Low HDL Cholesterol Efflux Capacity Indicates a Fatal Course of COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Laboratory Measurements
2.3. Preparation of apoB-Depleted Serum
2.4. Cholesterol Efflux Capacity
2.5. LCAT Activity
2.6. Arylesterase Activity of Paraoxonase
2.7. Anti-Oxidative Capacity
2.8. Anti-Inflammatory Activity by Inhibition of NFkB Expression
2.9. Quantification of Serum Amyloid A (SAA)
2.10. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Study Cohort
3.2. COVID-19 Is Associated with Alterations in HDL Metabolism and Parameters of HDL Function
3.3. HDL-Related Parameters and Treatment in COVID-19 Patients
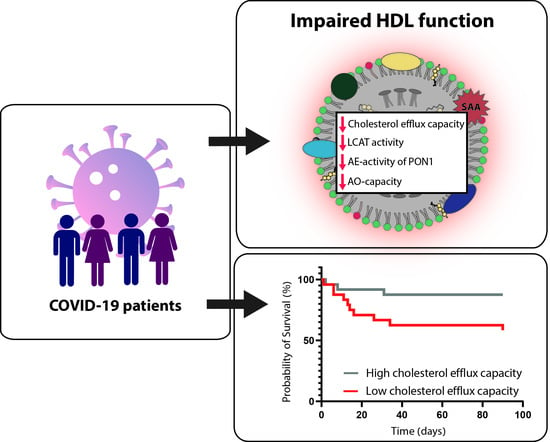
3.4. HDL Cholesterol Efflux Capacity Is Inversely Associated with Mortality Risk in COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Barter, P.J.; Rosenson, R.S.; Boden, W.E.; Chapman, M.J.; Cuchel, M.; D’Agostino, R.B.; Davidson, M.H.; Davidson, W.S.; Heinecke, J.W.; et al. High-Density Lipoproteins: A Consensus Statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 484–525. [Google Scholar] [CrossRef] [PubMed]
- Hewing, B.; Moore, K.J.; Fisher, E.A. HDL and Cardiovascular Risk. Circ. Res. 2012, 111, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, C.; Bizkarguenaga, M.; Gil-Redondo, R.; Diercks, T.; Arana, E.; García de Vicuña, A.; Seco, M.; Bosch, A.; Palazón, A.; San Juan, I.; et al. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience 2020, 23, 101645. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, D.; Wu, L.; He, G.; Ye, W. Declined Serum High Density Lipoprotein Cholesterol Is Associated with the Severity of COVID-19 Infection. Clin. Chim. Acta 2020, 510, 105–110. [Google Scholar] [CrossRef]
- Wei, X.; Zeng, W.; Su, J.; Wan, H.; Yu, X.; Cao, X.; Tan, W.; Wang, H. Hypolipidemia Is Associated with the Severity of COVID-19. J. Clin. Lipidol. 2020, 14, 297–304. [Google Scholar] [CrossRef]
- Tanaka, S.; De Tymowski, C.; Assadi, M.; Zappella, N.; Jean-Baptiste, S.; Robert, T.; Peoc’h, K.; Lortat-Jacob, B.; Fontaine, L.; Bouzid, D.; et al. Lipoprotein Concentrations over Time in the Intensive Care Unit COVID-19 Patients: Results from the ApoCOVID Study. PLoS ONE 2020, 15, e0239573. [Google Scholar] [CrossRef]
- Wang, G.; Deng, J.; Li, J.; Wu, C.; Dong, H.; Wu, S.; Zhong, Y. The Role of High-Density Lipoprotein in COVID-19. Front. Pharm. 2021, 12, 720283. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.-D.; Wang, J.; Wang, Y. Association Analysis Framework of Genetic and Exposure Risks for COVID-19 in Middle-Aged and Elderly Adults. Mech. Ageing Dev. 2021, 194, 111433. [Google Scholar] [CrossRef]
- Jin, H.; He, J.; Dong, C.; Li, B.; Ma, Z.; Li, B.; Huang, T.; Fan, J.; He, G.; Zhao, X. Altered Lipid Profile Is a Risk Factor for the Poor Progression of COVID-19: From Two Retrospective Cohorts. Front. Cell. Infect. Microbiol. 2021, 11, 712530. [Google Scholar] [CrossRef]
- Aung, N.; Khanji, M.Y.; Munroe, P.B.; Petersen, S.E. Causal Inference for Genetic Obesity, Cardiometabolic Profile and COVID-19 Susceptibility: A Mendelian Randomization Study. Front. Genet. 2020, 11, 586308. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, V.; Kumar, A.; Majella, M.G.; Seth, B.; Sivakumar, R.K.; Voruganti, D.; Bavineni, M.; Baghal, A.; Gates, K.; Kumari, A.; et al. HDL Cholesterol Levels and Susceptibility to COVID-19. eBioMedicine 2022, 82, 104166. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.; Celis-Morales, C.A.; Gray, S.R.; Katikireddi, S.V.; Niedzwiedz, C.L.; Hastie, C.; Ferguson, L.D.; Berry, C.; Mackay, D.F.; Gill, J.M.; et al. Modifiable and Non-Modifiable Risk Factors for COVID-19, and Comparison to Risk Factors for Influenza and Pneumonia: Results from a UK Biobank Prospective Cohort Study. BMJ Open 2020, 10, e040402. [Google Scholar] [CrossRef] [PubMed]
- Trakaki, A.; Marsche, G. Current Understanding of the Immunomodulatory Activities of High-Density Lipoproteins. Biomedicines 2021, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. HDL in Infectious Diseases and Sepsis. Handb. Exp. Pharm. 2015, 224, 483–508. [Google Scholar] [CrossRef]
- Norata, G.D.; Pirillo, A.; Catapano, A.L. HDLs, Immunity, and Atherosclerosis. Curr. Opin. Lipidol. 2011, 22, 410–416. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Pavel, M.A.; Jablonski, S.M.; Jablonski, J.; Hobson, R.; Valente, S.; Reddy, C.B.; Hansen, S.B. The Role of High Cholesterol in Age-Related COVID19 Lethality. bioRxiv 2021. [Google Scholar] [CrossRef]
- Palacios-Rápalo, S.N.; De Jesús-González, L.A.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; Martínez-Mier, G.; Quistián-Galván, J.; Muñoz-Pérez, A.; Bernal-Dolores, V.; del Ángel, R.M.; et al. Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry. Front. Immunol. 2021, 12, 796855. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in Innate and Adaptive Immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef]
- Guo, L.; Ai, J.; Zheng, Z.; Howatt, D.A.; Daugherty, A.; Huang, B.; Li, X.-A. High Density Lipoprotein Protects against Polymicrobe-Induced Sepsis in Mice*. J. Biol. Chem. 2013, 288, 17947–17953. [Google Scholar] [CrossRef] [Green Version]
- Holzer, M.; Schilcher, G.; Curcic, S.; Trieb, M.; Ljubojevic, S.; Stojakovic, T.; Scharnagl, H.; Kopecky, C.M.; Rosenkranz, A.R.; Heinemann, A.; et al. Dialysis Modalities and HDL Composition and Function. J. Am. Soc. Nephrol. 2015, 26, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Trieb, M.; Horvath, A.; Birner-Gruenberger, R.; Spindelboeck, W.; Stadlbauer, V.; Taschler, U.; Curcic, S.; Stauber, R.E.; Holzer, M.; Pasterk, L.; et al. Liver Disease Alters High-Density Lipoprotein Composition, Metabolism and Function. Biochim. Biophys. Acta 2016, 1861, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Wolf, P.; Curcic, S.; Birner-Gruenberger, R.; Weger, W.; Inzinger, M.; El-Gamal, D.; Wadsack, C.; Heinemann, A.; Marsche, G. Psoriasis Alters HDL Composition and Cholesterol Efflux Capacity. J. Lipid Res. 2012, 53, 1618–1624. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2019, 26, 1644–1664. [Google Scholar] [CrossRef]
- Chiesa, S.T.; Charakida, M. High-Density Lipoprotein Function and Dysfunction in Health and Disease. Cardiovasc. Drugs Ther. 2019, 33, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Souza Junior, D.R.; Silva, A.R.M.; Rosa-Fernandes, L.; Reis, L.R.; Alexandria, G.; Bhosale, S.D.; de Rose Ghilardi, F.; Dalçóquio, T.F.; Bertolin, A.J.; Nicolau, J.C.; et al. HDL Proteome Remodeling Associates with COVID-19 Severity. J. Clin. Lipidol. 2021, 15, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Begue, F.; Tanaka, S.; Mouktadi, Z.; Rondeau, P.; Veeren, B.; Diotel, N.; Tran-Dinh, A.; Robert, T.; Vélia, E.; Mavingui, P.; et al. Altered High-Density Lipoprotein Composition and Functions during Severe COVID-19. Sci. Rep. 2021, 11, 2291. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Stadler, J.T.; Lackner, S.; Mörkl, S.; Trakaki, A.; Scharnagl, H.; Borenich, A.; Wonisch, W.; Mangge, H.; Zelzer, S.; Meier-Allard, N.; et al. Obesity Affects HDL Metabolism, Composition and Subclass Distribution. Biomedicines 2021, 9, 242. [Google Scholar] [CrossRef]
- Trakaki, A.; Sturm, G.J.; Pregartner, G.; Scharnagl, H.; Eichmann, T.O.; Trieb, M.; Knuplez, E.; Holzer, M.; Stadler, J.T.; Heinemann, A.; et al. Allergic Rhinitis Is Associated with Complex Alterations in High-Density Lipoprotein Composition and Function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Norum, K.R.; Remaley, A.T.; Miettinen, H.E.; Strøm, E.H.; Balbo, B.E.P.; Sampaio, C.A.T.L.; Wiig, I.; Kuivenhoven, J.A.; Calabresi, L.; Tesmer, J.J.; et al. Lecithin:Cholesterol Acyltransferase: Symposium on 50 Years of Biomedical Research from Its Discovery to Latest Findings. J. Lipid Res. 2020, 61, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Kunnen, S.; Van Eck, M. Lecithin:Cholesterol Acyltransferase: Old Friend or Foe in Atherosclerosis? J. Lipid Res. 2012, 53, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Anastasius, M.; Kockx, M.; Jessup, W.; Sullivan, D.; Rye, K.-A.; Kritharides, L. Cholesterol Efflux Capacity: An Introduction for Clinicians. Am. Heart J. 2016, 180, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, A.C.; Schuller, M.; Holzer, M.; Stadler, J.T.; Hackl, G.; Posch, F.; Marsche, G.; Sourij, H.; Ekart, R.; Eller, K.; et al. Arylesterase Activity of HDL Associated Paraoxonase as a Potential Prognostic Marker in Patients with Sepsis and Septic Shock—A Prospective Pilot Study. Front. Med. 2020, 7, 579677. [Google Scholar] [CrossRef]
- Ripa, I.; Andreu, S.; López-Guerrero, J.A.; Bello-Morales, R. Membrane Rafts: Portals for Viral Entry. Front. Microbiol. 2021, 12, 631274. [Google Scholar] [CrossRef]
- Glende, J.; Schwegmann-Wessels, C.; Al-Falah, M.; Pfefferle, S.; Qu, X.; Deng, H.; Drosten, C.; Naim, H.Y.; Herrler, G. Importance of Cholesterol-Rich Membrane Microdomains in the Interaction of the S Protein of SARS-Coronavirus with the Cellular Receptor Angiotensin-Converting Enzyme 2. Virology 2008, 381, 215–221. [Google Scholar] [CrossRef]
- Tall, A.R. Cholesterol Efflux Pathways and Other Potential Mechanisms Involved in the Athero-Protective Effect of High Density Lipoproteins. J. Intern. Med. 2008, 263, 256–273. [Google Scholar] [CrossRef]
- Tanaka, S.; Begue, F.; Veeren, B.; Tran-Dinh, A.; Robert, T.; Tashk, P.; Lortat-Jacob, B.; Faille, D.; de Chaisemartin, L.; Zappella, N.; et al. First Recombinant High-Density Lipoprotein Particles Administration in a Severe ICU COVID-19 Patient, a Multi-Omics Exploratory Investigation. Biomedicines 2022, 10, 754. [Google Scholar] [CrossRef]
- Van Lenten, B.J.; Hama, S.Y.; de Beer, F.C.; Stafforini, D.M.; McIntyre, T.M.; Prescott, S.M.; La Du, B.N.; Fogelman, A.M.; Navab, M. Anti-Inflammatory HDL Becomes pro-Inflammatory during the Acute Phase Response. Loss of Protective Effect of HDL against LDL Oxidation in Aortic Wall Cell Cocultures. J. Clin. Investig. 1995, 96, 2758–2767. [Google Scholar] [CrossRef]
- Untersteller, K.; Meissl, S.; Trieb, M.; Emrich, I.E.; Zawada, A.M.; Holzer, M.; Knuplez, E.; Fliser, D.; Heine, G.H.; Marsche, G. HDL Functionality and Cardiovascular Outcome among Nondialysis Chronic Kidney Disease Patients. J. Lipid Res. 2018, 59, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Draganov, D.; Teiber, J.; Watson, C.; Bisgaier, C.; Nemzek, J.; Remick, D.; Standiford, T.; La Du, B. PON1 and Oxidative Stress in Human Sepsis and an Animal Model of Sepsis. Adv. Exp. Med. Biol. 2010, 660, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Teiber, J.F.; Horke, S.; Haines, D.C.; Chowdhary, P.K.; Xiao, J.; Kramer, G.L.; Haley, R.W.; Draganov, D.I. Dominant Role of Paraoxonases in Inactivation of the Pseudomonas Aeruginosa Quorum-Sensing Signal N-(3-Oxododecanoyl)-L-Homoserine Lactone. Infect. Immun. 2008, 76, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Ozer, E.A.; Pezzulo, A.; Shih, D.M.; Chun, C.; Furlong, C.; Lusis, A.J.; Greenberg, E.P.; Zabner, J. Human and Murine Paraoxonase 1 Are Host Modulators of Pseudomonas Aeruginosa Quorum-Sensing. FEMS Microbiol. Lett. 2005, 253, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cabana, V.G.; Siegel, J.N.; Sabesin, S.M. Effects of the Acute Phase Response on the Concentration and Density Distribution of Plasma Lipids and Apolipoproteins. J. Lipid Res. 1989, 30, 39–49. [Google Scholar] [CrossRef]
- Cabana, V.G.; Lukens, J.R.; Rice, K.S.; Hawkins, T.J.; Getz, G.S. HDL Content and Composition in Acute Phase Response in Three Species: Triglyceride Enrichment of HDL a Factor in Its Decrease. J. Lipid Res. 1996, 37, 2662–2674. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, Z.; Ai, J.; Huang, B.; Li, X.-A. Hepatic Scavenger Receptor BI Protects against Polymicrobial-Induced Sepsis through Promoting LPS Clearance in Mice. J. Biol. Chem. 2014, 289, 14666–14673. [Google Scholar] [CrossRef]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-Density Lipoprotein Binding to Scavenger Receptor-BI Activates Endothelial Nitric Oxide Synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef]
- Cockerill, G.W.; Rye, K.A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. High-Density Lipoproteins Inhibit Cytokine-Induced Expression of Endothelial Cell Adhesion Molecules. Arter. Thromb. Vasc. Biol. 1995, 15, 1987–1994. [Google Scholar] [CrossRef]
- Calabresi, L.; Franceschini, G.; Sirtori, C.R.; De Palma, A.; Saresella, M.; Ferrante, P.; Taramelli, D. Inhibition of VCAM-1 Expression in Endothelial Cells by Reconstituted High Density Lipoproteins. Biochem. Biophys. Res. Commun. 1997, 238, 61–65. [Google Scholar] [CrossRef]
- Bursill, C.A.; Castro, M.L.; Beattie, D.T.; Nakhla, S.; van der Vorst, E.; Heather, A.K.; Barter, P.J.; Rye, K.-A. High-Density Lipoproteins Suppress Chemokines and Chemokine Receptors in Vitro and in Vivo. Arter. Thromb. Vasc. Biol. 2010, 30, 1773–1778. [Google Scholar] [CrossRef] [Green Version]





| COVID-19 Patients (n = 48) | Non-COVID Pneumonia Patients (n = 32) | p-Value | |
|---|---|---|---|
| Age (years) | 68 (56–80) | 74 (53–81) | 0.529 |
| Female sex | 25 (52%) | 18 (56%) | 0.714 |
| Total cholesterol (mg/dL) | 158 (126–197) | 205 (162–244) | 0.004 |
| LDL-cholesterol | 89 (65–117) | 129 (93–160) | 0.005 |
| HDL-cholesterol (mg/dL) | 23.9 (17.6–38.6) | 53.0 (27.4–66.4) | <0.001 |
| Triglycerides (mg/dL) | 147 (101–228) | 138 (79–184) | 0.137 |
| CRP (mg/L) | 35.4 (12.3–77.0) | 52.4 (4.6–91.9) | 0.932 |
| IL-6 (ng/L) | 38.0 (15.7–116.0) | 42.1 (7.1–97.0) | 0.415 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadler, J.T.; Mangge, H.; Rani, A.; Curcic, P.; Herrmann, M.; Prüller, F.; Marsche, G. Low HDL Cholesterol Efflux Capacity Indicates a Fatal Course of COVID-19. Antioxidants 2022, 11, 1858. https://doi.org/10.3390/antiox11101858
Stadler JT, Mangge H, Rani A, Curcic P, Herrmann M, Prüller F, Marsche G. Low HDL Cholesterol Efflux Capacity Indicates a Fatal Course of COVID-19. Antioxidants. 2022; 11(10):1858. https://doi.org/10.3390/antiox11101858
Chicago/Turabian StyleStadler, Julia T., Harald Mangge, Alankrita Rani, Pero Curcic, Markus Herrmann, Florian Prüller, and Gunther Marsche. 2022. "Low HDL Cholesterol Efflux Capacity Indicates a Fatal Course of COVID-19" Antioxidants 11, no. 10: 1858. https://doi.org/10.3390/antiox11101858