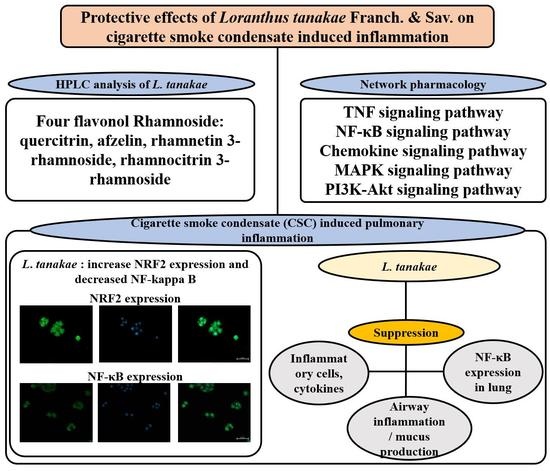
Loranthus tanakae Franch. & Sav. Suppresses Inflammatory Response in Cigarette Smoke Condensate Exposed Bronchial Epithelial Cells and Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Instrument
2.1.1. Plant Material
2.1.2. Extraction and Isolation
2.1.3. HPLC Analysis
2.2. Network Pharmacology Analysis
2.2.1. Small Molecules and Potential Target Genes
2.2.2. Protein–Protein Interaction (PPI)
2.2.3. Signal Pathway Analysis
2.3. In Vitro Experiment
2.3.1. Cell Viability
2.3.2. Evaluation of Inflammatory Cytokines in H292 Cells
2.3.3. Immunofluorescence for Nrf2 and NF-κB in H292 Cells
2.3.4. DPPH Radical Scavenging Activity
2.3.5. Measurement of ROS Production
2.4. In Vivo Experiment
2.4.1. Animals
2.4.2. Procedure of Animal Experiments
2.4.3. Measurement of Inflammatory Mediators in BALF
2.4.4. Histopathology of Lung Tissue
2.5. Statistical Analysis
3. Results
3.1. Isolation of Active Components
3.2. HPLC Analysis of LTE
3.3. Network of Active Molecules and Potential Target Genes
3.4. Protein–Protein Interaction (PPI)
3.5. Signal Pathway Analysis
3.6. Effects of LTE on the Generation of Inflammatory Cytokines in CSC Exposed H292 Cells
3.7. Effects of LTE on the Expression of Nrf2 and NF-κB in CSC Exposed H292 Cells
3.8. Effects of LTE and Its Components on ROS Production
3.9. Effects of LTE on Inflammatory Indexes in CSC+LPS Exposed Mice
3.10. Effects of LTE on Pathophysiological Alteration of Lung Tissue in CSC+LPS Exposed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taucher, E.; Mykoliuk, I.; Lindenmann, J.; Smolle-Juettner, F.M. Implications of the immune landscape in COPD and lung cancer: Smoking versus other causes. Front. Immunol. 2022, 13, 846605. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Seo, C.S.; Shin, N.R.; Kim, J.S.; Lee, S.I.; Kim, J.C.; Kim, S.H.; Shin, I.S. Modified mahuang-tang, a traditional herbal medicine suppresses inflammatory responses induced by cigarette smoke in human airway epithelial cell and mice. Phytomedicine 2019, 59, 152777. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Park, J.W.; Shin, N.R.; Jeon, C.M.; Kwon, O.K.; Lee, M.Y.; Kim, H.S.; Kim, J.C.; Oh, S.R.; Ahn, K.S. Melatonin inhibits MUC5AC production via suppression of MAPK signaling in human airway epithelial cells. J. Pineal Res. 2014, 56, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Greenlund, K.J.; VanFrank, B.; Xu, F.; Lu, H.; Croft, J.B. Smoking cessation among U.S. adult smokers with and without chronic obstructive pulmonary disease. 2018. Am. J. Prev. Med. 2022, 62, 492–502. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative stress in chronic obstructive pulmonary disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Barnes, P.J.; Anderson, G.P.; Fageras, M.; Belvisi, M.G. Chronic lung disease: Prospects for regeneration and repair. Eur. Respir. Rev. 2021, 30, 200213. [Google Scholar] [CrossRef]
- Bourbeau, J.; Bafadhel, M.; Barnes, N.C.; Compton, C.; Di Boscio, V.; Lipson, D.A.; Jones, P.W.; Martin, N.; Weiss, G.; Halpin, D.M.G. Benefit/risk profile of single-inhaler triple therapy in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 499–517. [Google Scholar] [CrossRef]
- Joo, S.W.; Kim, H.G.; Oh, E.J.; Ko, J.H.; Lee, Y.G.; Kang, S.C.; Lee, D.Y.; Baek, N.I. Cyclofarnesane sesquiterpene glucoside from the whole plant of Loranthus tanakae and its cytotoxicity. J. Appl. Biol. Chem. 2019, 62, 7–10. [Google Scholar] [CrossRef]
- Zhou, J.T.; Ren, K.D.; Hou, J.; Chen, J.; Yang, G. α-rhamnrtin-3-α-rhamnoside exerts anti-inflammatory effects on lipopolysaccharide-stimulated RAW264.7 cells by abrogating NF-κB and activating the Nrf2 signaling pathway. Mol. Med. Rep. 2021, 24, 799. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.S.; Choi, S.U.; Ryu, S.Y. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch. Pharm. Res. 2004, 27, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yan, H.; Bi, L.; Geng, Z.; Wu, X.; Yang, G. Biotransformation characteristics of Loranthus tanakae by Rhodopseudomonas palustris. Cellulose Chem. Technol. 2016, 50, 819–829. Available online: https://www.cellulosechemtechnol.ro/pdf/CCT7-8(2016)/p.819-829.pdf (accessed on 20 September 2022).
- Noor, R.; Qamar, M.T.; Ashfaq, U.A.; Albutti, A.; Alwahmi, A.S.S.; Alhasir, M.A. Network pharmacology approach for medicinal plants: Review and assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Lee, J.Y.; Chun, J.M. Exploring the mechanism of Gyejibokryeong-hwan against atherosclerosis using network pharmacology and molecular docking. Plants 2020, 9, 1750. [Google Scholar] [CrossRef]
- Huang, D.; Lv, Y.; Lu, C.S.; Zhang, B.; Fu, Z.J.; Huang, Y.L. Mechanism of Rhizoma Cpotidis in epilepsy with network pharmacology. Allergol. Immunopathol. 2022, 50, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Kim, J.S.; Lee, J.; Seo, Y.H.; Kim, H.S.; Ryu, S.M.; Choi, G.; Moon, B.C.; Lee, A.Y. Pharmacological effects of Agastache rugosa against gastritis using a network pharmacology approach. Biomolecules 2020, 10, 1298. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, C.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acid Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Huang, X.F.; Cheng, W.B.; Jiang, Y.; Liu, Q.; Liu, X.H.; Xu, W.F.; Huang, H.T. A network pharmacology-based strategy for predicting anti-inflammatory targets of ephedra in treating asthma. Int. Immunopharmacol. 2020, 83, 106423. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.W.; Lee, A.Y.; Seo, Y.S.; Lee, S.J.; Kim, W.I.; Shin, D.H.; Kim, J.C.; Kim, J.S.; Lim, J.O.; Shin, I.S. Anti-asthmatic effects of Phlomis umbrosa Turczaninow using ovalbumin induced asthma murine model and network pharmacology analysis. Biomed. Pharmacother. 2022, 145, 112410. [Google Scholar] [CrossRef]
- Shin, I.S.; Shin, N.R.; Park, J.W.; Jeon, C.M.; Hong, J.M.; Kwon, O.K.; Kim, J.S.; Lee, I.C.; Kim, J.C.; Oh, S.R.; et al. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary disease via the suppression of Erk-Sp1 signaling. J. Pineal Res. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Lim, J.O.; Song, K.H.; Lee, I.S.; Lee, S.J.; Kim, W.I.; Pak, S.W.; Shin, I.S.; Kim, T. Cimicifugae Rhizoma extract attenuates oxidative stress and airway inflammation via the upregulation of Nrf2/HO-1/NQO1 and downregulation of NF-B phosphorylation in ovalbumin-induced asthma. Antioxidants 2021, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Kosakowska, W.; Baczek, K.; Przybyl, J.L.; Pioro-Jabrucka, E.; Czupa, W.; Synowiec, A.; Gniewosz, M.; Costa, R.; Mondello, L.; Weglarz, Z. Antioxidant and antibacterial activity of Roseroot (Rhodiola rosea L.) dry extracts. Molecules 2018, 23, 1767. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pelgrim, C.E.; Peralta Marzal, L.N.; Korver, S.; van Ark, I.; Leusink-Muis, T.; van Helvoort, A.; Keshavarzian, A.; Kraneveld, A.D.; Garssen, J.; et al. Changes in intestinal homeostasis and immunity in a cigarette smoke- and LPS-induced murine model of COPD: The lung-gut axis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 323, L266–L280. [Google Scholar] [CrossRef] [PubMed]
- Hardaker, E.L.; Freeman, M.S.; Dale, N.; Bahra, R.; Raza, F.; Banner, K.H.; Poll, C. Exposing rodents to a combination of tobacco smoke and lipopolysaccharide result in an exaggerated inflammatory response in the lung. Br. J. Pharmacol. 2010, 160, 1985–1996. [Google Scholar] [CrossRef]
- Bose, S.; Maji, S.; Chakraborty, P. Quercitrin from Ixora coccinea leaves and its anti-oxidant activity. J. PharmaSciTech 2013, 2, 72–74. Available online: www.pharmascitech.in/admin/php/uploads/45_pdf.pdf (accessed on 13 April 2022).
- Lee, S.Y.; So, Y.J.; Shin, M.S.; Cho, J.Y.; Lee, J. Antibacterial effects of afzelin isolated from Cornus macrophylla on Pseudomonas aeruginosa, a leading cause of illness in immunocompromised individuals. Molecules 2014, 19, 3173–3180. [Google Scholar] [CrossRef]
- Chung, S.K.; Kim, Y.C.; Takaya, Y.; Terashima, K.; Niwa, M. Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. J. Agric. Food Chem. 2004, 52, 4664–4668. [Google Scholar] [CrossRef]
- Shanmugam, G.; Narasimhan, M.; Sakthivel, R.; Kumar, R.R.; Davidson, C.; Palaniappan, S.; Claycomb, W.W.; Hoidal, J.R.; Darley-Usmar, V.M.; Rajasekaran, N.S. A biphasic effect of TNF-α in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox Biol. 2016, 9, 77–89. [Google Scholar] [CrossRef]
- Helou, D.G.; Martin, S.F.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Romer, S. Nrf2 involvement in chemical-induced skin innate immunity. Front. Immunol. 2019, 10, 1004. [Google Scholar] [CrossRef]
- van der Horst, A.; Burgering, B.M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, P.; Wang, Q.; Sun, F.; Liu, F. Sulforaphane attenuates H2O2-induced oxidant stress in human trabecular meshwork cells (HTMCs) via the phosphatidylinositol3-kinase (PI3K)/serin/threonine kinase (Akt)-mediated factor-E2-related factor 2 (Nrf2) signaling activation. Med. Sci. Monit. 2019, 25, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Song, H.H.; Shin, I.S.; Cho, B.O.; Jeong, S.H.; Kim, D.Y.; Oh, S.R. Suffruticosol A isolated from Paeonia lactiflora seedcases attenuates airway inflammation in mice induced by cigarette smoke and LPS exposure. J. Funct. Foods 2015, 17, 774–784. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.; Kim, J.M.; Lee, M.K.; Se, S.J.; Park, K.Y. Afzelin suppresses proinflammatory responses in particulate matter-exposed human keratinocytes. Int. J. Mol. Med. 2019, 43, 2516–2522. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Park, S.M.; Yang, S.R. An update on the role of Nrf2 in respiratory disease: Molecular mechanisms and therapeutic approaches. Int. J. Mol. Sci. 2021, 22, 8406. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Sapey, E. Oxidative stress in COPD: Sources, markers, and potential mechanisms. J. Clin. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- Krajka-Kuzniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.Y.; Bach, F.H. Hemeoxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef]
- Yerra, V.G.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef]
- Dai, X.; Ding, Y.; Zhang, Z.; Cai, X.; Li, Y. Quercetin and quercitrin protect against cytokine-induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int. J. Mol. Med. 2013, 31, 265–271. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, Y.; Zhao, Y.; Xie, X.; Kuang, N.; Wei, Y.; Yu, M.; Hu, T. Intervening effects and molecular mechanism of quercitrin on PCV2-induced histone acetylation, oxidative stress and inflammatory response in 2D4/2 cells. Antioxidants 2022, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.W.; Jung, E.; Kim, S.; Kim, J.H.; Kim, E.G.; Lee, J.; Park, D. Antagonizing effects and mechanisms of afzelin against UVB-induced cell damage. PLoS ONE 2013, 8, e61971. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kang, M.K.; Lee, E.J.; Kim, D.Y.; Oh, H.; Kim, S.I.; Oh, S.Y.; Kim, K.H.; Park, S.J.; Choi, Y.J.; et al. Dried yeast extracts curtails pulmonary oxidative stress, inflammation and tissue destruction in a model of experimental emphysema. Antioxidants 2019, 8, 349. [Google Scholar] [CrossRef] [Green Version]









| Sample | DPPH IC50 (μM) | ABTS IC50 (μM) |
|---|---|---|
| Gallic acid (positive control) | 20.43 | 13.81 |
| LTE (μg/mL) | 124.37 | 226.1 |
| Rhamnetin 3-ramnoside | 17.90 | 36.36 |
| Rhamnocitrin 3-rhamnoside | - | - |
| Afzelin | - | - |
| Quercitrin | 23.72 | 64.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-W.; Lee, A.Y.; Lim, J.-O.; Lee, S.-J.; Kim, W.-I.; Yang, Y.-G.; Kim, B.; Kim, J.-S.; Chae, S.-W.; Na, K.; et al. Loranthus tanakae Franch. & Sav. Suppresses Inflammatory Response in Cigarette Smoke Condensate Exposed Bronchial Epithelial Cells and Mice. Antioxidants 2022, 11, 1885. https://doi.org/10.3390/antiox11101885
Park S-W, Lee AY, Lim J-O, Lee S-J, Kim W-I, Yang Y-G, Kim B, Kim J-S, Chae S-W, Na K, et al. Loranthus tanakae Franch. & Sav. Suppresses Inflammatory Response in Cigarette Smoke Condensate Exposed Bronchial Epithelial Cells and Mice. Antioxidants. 2022; 11(10):1885. https://doi.org/10.3390/antiox11101885
Chicago/Turabian StylePark, So-Won, A Yeong Lee, Je-Oh Lim, Se-Jin Lee, Woong-Il Kim, Yea-Gin Yang, Bohye Kim, Joong-Sun Kim, Sung-Wook Chae, Kun Na, and et al. 2022. "Loranthus tanakae Franch. & Sav. Suppresses Inflammatory Response in Cigarette Smoke Condensate Exposed Bronchial Epithelial Cells and Mice" Antioxidants 11, no. 10: 1885. https://doi.org/10.3390/antiox11101885