The Sodium–Glucose Co-Transporter-2 (SGLT2) Inhibitors Reduce Platelet Activation and Thrombus Formation by Lowering NOX2-Related Oxidative Stress: A Pilot Study
Abstract
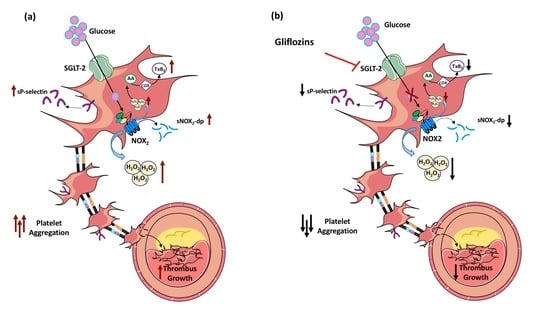
:1. Introduction
2. Materials and Methods
2.1. Human Study
2.2. In Vitro Study
2.2.1. Platelet Preparation
2.2.2. Platelet Aggregation
2.2.3. Serum and Platelet TxB2 Production
2.2.4. Plasma and Platelet sP-Selectin Levels
2.2.5. Plasma and Platelets Soluble CD40 Ligand Levels
2.2.6. Serum and Platelet sNOX2-dp
2.2.7. Serum and Platelets H2O2 Production
2.2.8. Determination of % HBA
2.2.9. Thrombus Formation
2.2.10. Statistical Analysis
3. Results
3.1. In Vivo Study
3.1.1. Oxidative Stress Evaluation
3.1.2. Platelet Function
3.1.3. Linear Correlation
3.1.4. Thrombus Formation
3.2. In Vitro Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3047. [Google Scholar] [CrossRef] [PubMed]
- Tancredi, M.; Rosengren, A.; Svensson, A.M.; Kosiborod, M.; Pivodic, A.; Gudbjornsdottir, S.; Wedel, H.; Clements, M.; Dahlqvist, S.; Lind, M. Excess Mortality among Persons with Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Marzotti, S.; Guglielmini, G.; Momi, S.; Giannini, S.; Minuz, P.; Lucidi, P.; Bolli, G.B. Hyperglycemia-induced platelet activation in type 2 diabetes is resistant to aspirin but not to a nitric oxide-donating agent. Diabetes Care 2010, 33, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Nusca, A.; Tuccinardi, D.; Proscia, C.; Melfi, R.; Manfrini, S.; Nicolucci, A.; Ceriello, A.; Pozzilli, P.; Ussia, G.P.; Grigioni, F.; et al. Incremental role of glycaemic variability over HbA1c in identifying type 2 diabetic patients with high platelet reactivity undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 2019, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Adjedj, J.; Varenne, O. Diabetes Mellitus, a prothrombotic disease. Ann. Cardiol. Angeiol. 2017, 66, 385–392. [Google Scholar] [CrossRef]
- Cangemi, R.; Pignatelli, P.; Carnevale, R.; Nigro, C.; Proietti, M.; Angelico, F.; Lauro, D.; Basili, S.; Violi, F. Platelet isoprostane overproduction in diabetic patients treated with aspirin. Diabetes 2012, 61, 1626–1632. [Google Scholar] [CrossRef]
- Carnevale, R.; Loffredo, L.; Sanguigni, V.; Plebani, A.; Rossi, P.; Pignata, C.; Martire, B.; Finocchi, A.; Pietrogrande, M.C.; Azzari, C.; et al. Different degrees of NADPH oxidase 2 regulation and in vivo platelet activation: Lesson from chronic granulomatous disease. J. Am. Heart Assoc. 2014, 3, e000920. [Google Scholar] [CrossRef]
- Matsushima, S.; Tsutsui, H.; Sadoshima, J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Med. 2014, 24, 202–205. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Ueno, M. Optimizing platelet inhibition in clopidogrel poor metabolizers: Therapeutic options and practical considerations. JACC Cardiovasc. Interv. 2011, 4, 411–414. [Google Scholar] [CrossRef]
- Dave, C.V.; Kim, S.C.; Goldfine, A.B.; Glynn, R.J.; Tong, A.; Patorno, E. Risk of Cardiovascular Outcomes in Patients with Type 2 Diabetes after Addition of SGLT2 Inhibitors versus Sulfonylureas to Baseline GLP-1RA Therapy. Circulation 2021, 143, 770–779. [Google Scholar] [CrossRef]
- Lee, M.M.Y.; Kristensen, S.L.; Gerstein, H.C.; McMurray, J.J.V.; Sattar, N. Cardiovascular and mortality outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A meta-analysis with the FREEDOM cardiovascular outcomes trial. Diabetes Metab. Syndr. 2022, 16, 102382. [Google Scholar] [CrossRef] [PubMed]
- Cameron-Vendrig, A.; Reheman, A.; Siraj, M.A.; Xu, X.R.; Wang, Y.; Lei, X.; Afroze, T.; Shikatani, E.; El-Mounayri, O.; Noyan, H.; et al. Glucagon-Like Peptide 1 Receptor Activation Attenuates Platelet Aggregation and Thrombosis. Diabetes 2016, 65, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Esposito, K.; Giugliano, D.; Genovese, S. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013, 36, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Oeseburg, H.; de Boer, R.A.; Buikema, H.; van der Harst, P.; van Gilst, W.H.; Sillje, H.H. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.M.; Alsahli, M.; Gerich, J.E. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 104, 297–322. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013, 1, 140–151. [Google Scholar] [CrossRef]
- Bailey, C.J.; Gross, J.L.; Pieters, A.; Bastien, A.; List, J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 2223–2233. [Google Scholar] [CrossRef]
- Roden, M.; Weng, J.; Eilbracht, J.; Delafont, B.; Kim, G.; Woerle, H.J.; Broedl, U.C.; EMPA-REG MONO Trial Investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013, 1, 208–219. [Google Scholar] [CrossRef]
- Stenlof, K.; Cefalu, W.T.; Kim, K.A.; Alba, M.; Usiskin, K.; Tong, C.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes. Metab. 2013, 15, 372–382. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Lescano, C.H.; Leonardi, G.; Torres, P.H.P.; Amaral, T.N.; de Freitas Filho, L.H.; Antunes, E.; Vicente, C.P.; Anhe, G.F.; Monica, F.Z. The sodium-glucose cotransporter-2 (SGLT2) inhibitors synergize with nitric oxide and prostacyclin to reduce human platelet activation. Biochem. Pharmacol. 2020, 182, 114276. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Lee, M.K.; Al-Sharea, A.; Dragoljevic, D.; Barrett, T.J.; Montenont, E.; Basu, D.; Heywood, S.; Kammoun, H.L.; Flynn, M.; et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J. Clin. Investig. 2017, 127, 2133–2147. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. (2) Classification and diagnosis of diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef]
- Pignatelli, P.; Carnevale, R.; Cangemi, R.; Loffredo, L.; Sanguigni, V.; Stefanutti, C.; Basili, S.; Violi, F. Atorvastatin inhibits gp91phox circulating levels in patients with hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 360–367. [Google Scholar] [CrossRef]
- Born, G.V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Pastori, D.; Pignatelli, P.; Sanguigni, V.; Violi, F.; Carnevale, R. A novel role of MMP2 in regulating platelet NOX2 activation. Free Radic. Biol. Med. 2020, 152, 355–362. [Google Scholar] [CrossRef]
- Kaikita, K.; Hosokawa, K.; Dahlen, J.R.; Tsujita, K. Total Thrombus-Formation Analysis System (T-TAS): Clinical Application of Quantitative Analysis of Thrombus Formation in Cardiovascular Disease. Thromb. Haemost. 2019, 119, 1554–1562. [Google Scholar] [CrossRef] [Green Version]
- Kohlmorgen, C.; Gerfer, S.; Feldmann, K.; Twarock, S.; Hartwig, S.; Lehr, S.; Klier, M.; Kruger, I.; Helten, C.; Keul, P.; et al. Dapagliflozin reduces thrombin generation and platelet activation: Implications for cardiovascular risk reduction in type 2 diabetes mellitus. Diabetologia 2021, 64, 1834–1849. [Google Scholar] [CrossRef] [PubMed]
- Spigoni, V.; Fantuzzi, F.; Carubbi, C.; Pozzi, G.; Masselli, E.; Gobbi, G.; Solini, A.; Bonadonna, R.C.; Dei Cas, A. Sodium-glucose cotransporter 2 inhibitors antagonize lipotoxicity in human myeloid angiogenic cells and ADP-dependent activation in human platelets: Potential relevance to prevention of cardiovascular events. Cardiovasc. Diabetol. 2020, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Krotz, F.; Sohn, H.Y.; Pohl, U. Reactive oxygen species: Players in the platelet game. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1988–1996. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Batzias, K.; Antonopoulos, A.S.; Oikonomou, E.; Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Mistakidi, C.V.; Noutsou, M.; Katsiki, N.; Karopoulos, P.; et al. Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2018, 2018, 1232583. [Google Scholar] [CrossRef]
- Sposito, A.C.; Breder, I.; Soares, A.A.S.; Kimura-Medorima, S.T.; Munhoz, D.B.; Cintra, R.M.R.; Bonilha, I.; Oliveira, D.C.; Breder, J.C.; Cavalcante, P.; et al. Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: A randomized active-controlled trial. Cardiovasc. Diabetol. 2021, 20, 74. [Google Scholar] [CrossRef]
- Avila, C.; Huang, R.J.; Stevens, M.V.; Aponte, A.M.; Tripodi, D.; Kim, K.Y.; Sack, M.N. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp. Clin. Endocrinol. Diabetes 2012, 120, 248–251. [Google Scholar] [CrossRef]




| GLP1-RA Group A (n = 16) | Gliflozins Group B (n = 16) | p-Value | |
|---|---|---|---|
| Age (years) | 59.9 ± 10.2 | 57.5 ± 5.7 | 0.432 |
| Women (n, %) | 4, 25.0% | 3, 18.8% | 0.669 |
| Diabetes duration (years) | 4.0 [1.0–7.0] | 4.5 [3.0–9.0] | 0.426 |
| BMI (kg/m2) | 29.9 ± 4.2 | 30.7 ± 4.7 | 0.597 |
| Smoking (n, %) | 4, 25.0% | 4, 25.0% | - |
| Arterial hypertension (n, %) | 10, 62.5% | 11, 68.8% | 0.710 |
| Statin (n, %) | 11, 68.8% | 11, 68.8% | - |
| Acetylsalicylic acid (n, %) | 4, 25.0% | 3, 18.8% | 0.669 |
| Metformin Dose (g) | 2.0 ± 0.2 | 1.8 ± 0.6 | 0.317 |
| Glycaemia (mg/dL) | 150.0 [123.0–167.7] | 122.2 [108.7–161.0] | 0.184 |
| Hb1Ac (%) | 7.7 [7.0–8.7] | 6.9 [5.9–7.8] | 0.023 |
| Total Cholesterol (mg/dL) | 197.0 [157.5–217.0] | 175.0 [134.0–193.0] | 0.123 |
| HDL-c (mg/dL) | 48.5 [38.7–60.2] | 43.0 [36.0–51.0] | 0.102 |
| Triglycerides (mg/dL) | 129.5 [97.5–172.0] | 162.0 [103.0–190.0] | 0.310 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignatelli, P.; Baratta, F.; Buzzetti, R.; D’Amico, A.; Castellani, V.; Bartimoccia, S.; Siena, A.; D’Onofrio, L.; Maddaloni, E.; Pingitore, A.; et al. The Sodium–Glucose Co-Transporter-2 (SGLT2) Inhibitors Reduce Platelet Activation and Thrombus Formation by Lowering NOX2-Related Oxidative Stress: A Pilot Study. Antioxidants 2022, 11, 1878. https://doi.org/10.3390/antiox11101878
Pignatelli P, Baratta F, Buzzetti R, D’Amico A, Castellani V, Bartimoccia S, Siena A, D’Onofrio L, Maddaloni E, Pingitore A, et al. The Sodium–Glucose Co-Transporter-2 (SGLT2) Inhibitors Reduce Platelet Activation and Thrombus Formation by Lowering NOX2-Related Oxidative Stress: A Pilot Study. Antioxidants. 2022; 11(10):1878. https://doi.org/10.3390/antiox11101878
Chicago/Turabian StylePignatelli, Pasquale, Francesco Baratta, Raffaella Buzzetti, Alessandra D’Amico, Valentina Castellani, Simona Bartimoccia, Antonio Siena, Luca D’Onofrio, Ernesto Maddaloni, Annachiara Pingitore, and et al. 2022. "The Sodium–Glucose Co-Transporter-2 (SGLT2) Inhibitors Reduce Platelet Activation and Thrombus Formation by Lowering NOX2-Related Oxidative Stress: A Pilot Study" Antioxidants 11, no. 10: 1878. https://doi.org/10.3390/antiox11101878