, including fever, chills, dyspnea, headache, cough, fatigue, and myalgia in the group administered ivermectin compared to the control group. In addition, the odds ratio of the above symptoms was significantly lower in patients who received ivermectin than in patients who did not receive the drug (OR = 0.16, 95% CI = 0.09, 0.27).
Similar content being viewed by others
1 Introduction
The COVID-19 disease, which is caused by the severe acute respiratory syndrome coronavirus and has a high prevalence, has harmed a significant number of people. Approximately five million people have died worldwide from this virus to date (Rivera et al. 2020). Along with inviting people to consider physical contact restrictions and providing comprehensive health advice, various drugs with antiviral and anti-inflammatory properties are used to prevent virus transmission and control. Several studies have demonstrated that chloroquine phosphate and hydroxychloroquine (HCQ) inhibit COVID-19 in-vitro(Andreani et al. 2020; Gautret et al. 2020; Million et al. 2020). Other treatments, such as nitazoxanide(Kelleni 2020), tocilizumab(Ignatius et al. 2021), stem cell therapy(Sahu et al. 2021), and plasma therapy(Sahu et al. 2020), are prohibitively expensive, necessitating the use of widely available medications such as Ivermectin and HCQ. Also, several drugs, including Remdesivir, Oseltamivir, and HCQ, have been tested in extensive comparative studies(Geleris et al. 2020; Katzen et al. 2019; Lagier et al. 2020; Sturrock and Chevassut 2020; Wang et al. 2020; Yu et al. 2020). Some studies have shown that Lopinavir, Ritonavir, and Remdesivir have no tangible effects on the COVID-19 virus while being associated with many adverse effects(Beigel et al. 2020; Cao et al. 2020). Therefore, most guidelines recommended using Dexamethasone and Remdesivir as standard-of-care in hospitalized adults(Bhimraj et al. 2020; Pau et al. 2020). However, the WHO recommendation is against the use of Remdesivir in hospitalized patients with COVID-19(Bhimraj et al. 2020). While mRNA and adenovirus-vector vaccines are extensively employed in developing countries, global COVID-19 vaccination rates are modest(Janik et al. 2021). Consequently, it may be beneficial to use a drug that shows promise in clinical trials. A cross-sectional study to assess the acceptance of the vaccine by the Indonesian public found that it was strongly influenced by the vaccine's baseline effectiveness. Therefore, preparing the general population to accept a vaccine with relatively low efficacy may be associated with challenges.
Furthermore, it is unclear when these vaccines are used publicly; therefore, choosing a drug that has shown promising effects can prove helpful (Cohen 2020; Harapan et al. 2020). Ivermectin, a mixture of two closely related macrocyclic lactones, is a relatively affordable anti-parasitic medicine that has previously been used successfully in humans. It has a broad antiviral range and suppresses the proliferation of COVID-19 in the cell culture(Heidary and Gharebaghi 2020; Lehrer and Rheinstein 2020; Wagstaff et al. 2012). Moreover, ivermectin showed antiviral activity against various RNA and DNA-based viruses(Heidary and Gharebaghi 2020). Ivermectin's three proposed mechanisms in reducing the load of the SARS-CoV-2 virus include inhibition of nuclear import of host cell (Wagstaff et al. 2012) and interfering with attachment of spike protein to the human cell membrane(de Oliveira, Rocha, Paluch, and Costa, 2020; Xuemei Zhang et al. 2009). The hypotheses show that viral replication in later stages is affected by cytopathic changes because virus replication and culture are minimized. Also, cytopathic changes confirmed the findings of Lee et al.(Lee et al. 2020). Therefore, these unstable RNA (SARS-CoV-2) fragments, as a result of an inflammatory response, severely destroys COVID-19. Laboratory studies showed that ivermectin has the potential of inflammation inhibition and its anti-inflammatory properties inhibit cytokine production, regulating NF-κB transcription while limiting the production of nitric oxide and prostaglandin E2(Ci et al. 2009; X Zhang et al. 2008). The purpose of this study is to determine the effect of ivermectin on out patients with COVID-19 and to answer the question of whether this drug can reduce the symptoms of the disease or not?
2 Materials and Methods
2.1 Study Design
This study is designed to answer the following question:
Whether oral ivermectin ( 200 µg/kg) can be used for the reduction symptoms of COVID-19 disease?
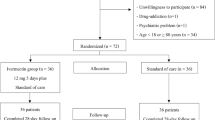
Therefore, we designed descriptive-analytical cross-sectional study to compare reducing the disease symptoms and its progression. This epidemiological investigation was conducted on a target population in Iran's south and northwestern provinces (Qazvin and Khuzestan). In this study, patients reported fever or respiratory symptoms or close contact with the patient at home or in public places were tested for SARS-CoV-2 infection.
A standardized method was used to select participants. Trained doctors or laboratory technicians obtained the nasopharyngeal swabs. Also, diagnosis of COVID-19 was confirmed by real-time PCR.
Patients with positive RT-PCR test was employed from July 1 to 30,2020. Structured interviews were conducted to document symptoms for all patients with confirmed COVID-19. Data collection was performed according to the criteria established for demographics, underlying disease, disease progression, and clinical symptoms. We used a checklist for all patients to provide information on demographic, symptoms and signs and any comorbidities that could been related to the disease. In this study, we tried to keep the data anonymous.
2.2 Patients
Inclusion criteria included being able to provide informed consent, not participating in any other clinical study, age > 18 years and the positive confirmation of real-time reverse transcription polymerase chain reaction (RT-PCR). Additionally, we excluded children, pregnant and lactating women, subjects with immunodeficiency and participants with no RT-PCR test result from the study. The sample size was determined using with p1 = 0.09, p2 = 0.02, α error of 0.05, a power of 0.8 and a 95% confidence level. The sample size was estimated to be 167 pairs, i.e., 334 individuals. With considering 5% drop-out rate, finally, 345 patients were enrolled according to the inclusion criteria.
2.3 Procedure
We studied patients and their medication prescriptions in this study by administering ivermectin. Patients administered ivermectin tablets (at least 200 μg /kg) during the first week of disease were termed Ivermectin users. All patients received standard outpatient care based on the "Iranian guideline of outpatients COVID-19 patients' management were considered as comparison group(Rahmanzade et al. 2020). We also categorized their demographic characteristics such as sex, age, marital status, body mass index (BMI), and history of chronic disease groups (diabetes, hypertension, pulmonary, cardiovascular, kidney and hepatic diseases, and auto-immune) in two groups, separately.
2.4 Ethics Statement and Informed Consent
The ethics committee of Qazvin University of Medical Sciences (IR.QUMS.REC.1399.261) approved all regulations and methods, following the Declaration of Helsinki and its amendments. In addition, all patients signed an informed consent form before inclusion.
2.5 Statistical Analysis
We comprised the data of the all patients in two groups of the demographic characteristics such as sex, age, marital status and comorbidity. Normality of data distribution was test with Kolmogorov–Smirnov. We done descriptive analysis for categorical and continuous variables with number (percentage) and mean (standard deviation), respectively. For analytical analysis we used t-test, Chi-square test for continues and discrete variables, respectively. Also, we used bivariate logistic regression for measuring the effect size of ivermectin use to decrease in symptoms of COVID-19 diseases and disease symptoms. Dependent variables consist of symptoms of COVID-19 diseases such as fever, chills, shortness breath, headache, dry cough, weakness, muscle pain, diarrhea and vomiting and symptoms reduction, totally. Independent variable considered as ivermectin use. We used SPSS 23.0 (IBM, Armonk, NY) for doing statistical analysis. We considered p value < 0.05 for statistical significance.
3 Results
In this cross-sectional study, we enrolled 347 patients with COVID-19. Sex ratio was 0.64 (female/male: 37.9/59.8). Two-third of the patients were married (70.8%). The mean age of patients was 52.25 ± 17.36, with the range of 19–85 years. Most patients were under 50 years old (72.8%). Demographic and clinical characteristics of the patients with COVID-19 stratified by being administered ivermectin are shown in (Table 1). Based on Table 1, there was no a high risk of heterogeneity of the participants. Approximately half of the patients received ivermectin (48.7%). Thirty-seven percent of the participants had a normal BMI. The most common non-communicable diseases among the patients were hypertension and diabetes at 10.4 and 6.4%. This study discovered no statistically significant difference between the ivermectin-taking and non-taking group considering age, sex, BMI, plus comorbidity with chronic disease groups (diabetes, hypertension, pulmonary, auto-immune, cardiovascular, kidney, and hepatic diseases), that decrease the risk of the heterogeneity of two groups in main confounders.
According to the results of the bivariate logistic regression (Table 2), symptoms including fever (p = 0.006), chills (p = 0.015), dyspnea (p = 0.001), headache (p < 0.001), cough (p < 0.001), fatigue (p = 0.230) and myalgia (p < 0.001) were associated with taking ivermectin. The odds ratio for the above symptoms was significantly lower in patients with COVID-19 than patients taking ivermectin. There was no significant difference between the two groups in terms of diarrhea (p = 0.447) and vomiting (p = 0.555).
Our results indicated that the odds ratio for symptom reduction was significantly lower in patients without a history of ivermectin use (OR = 0.16, 95% CI = 0.09, 0.27) than in patients who had used ivermectin previously. Also, the odds of symtoms reduction in ivermectin taking group was 0.16 times higher than comparison group.
4 Discussion
COVID-19 has recently spread throughout the world, altering human lives, and researchers are continuing to identify a suitable drug to treat patients affected by COVID-19. As previously stated, numerous drugs have been evaluated, but ivermectin was concluded to be effective in several clinical trials(Marik et al. 2020; Niaee et al. 2020; Turkia). The evidence for ivermectin's antiviral activity from in-vitro and animal studies supports its efficacy in preventing and treating infections in their early stages. The concentrations tested in these in-vitro assays are equivalent to more than 50-fold the normal Cmax achieved with a standard single dose of IVM 200 μg/kg, raising concerns about the effective dose of IVM for treating COVID-19 in humans and its tolerability(Chaccour et al. 2020). The results of a clinical trial study of this research team aimed at determining the dose of ivermectin in mild COVID-19 patients showed that the effect of a single dose is better than the interval dose compared to the control group (Niaee et al. 2021). And another similar study performed on outpatients with mild symptoms of COVID-19 at a dose of 200 µg /kg and with good results, justifies the claim that the clinically appropriate dose is different from the dose of this in vitro (Biber et al. 2021). Therefore, to further confirm the previous results in this cross-sectional study, we prescribed ivermectin 200 μg / kg tablets. Also, our previous study established the effect of ivermectin on length of stay in the hospital and intensive care unit(Niaee et al. 2021). In our previous clinical trial, immunomodulatory effects were referred through promotion in C-Reactive protein, absolute Lymphocyte count, blood urea Nitrogen, Creatinine, Erythrocyte sedimentation rate, and white blood cells in comparison with the placebo and control. Numerous cross-sectional studies on COVID-19 have been conducted in various countries using a variety of approaches, including a cross-sectional study on the role of public awareness in preventing the spread of the COVID-19 outbreak in India or on COVID-19 perceptions, knowledge, and behaviors among social media users, plus a cross-sectional study and a community-based cross-sectional study of the epidemiology of onchocerciasis in unmapped villages in Jimma Zone, Southwestern Ethiopia, for community-directed treatment with ivermectin, among others. However, this study appeared to be the first cross-sectional study of ivermectin and coronavirus conducted in outpatients and included an analysis of the most common COVID-19 symptoms, including fever, chills, dyspnea, headache, cough, fatigue, myalgia, diarrhea, and vomiting(Ali et al. 2020; Dana et al. 2015; Harapan et al. 2020; Jin-Wei Ai1 J-WC; Kaushik, Agarwal, and Gupta, 2020; Swapna Mandal JB), (Table 2). In general, significant differences in symptom reduction were observed between the two selected groups of ivermectin users and non-users. Other manifestations, such as conjunctivitis, hyposmia, hypogeusia, loss of sense of smell, abnormal eye movements, and others, were not assessed in this study. However, during a clinical trial, additional symptoms such as bilateral conjunctival injection without associated secretions, hypogeusia, skin rash, and hyposmia were reported(Chen et al. 2020). Overall, this study established an appropriate relationship between the drug and the patient to prevent the severity of illness and promote health through a prompt recovery. After treatment with ivermectin, disease monitoring revealed a significant reduction in symptoms in the mild COVID-19 patients with a positive RT-PCR. Finally, our results show that the odds of symptoms reduction in ivermectin taking group were 0.16 times higher than comparison group. Given the high prevalence of this disease in many countries, ivermectin may be an excellent choice for relieving patient symptoms such as fever, chills, dyspnea, headache, cough, fatigue, and myalgia with a single dose (200 µg/kg). However, the effects of ivermectin on chemoprophylaxis and viral transmission prevention in communities must be thoroughly investigated.
5 Conclusion
The results of this study showed that a single dose of ivermectin (200 µg/kg) reduced the severity of illness symptoms in COVID-19 patients receiving ambulatory care.
6 Limitations
Our study was cross-sectional, which had limitations such as the impossibility of determining the temporality of variables and inferring causality. Additionally, information bias may have occurred during data collection, such as responder bias, recall bias, interviewer bias, and social acceptability bias. Additionally, our study included only mild cases and immunocompetent patients. Therefore, ongoing studies with larger sample sizes, focusing on strategies to increase ivermectin's antiviral potency and its combination with other antivirals, and on severe COVID-19 cases, as well as interventional studies, are recommended. Also, probability there were some unstudied factors that could be as confounding variable and should be considered in future studies.
Availability of Data and Materials
The dataset used in this study is only available upon request from the corresponding author.
References
Ali KF, Whitebridge S, Jamal MH, Alsafy M, Atkin SL (2020) Perceptions, knowledge, and behaviors related to COVID-19 among social media users: cross-sectional study. J Med Internet Res 22(9):e19913
Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, La Scola B (2020) In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog 145:104228
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Kline S (2020) Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med 583(19):1813–1826
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VCC, O’Horo JC (2020) Infectious diseases society of america guidelines on the treatment and management of patients with COVID-19. Clin Infect Diseases. Doi: https://doi.org/10.1093/cid/ciaa478
Biber A, Mandelboim M, Harmelin G, Lev D, Ram L, Shaham A, Schwartz E (2021). Favorable outcome on viral load and culture viability using Ivermectin in early treatment of non-hospitalized patients with mild COVID-19, A double-blind, randomized placebo-controlled trial. medRxiv. Doi: https://doi.org/10.1101/2021.05.31.21258081
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Wei M (2020) A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799
Chaccour C, Hammann F, Ramón-García S, Rabinovich NR (2020) Ivermectin and COVID-19: keeping rigor in times of urgency. Am J Trop Med Hyg 102(6):1156
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Yu H (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig 130(5):2620–2629
Ci X, Li H, Yu Q, Zhang X, Yu L, Chen N, Deng X (2009) Avermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam Clin Pharmacol 23(4):449–455
Cohen J (2020) Vaccine designers take first shots at COVID-19. Am Assoc Adv Sci 368(6486):14–16
Dana D, Debalke S, Mekonnen Z, Kassahun W, Suleman S, Getahun K, Yewhalaw D (2015) A community-based cross-sectional study of the epidemiology of onchocerciasis in unmapped villages for community directed treatment with ivermectin in Jimma Zone, southwestern Ethiopia. BMC Public Health 15(1):1–7
de Oliveira OV, Rocha GB, Paluch AS, Costa LT (2021) Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening. J Biomol Struct Dyn 39(11):3924–3933
Gautret P, Lagier J-C, Parola P, Meddeb L, Sevestre J, Mailhe M, Seng P (2020) Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis 34:101663
Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Barr RG (2020) Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 382(25):2411–2418
Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, Mudatsir M (2020) Acceptance of a COVID-19 vaccine in southeast Asia: A cross-sectional study in Indonesia. Front Public Health 8. https://doi.org/10.3389/fpubh.2020.00381
Heidary F, Gharebaghi R (2020) Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot 73(9):593–602
Ignatius EH, Wang K, Karaba A, Robinson M, Avery RK, Blair P, Siddiqui Z (2021) Tocilizumab for the treatment of COVID-19 among hospitalized patients: a matched retrospective cohort analysis. Paper Presented at the Open Forum Infectious Diseases 8(1):598
Janik E, Niemcewicz M, Podogrocki M, Majsterek I, Bijak M (2021) The Emerging Concern and Interest SARS-CoV-2 Variants. Pathogens 10(6):633
Jin-Wei Ai1 JWC, Liu X-Y, Fan W-F, Qu G-J, Zhang M-L, Pei S-D, Tang B-W, Yuan S, Li Y, Wang L-S, Huang G-X, Pei B (2019) The cross-sectional study of hospitalized coronavirus disease 2019 patients in Xiangyang. Hubei province
Katzen J, Kohn R, Houk JL, Ison MG (2019) Early oseltamivir after hospital admission is associated with shortened hospitalization: a 5-year analysis of oseltamivir timing and clinical outcomes. Clin Infect Dis 69(1):52–58
Kaushik M, Agarwal D, Gupta AK (2020) Cross-sectional study on the role of public awareness in preventing the spread of COVID-19 outbreak in India. Postgraduate Med J, 138349
Kelleni MT (2020) Nitazoxanide/azithromycin combination for COVID-19: A suggested new protocol for early management. Pharmacol Res 157:104874
Lagier J-C, Million M, Gautret P, Colson P, Cortaredona S, Giraud-Gatineau A, Tissot-Dupont H (2020) Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med Infect Dis 36:101791
Lee JS, Park S, Jeong HW, Ahn JY, Choi SJ, Lee H, Kwon JS (2020) Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol 5(49):eabd1554
Lehrer S, Rheinstein PH (2020) Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. in vivo 34(5):3023–3026
Marik PE, Kory P, Varon J, Iglesias J, Meduri GU (2020) MATH+ protocol for the treatment of SARS-CoV-2 infection: the scientific rationale. Expert Rev Anti Infect Ther 19(2):129–135
Million M, Lagier J-C, Gautret P, Colson P, Fournier P-E, Amrane S, Doudier B (2020) Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille. France Travel Med Infectious Dis 35:101738
Niaee MS,Namdar P, Allami A, ZolghadrL (2021) Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial. Asian Pacific J Trop Med 14(6):266–273
Pau AK, Aberg J, Baker J, Belperio PS, Coopersmith C, Crew P, Harrison C (2020) Convalescent plasma for the treatment of COVID-19: perspectives of the National Institutes of Health COVID-19 Treatment Guidelines Panel. Ann Internal Med 174(1):93–95
Rahmanzade R, Rahmanzadeh R, Hashemian SM, Tabarsi P (2020) Iran’s Approach to COVID-19: Evolving Treatment Protocols and Ongoing Clinical Trials. Front Public Health 8:523
Rivera A, Ohri N, Thomas E, Miller R, Knoll MA (2020) The impact of COVID-19 on radiation oncology clinics and patients with cancer in the United States. Adv Radiat Oncol 5(4):538–543
Sahu KK, Mishra AK, Raturi M, Lal A (2020) Current perspectives of convalescent plasma therapy in COVID-19. Acta Bio Medica: Atenei Parmensis 91(4):e2020155
Sahu KK, Siddiqui AD, Cerny J (2021) Mesenchymal stem cells in COVID-19: a journey from bench to bedside. Lab Med 52(1):24–35
Sturrock BR, Chevassut TJ (2020) Chloroquine and COVID-19–a potential game changer? Clin Med 20(3):278
Swapna Mandal JB, S. E. B., Jeremy S Brown, 4Emma K Denneny, Samanjit S Hare,MelissaHeightman, Toby E Hillman, Joseph Jacob, Hannah C Jarvis, Marc C I Lipman, Sindhu B Naidu,Arjun Nair, Joanna C Porter, Gillian S Tomlinson, John R Hurst ARC Study Group.(2020), across-sectional study of persisting symptoms, biomarker andimaging abnormalities following hospitalisation for COVID-19. doi:101136/thoraxjnl-2020–215818. Thorax 1–3.
Turkia, M.(2021), A timeline of ivermectin-related events in the COVID-19 pandemic. DOI:https://doi.org/10.13140/RG.2.2.23081.72805
Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA (2012) Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochemical Journal 443(3):851–856
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Lu Q (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet 395(10236):1569–1578
Yu B, Li C, Chen P, Zhou N, Wang L, Li J, Wang D-W (2020) Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Science China Life Sciences 63(10):1515–1521
Zhang X, Song Y, Ci X, An N, Ju Y, Li H, Deng X (2008) Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res 57(11):524–529
Zhang X, Song Y, Xiong H, Ci X, Li H, Yu L, Deng X (2009). Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int Immunopharm 9(3):354–359
Acknowledgements
The support of the deputy of research and technology of Qazvin University of Medical Sciences is acknowledged. Furthermore, we appreciate the support and cooperative attitudes of the Qazvin University of Medical Sciences' Cellular and Molecular Research Center and Infection Prevention Center and all clinical, technical, and paramedical staff on the wards and laboratories.
Funding
None.
Author information
Authors and Affiliations
Contributions
MSN: resources, supervision, project administration. LZ: writing the original draft, data curation, design Fig. 1 and Tables, data analysis. ZH: data analysis, editing. PN: resources, supervision physician, project administration, supervision physician. AA: resources, methodology, supervision physician, project administration. F A: physician, patient care, recording, and collection of clinical data. MV: supervision, patient care, recording, and collection of clinical data. NG: conceptualization, methodology, supervision, project administration, writing, review and editing, resources.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics Approval and Consent to Participate
The ethics committee of Qazvin University of Medical Sciences approved the study (IR.QUMS.REC.1399.261) as per the Declaration of Helsinki and its amendments. Before inclusion, all patients signed an informed consent form.
Consent for Publication
Not applicable.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niaee, M.S., Zolghadr, L., Hosseinkhani, Z. et al. Ivermectin-Induced Clinical Improvement and Alleviation of Significant Symptoms of COVID-19 Outpatients: A Cross-Sectional Study. Iran J Sci Technol Trans Sci 46, 1369–1375 (2022). https://doi.org/10.1007/s40995-022-01349-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-022-01349-8